Clinical characteristics and outcomes after new-onset seizure among Zambian children with HIV during the antiretroviral therapy era
Abstract
Objective
This study describes clinical profiles including human immunodeficiency virus (HIV) disease history and seizure etiology among children living with HIV presenting with new-onset seizure during the era of antiretroviral therapy (ART) in Zambia. 30-day mortality and cause of death are also reported.
Methods
Children living with HIV (CLWHIV) with new-onset seizures were prospectively evaluated at one large urban teaching hospital and two non-urban healthcare facilities. Interviews with family members, review of medical records, and where needed, verbal autopsies were undertaken. Two clinicians who were not responsible for the patients' care independently reviewed all records and assigned seizure etiology and cause of death with adjudication as needed.
Results
From April 2016 to June 2019, 73 children (49 urban, 24 rural) were identified. Median age was 6 years (IQR 2.2-10.0) and 39 (53%) were male children. Seizures were focal in 36 (49%) and were often severe, with 37% presenting with multiple recurrent seizures in the 24 hours before admission or in status epilepticus. Although 36 (49%) were on ART at enrollment, only 7 of 36 (19%) were virally suppressed. Seizure etiologies were infectious in over half (54%), with HIV encephalitis, bacterial meningitis, and tuberculous meningitis being the most common. Metabolic causes (19%) included renal failure and hypoglycemia. Structural lesions identified on imaging accounted for 10% of etiologies and included stroke and non-accidental trauma. No etiology could be identified in 12 (16%) children, most of whom died before the completion of clinical investigations. Twenty-two (30%) children died within 30 days of the index seizure.
Significance
Despite widespread ART roll out in Zambia, new-onset seizure in CLWHIV occurs in the setting of advanced, active HIV disease. Seizure severity/burden is high as is early mortality. Enhanced programs to assure early ART initiation, improve adherence, and address ART failure are needed to reduce the burden of neurological injury and premature death in CLWHIV.
Key points
- Despite widely available ART and PMCTC programs in Zambia, new-onset seizure in children with HIV continues to occur primarily as a manifestation of advanced HIV disease.
- Seizure burden, in terms of seizure severity at presentation, is high and common seizure etiologies include CNS OIs and metabolic abnormalities.
- CLWHIV with new-onset seizure suffer from high 30-day mortality rates.
- Programs to support ART access and adherence, especially for vulnerable children such as orphans and those living in poor households, are needed to improve the neurological health and well-being of CLWHIV in Zambia.
1 INTRODUCTION
Underlying neurological disease in people living with human immunodeficiency virus (PLWHIV) often manifests as a new-onset seizure, especially prior to widespread availability of antiretroviral therapies (ART). Between 1993 and 2003, among Indian populations with HIV, 20% of adults with neurological disorders related to advanced stages of immunosuppression presented with new-onset seizure.1 Early in the HIV pandemic, seizures were commonly identified as part of the clinical phenomenon of severe immunosuppression though often without a clear underlying etiology.2, 3 Common seizure etiologies in this pre-ART era included opportunistic infections (OI),4, 5 other systemic illnesses secondary to HIV, adverse drug reactions, and metabolic derangements.6
The common co-occurrence of seizures in PLWHIV extend to children as well.7, 8 Similar to adults, children with HIV (CLWHIV) are also at risk of seizures secondary to OIs9 and stroke.10 Per the UNAIDS 2020 report, the incidence of CLWHIV (aged 0-14 years) in 2019 accounted for 150 000 (9%) of the estimated total 1.7 million cases worldwide. Of those children, 84% of the cases originated from sub-Saharan Africa.11 In this region, expeditious diagnosis and treatment of new-onset seizures may be challenging due to limited resources for diagnostic assessments and lack of clear guidance on long-term management.10, 12
Treating seizures in PLWHIV is particularly hazardous in resource-limited African settings, where enzyme-inducing antiseizure medications (EI-ASMs) are heavily relied on for epilepsy management. The co-usage of EI-ASM with ART is not recommended due to concerns of drug-drug interactions, which may lead to toxicity and/or failure of either agent.10, 13-15 As seizures in PLWHIV are strongly associated with advanced stages of HIV disease, early ART initiation is likely critical to avoiding this conundrum.7
Antiretroviral therapy rolled out nationwide in Zambia in 2005, and today, HIV testing and treatment programs utilizing “test and treat” policies and including programs for the prevention of mother to child transmission (PMTCT) are ubiquitous. To examine clinical characteristics and outcomes of new onset seizures in CLWHIV during this ART era, we identified CLWHIV with new-onset seizures in both urban and rural settings in Zambia. We examined clinical and demographic data including HIV treatment history, seizure etiology, semiology, and seizure severity. Lastly, due to identification of the high burden of early mortality, 30-day cause of death was also assessed.
