The efficacy and safety of adjunctive perampanel for the treatment of refractory focal-onset seizures in patients with epilepsy: A meta-analysis
Abstract
Objective
The last decade has seen an increase in the use of anti-seizure medications (ASMs); however, the burden of treating drug-resistant epilepsy has not fallen. We performed this meta-analysis to evaluate the optimal dose of Perampanel (PER) as a new adjunctive treatment for drug-resistant seizures.
Methods
We searched for studies published from inception to February 1, 2021 from PubMed, Central Register of Controlled Trials (CENTRAL), and ScienceDirect. Research characteristics, patients' characteristics, and treatment regimen, concomitant ASMs, clinical outcomes were extracted. The practical outcome included a reduction in seizures frequency ≥50%, ≥75%, and ≥100% from baseline convulsive seizure frequency, and the safety outcome included the proportion of drug withdrawal and adverse reactions. Odds ratios (OR) for 95% confidence intervals (