2 METHODS
2.1 Study sites
This research was completed as part of the Cohort of HIV-Associated Seizure and Epilepsy Study. From April 12, 2016 to June 30, 2019, CLWHIV presenting with new-onset seizures were prospectively identified at Zambia's University Teaching Hospitals (UTH) in the capital city, Lusaka, and at three non-urban sites (referred to collectively as Rural Health Center [RHC])—a rural faith-based hospital (Chikankata Hospital), a peri-urban, faith-based hospital (Monze Mission Hospital), and an employer-sponsored hospital (Nakambala Hospital) established to provide care for factory workers and their families. At UTH, screening for eligible patients was undertaken in the Pediatric Emergency Department, Pediatric Intensive Care Unit, and on the General Pediatric inpatient services including the malnutrition unit. At the non-urban hospitals, research staff screened for potential participants through the outpatient filter clinics, Accident and Emergency Departments, and by review of new admissions to the hospital at least 6 days per week during the enrollment period. At all enrolling study sites, acute seizure treatment options were limited to benzodiazepines and phenobarbital without recourse to intubation or ventilation.
University Teaching Hospitals is a tertiary care, teaching hospital, and within this facility, all clinicians were physicians, including pediatricians and postgraduate registrars training in pediatrics. At the rural sites, healthcare providers were physicians, medical licentiates, and clinical officers. The level of clinician expertise varies across the type of facility in Zambia, and the expertise within the study site facilities were typical of hospitals in Zambia meaning other tertiary care facilities would have similar expertise to UTH and other rural hospitals would have similar staffing to those at the rural study sites.
2.2 Inclusion criteria and data collection
Children were identified as potential participants based upon presentation with an event considered to be possible seizure. Inclusion criteria were as follows: (1) 1 month-<18 years of age, (2) HIV+, (3) having experienced the index seizure <6 weeks prior to screening, and (4) written consent from parent/guardian. For children under 1 year of age, readily available HIV antibody tests were used for initial screening and inclusion with HIV PCR studies sent as well. When these were available (~1 month later), infants enrolled based upon a positive antibody test but found to be HIV PCR negative were withdrawn from the study. Where appropriate, assent was sought from enrolled children after recovery from their acute illness. Ethics approvals were granted by the University of Zambia's Biomedical Research Ethics Committee and the appropriate associated US institutions. If HIV status was not documented, HIV testing was offered as part of screening.
All care for enrolled children was delivered by the healthcare providers routinely caring for such patients within the enrolling facilities. To optimize diagnostic evaluations, the research team assured consumables for routine care were in stock throughout the enrollment period, including rapid malaria diagnostic tests, point-of-care glucose measures, Cryptococcal antigen (CrAg) test kits for serum and cerebrospinal fluid (CSF), and consumables for lumbar puncture completion. If CSF was obtained, extensive diagnostic evaluations not routinely available in the public sector were made available (detailed below). In the urban setting only, the costs of electroencephalographs (EEG) and neuroimaging studies were covered by the research budget if these studies were requested by the care team. Imaging and EEG were not available to patients outside of Lusaka.
Structured data collection tools captured HIV treatment history, World Health Organization (WHO) HIV stage at enrollment, epilepsy history in first-degree relatives, any seizure provocations or current illnesses at presentation (including central nervous system [CNS] infections, metabolic derangements), seizure semiology, and seizure severity. An index seizure was deemed focal if the child had one or more of the following: (1) a focal neurological examination at presentation that fluctuated with recovery from the seizure, (2) focal seizure semiology by patient or witness description, or (3) focal EEG findings. To complement the basic diagnostic evaluations available at each site, expanded neurodiagnostics included venereal disease research laboratory (VDRL), rapid plasma reagin (RPR) and Treponema pallidum hemagglutination assays, CSF studies including for tuberculosis (TB) GeneXpert and cultures, cryptococcal antigen tests (serum and CSF), and polymerase chain reaction (PCR) studies for Cytomegalovirus (CMV), JC virus (JCV), Herpes Simplex Virus 1 and 2 (HSV-1, HSV-2), Varicella Zoster Virus (VZV), Epstein Bar Virus (EBV) and Human Herpesvirus-6a and b (HHV-6A, HHV-6B). With “test and treat” strategies in place,16 children without previous plasma viral load assessment, but with parental consent, had this determined. Neuroimaging was performed on a Siemens 64 slice CT scanner or a Siemens 1.5T MRI with standard clinical reports for care purposes and detailed structured research reports using NeuroInterp17 provided by neuroradiologists. Except for neuroimaging, data were collected on paper forms and with dual data entry into Research Electronic Data Capture hosted at the University of Rochester Medical Center.18, 19
2.3 Seizure etiology and cause of death outcomes
Two clinicians (GLB, CMB) with full access to all clinical and demographic data independently assigned seizure etiologies and causes of death. Study participants were classified as having a CNS OI with either (1) positive CSF CrAg or serum CrAg with signs/symptoms of meningitis, (2) viral deoxyribonucleic acid (DNA) amplification of CSF PCR for an OI including CMV, JCV, HSV, EBV, HHV, and VZV, (3) TB meningitis via culture or GeneXpert, or (4) an invasive bacterial meningitis. For cases of meningitis, clinical signs were also used to determine acute infection. For seizure etiology, if more than one potential etiology was identified, the etiology more proximate to a brain-specific cause (ie, focal brain lesion) rather than a non-specific cause (eg, sepsis) was assigned. Given the lack of ventilator support and the reliance upon benzodiazepines and phenobarbital for acute seizure management, cause of death reviews included consideration of respiratory suppression from ASM as a possible cause of death with attention to doses and timing of ASMs relative to the time of death.
3 RESULTS
A total of 365 children were screened at UTH, with 290 deemed ineligible. See Figure 1 for enrollment details. Reasons for ineligibility were not mutually exclusive and included negative HIV status (184, 63%), event not new-onset seizure (83, 30%), a known history of epilepsy (38, 13%), age (29, 10%), a seizure onset time >6 weeks prior to screening (5, 2%) and death (4, 1%). Of the 108 eligible children, 8 (11%) parents declined to participate, 11 (15%) died before consent could be obtained, and 2 (3%) were lost to follow-up (absconded or were transferred to another unit of the hospital and untraceable). Fifty-four (72%) urban children were enrolled.
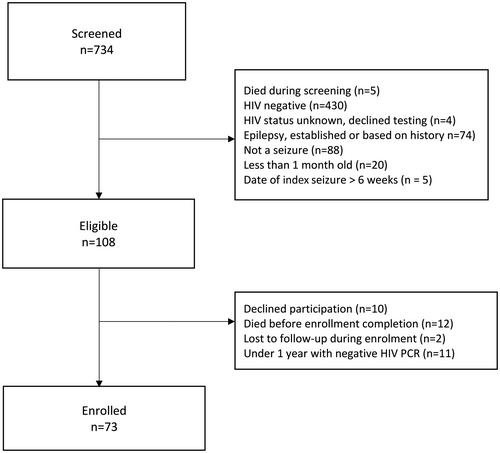
At the three non-urban sites, 369 children were screened, with 337 deemed ineligible. Reasons for ineligibility included negative HIV status (319, 95%), event not new-onset seizure (5, 1.5%), a known history of epilepsy (36, 11%), age (2, 0.6%) and death (1, 0.3%). Two (6%) of the 32 eligible children's parents declined to participate and 30 children were enrolled. After 6 months of screening, no eligible patients had been identified at Nakambala Hospital, with an external retrospective chart review confirming the lack of any children with HIV presenting with new-onset seizure, so this site was closed for enrollment. Across all sites, 11 consented infants with a positive HIV antibody test but subsequent negative DNA PCR were withdrawn. Among HIV-positive children screened for enrollment, 20% of the urban children and 72% of the rural children did not have new-onset seizure and instead were either known to have epilepsy or had epilepsy by history but without prior diagnosis and as such were excluded from enrollment. Ultimately, 49 urban and 24 non-urban children provided data for this analysis.
Demographic data for the 73 children are provided in Table 1. Median age was 6 years (IQR 2.2-10). Just over half (39, 53%) were male children. Several children were orphans being cared for by guardians other than parents and these individuals could only provide limited family and medical history. Prior to the index seizure, a quarter of children had a history of a CNS OI and 6 (8%) had a history of a prior CNS insult including traumatic brain injury, cerebral malaria, and/or stroke. Almost half of the index seizures were focal, and over a third were multiple or prolonged.
| Characteristic | Participant number |
|---|---|
| Age, years | |
| Median (IQR) | 6 (2.2-10) |
| Sex | |
| Male | 39 (53%) |
| Female | 34 (47%) |
| CD4 cell count | |
| CD4 T-cell counts available, n (%) | 64 (88) |
| CD4 Count Median (IQR)a | 410 (130-835) |
| CD4% available, n (%) | 54 (74) |
| CD4 <200, n (%) | 20 (31) |
| CDC HIV stage based on age-specific CD4 percentages among n = 54, n (%) | |
| Stage 1 | 14 (26) |
| Stage 2 | 15 (28) |
| Stage 3 | 25 (46) |
| WHO stage at enrollment among n = 73 | |
| Stage 1 | 14 (19%) |
| Stage 2 | 4 (5%) |
| Stage 3 | 11 (15%) |
| Stage 4 | 44 (60%) |
| Relevant history | |
| Time between HIV diagnosis and seizure in days n=72 | |
| Median (IQR) | 100 (0-1542) |
| Mean (SD) | 847 (1352) |
| Plasma viral load copies/mL Mean (SD)b | 152,824 (297,783) |
| Family history of epilepsyc | 10 (15%) |
| History of opportunistic infection | 18 (25%) |
| Pre-existing CNS insultsd | 6 (8%†) |
| Seizure characteristicse | |
| Focality | 36 (49%) |
| Brief | 18 (25%) |
| Multiple | 28 (38%) |
| Status epilepticus | 27 (37%) |
Note
- Considered focal if (1) a focal neurological exam at presentation that fluctuated with recovery from the seizure, (2) focal seizure semiology by patient or witness description, or (3) focal EEG findings.
- Abbreviations: CNS, Central Nervous System; IQR, Interquartile Range; SD, Standard Deviation.
- a Data available for n = 33 within 60 d of enrollment.
- b Data available for n = 54 within 60 d of enrollment.
- c Due to large number of orphaned children, family history was only available for 66 of the 75.
- d Represents history of traumatic brain injury, cerebral malaria, stroke, meningitis/encephalitis.
- e Brief: <5 min or a single seizure Multiple: 1-4 events or prolonged (>5-30 min) Status Epilepticus: more than 5 seizures and/or seizing for more than 30 min.
Over half of those enrolled (44, 60%) had WHO stage 4 HIV disease at the time of presentation. Less than half (36, 49%) of children enrolled were on ART. HIV treatment histories for these children are detailed in Table 2. Among those on ART, 19 (56%) had been on treatment for >1 year. Unfortunately, viral suppression (defined as having <200 copies per mL) was evident in only 7 children representing 19% of children on ART and 10% of the overall study population. For children on ART for >3 months without viral suppression, study staff reviewed ART clinic files and interviewed families to determine whether formal investigations for ART failure were warranted. Among children without viral suppression on ART for at least 3 months, adherence was universally very poor. Based upon review of ART clinic records and parental/guardian interviews, all of these children had prolonged and often recurrent periods in which ART were not collected and/or administered. Long-time intervals (median 182 days, mean 383 days) between HIV diagnosis and ART initiation were notable. ART regimen was varied, with 31% of children on efavirenz (EFV).
| Characteristics | Participant number |
|---|---|
| Admitted on ART | 36 (49%) |
| Current combined ART (cART) use | N = 34 |
| <2 mo | 6 (18%) |
| 2-6 mo | 4 (12%) |
| 6 mo–1 y | 5 (15%) |
| >1 y | 19 (56%) |
| Time between HIV diagnosis and ART initiation in days1 | N = 34 |
| Median (IQR) | 123 (0-182) |
| Mean (SD) | 309 (669) |
| Viral suppression for those on ART (n = 36) | 7 (19%) |
| cART regimen at enrollment | |
| TDF/3TC/EFV | 3 (8%) |
| ABC/3TC/LPV/r | 12 (33%) |
| ABC/3TC/EFV | 8 (22%) |
| ABC/3TC/NVP | 5 (14%) |
| ZDV/3TC/LPV/r | 4 (11%) |
| TDF/3TC/NVP | 2 (6%) |
| TDF/3TC/RAL/ATV | 1 (3%) |
| ZDV/3TC/NVP | 1 (3%) |
| Regimen with EFV | 11 (31%) |
- Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; cART, combined ART; EFV, efavirenz; LPV/r, lopinavir/ritonavir; NVP, nevirapine; RAL, raltegravir; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
- a Calculated based on ART initiation date and date of first seizure. Date of ART initiation was not available for two children.
Table 3 summarizes cause of death within 30 days of the index seizure. A total of 22 (30%) children died, and no children were lost to follow-up. Causes of death identified included advanced HIV, meningitis, sepsis, disseminated TB, and status epilepticus.
| Etiology | UTH (n = 17) | RHC ( n = 5) |
|---|---|---|
| Advanced HIV | – | 1 (20%) |
| Cryptococcal meningitis | 1 (6%) | – |
| Disseminated TB | 2 (11%) | – |
| Malnutrition | 1 (6%) | – |
| Meningitis | 1 (6%) | 1 (20%) |
| Pneumocystis Pneumonia | 1 (6%) | – |
| Transfusion reaction | 1 (6%) | – |
| TB meningitis | 1 (6%) | – |
| Renal failure | 2 (11%) | 1 (20%) |
| Sepsis | 2 (11%) | – |
| Status epilepticus | 3 (18%) | – |
| Steven-Johnson Syndrome | 1 (6%) | – |
| Super-refractory status | 1 (6%) | – |
| Unknown | – | 2 (40%) |
- Abbreviation: TB, Tuberculosis.
Table 4 outlines seizure etiologies stratified by urbanicity. Diagnostic evaluations were particularly limited in the non-urban settings with neuroimaging and EEG only available to urban children. Across all sites, lumbar puncture (LP) uptake was poor despite the assurance of supplies for LP completion and the availability of extensive, relevant diagnostic investigations for study participants. Only a third of children underwent lumbar puncture for CSF diagnostics. Neuroimaging was performed on 39 of 49 urban children, with 18 of 39 getting an MRI, 17 of 39 receiving a CT, and 4 of 39 receiving both. An EEG was obtained in 12 of 49 urban children.
| Seizure etiology | Total (n = 73) | UTH (n = 49) | RHC (n = 24) |
|---|---|---|---|
| Infectious | |||
| Cerebritis | 1 (1%) | 1 (2%) | – |
| CMV-Encephalitis | 2 (3%) | 2 (4%) | – |
| Cryptococcal Meningitis | 2 (3%) | 1 (2%) | 1 (4%) |
| HIV-Encephalitis | 8 (11%) | 8 (16%) | – |
| HIV-Vasculitis | 1 (1%) | 1 (2%) | – |
| Neurocysticercosis | 2 (3%) | 2 (4%) | – |
| PML | 2 (3%) | 2 (4%) | – |
| CNS TB | 7 (10%) | 5 (8%) | 2 (8%) |
| Toxoplasma Encephalitis | 1 (1%) | 1 (2%) | – |
| Meningitis | 10 (14%) | 7 (14%) | 3 (12%) |
| Malaria | 2 (3%) | – | 2 (8%) |
| Brain abscess | 1 (1%) | 1 (2%) | – |
| Total infections | 39 (54%) | 31 (63%) | 8 (33%) |
| Systemic | |||
| Renal failure | 4 (5%) | 3 (6%) | 1 (4%) |
| Sepsis | 1 (1%) | – | 1 (4%) |
| Parameningeal sinus infection | 1 (1%) | 1 (2%) | – |
| Fever (febrile seizure) | 5 (7%) | 1 (2%) | 4 (16%) |
| Hypoglycemia | 1 (1%) | 1 (2%) | – |
| Other metabolic abnormality | 2 (3%) | 2 (4%)b | – |
| Total systemic causes | 14 (19%) | 8 (16%) | 6 (24%) |
| Structural | |||
| Acute stroke | 3 (4%) | 3 (6%) | – |
| PRES | 1 (1%) | 1 (2%) | – |
| Cerebral palsy | 1 (1%) | 1 (2%) | – |
| Prior stroke | 1 (1%) | 1 (2%) | – |
| Traumatic brain injury | 2 (3%) | 2 (4%) | – |
| Total structural causes | 8 (11%) | 8 (16%) | – |
| Unknown | 12 (16%) | 2 (4%) | 10 (42%) |
- Abbreviations: CMV, cytomegalovirus; CNS, central nervous system; PML, progressive multifocal leukoencephalopathy; PRES, posterior reversible encephalopathy syndrome; TB, tuberculosis.
- a Only urban children had access to EEG and neuroimaging.
- b 1(2%) due to malnutrition, 1(2%) due to gastroenteritis.
Infectious etiologies were identified in 53% of the population, representing a combination of both opportunistic and non-opportunistic infections. Bacterial meningitis, tuberculous meningitis, and HIV encephalitis 1 were the most common infectious etiologies identified. Renal failure with frank uremia was present in 6% (3) of children in the urban setting where kidney function could be ascertained relatively easily. Renal failure occurred in the setting of both treated and untreated HIV and among those on treatment was often in the setting of ART regimen with known renal toxicity (primarily tenofovir). Only 1 child had a seizure that was deemed febrile after full data review. Given the dearth of available diagnostic data, more children in the rural setting had an unknown seizure etiology compared with the urban children.
4 DISCUSSION
This study evaluated new-onset seizure in Zambian children during a period in which HIV testing and treatment options were ubiquitous and readily available for those children who had carers willing and able to seek and retain engagement with the healthcare system. Sadly, seizure etiologies and outcomes in this ART era differed little from studies conducted in South Africa before HIV treatment were widely available.8 Despite over a decade of widespread ART availability including PMTCT programs, among CLWHIV and new-onset seizures, seizures continue to largely reflect the pathophysiology of long-standing, advanced, and often untreated HIV disease.
This study was not designed to determine whether successful PMTCT and ART outreach programs reduce the burden of HIV-associated seizures in children; however, the absence of any identifiable eligible cases at Nakambala Hospital after 6 months of active screening indirectly support this supposition. HIV care for the Nakambala catchment population includes HIV services embedded within the routine antenatal care provided to all covered women, ART services close to people's residence, time off work for seeking monthly HIV care for oneself or one's child, and active tracking of infants identified to be HIV exposed or infected to assure early treatment. Work in Botswana further supports the role of early ART initiation in preventing seizures and seizure-related morbidity and mortality in CLWHIV.7
Interestingly, febrile seizures were determined to be the seizure etiology in only one child despite febrile seizures being one of the most common etiologies for acute seizures in Zambian children.20-22 Over half the cohort presented with multiple recurrent seizures or status epilepticus. This may reflect delays in accessing care and may also have contributed to the high inpatient mortality. It is also possible that children with a brief single seizure may not have been brought for care if they seemed otherwise well given that febrile seizures are extremely common in these setting and pre-existing barriers in travel and cost. Other work in the region has shown that children with simple febrile seizures are often not brought for care.20 Renal failure was responsible for 3 deaths total and 1 in the rural centers, but this is likely an underestimation as kidney function studies are difficult to acquire at the non-urban sites. Possible reasons for renal failure include ART side effects, underlying HIV disease, and acute kidney injury as a result of rhabdomyolysis from prolonged seizures. Malaria did not play a large role as the etiology for epilepsy in this population. Malaria remains a major cause of death in some regions of Zambia but not in Lusaka or the Southern Province where this study was conducted. Moreover, implementation of programs like the US President's Malaria initiative has contributed to significant reduction in infection rate for children under 5 in the last decade. Thus, a history of severe malaria as expected should be fairly uncommon in this age group and region.23
Only half the patients in this study were on ART, and the majority of those on ART showed poor viral suppression in the setting of very poor adherence with multiple and prolonged periods of defaulting from treatment. Zambia met the 90:90:90 goals24 as early as 2011. In general, ART enrollment and adherence among children in Zambia is likely much better than was observed in the children in this study who presented with new-onset seizure.25 The most recent reports indicate that 84% of Zambian children with HIV are on ART with 87% of these being virally suppressed.24 This is in significant contrast to the situation of children in this study of new-onset seizure who appear to “slip through the cracks” of HIV care and treatment services—a situation previously described among adults in Zambia.26 Caregivers interviewed reported multiple barriers to ART adherence including socioeconomic factors and household instability particularly in homes that have assumed care of orphaned children. Additional factors included stigma and limited understanding of medication benefits. These reports are congruent with studies of ART adherence27, 28 and underscore the imperative to provide extensive support services for these vulnerable populations. Poor outcomes in this population undoubtedly occur due to a number of factors including delayed treatment initiation after diagnosis, poor adherence, lack of appropriate diagnostic resources, and delayed care-seeking for acute illness.
Despite assuring all study sites had the material resources for LP completion and offering extensive diagnostic evaluations if CSF was acquired, LP uptake was quite poor with only a third of children undergoing an LP. The poor uptake of LP in the region has previously been described in PLWHIV with new-onset seizure in Zambia.26 This issue is increasingly recognized and appears to be a multifactorial problem warranting further study.29-31 In the context of this study, the constrained diagnostic data negatively impacted the identification of seizure etiology and cause of death and may have contributed to the high 30-day mortality. Nonetheless, diagnostic data in this cohort are likely more extensive than are usually available. Imaging requires some user fees unless waived by social work, EEG consumables are often out of stock and indirect costs of bringing children from outside the capital city into the tertiary care centers for diagnostic assessments are prohibitive for most families.
There are a number of other study limitations. These includes the single-country setting, small sample size, and heterogeneity of the study population.5, 32 Rural and urban populations differed in terms of their access to imaging and EEG, but also differed in terms of demographic characteristics. Children in the rural areas were accessing care at the ART clinic associated with their residential catchment area. In contrast, the urban cohort were children seeking care at Zambia's largest tertiary care children's hospital and previous work has shown that children accessing care at this site are among the most economically deprived in the city.33, 34 Seizure severity burden was high with children presenting many hours after the index seizure onset with fulminant status epilepticus and/or coma. Acute seizure management options are quite constrained in Zambia relative to high-income settings and intubation for ventilation is not de facto available even at the Teaching Hospitals for such cases. A comparative evaluation of quality of care delivery for these children, in terms of acute seizure management, to determine whether care quality contributed to mortality was not possible due to limited documentation even in the context of this prospective study. Certainly, active seizures did not always result in escalation of acute ASMs, but given the need for clinicians to balance risks and benefits with one of the primary risks being death from ASM-induced respiratory failure, this may reflect sound clinical judgment. None of the deaths were attributed to respiratory failure from ASMs, suggesting that the Zambian clinicians caring for these children choose less aggressive seizure management with less optimal seizure control over the risk of an iatrogenic death. The limitations to conduct diagnostic work and research speak to the clinical challenges that remain present. Nonetheless, the findings provide insights into the etiology and burden of seizures among CLWHIV in a large sub-Saharan African country and offers some direction for program development and future research. With fewer than 1 neurologist per million people in Zambia, there are not enough specialists in neurology to be directly involved in the care of children with HIV and seizures, but clear care pathways developed with local resource and expertise in mind could prove extremely valuable.35
Despite widespread HIV testing and treatment availability in Zambia, new-onset seizure in CLWHIV still occurs largely in the setting of advanced, active HIV disease. Enhanced programs to assure early ART initiation, improve adherence and expeditiously identify and address ART failure are needed to reduce the burden of neurological injury and premature death in CLWHIV. These supportive efforts are especially needed among the particularly vulnerable including the poor, rural populations, and orphans.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to disclose.
ETHICAL APPROVAL
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ENDNOTE
REFERENCES
- 1 Date of ART initiation was no available for two children.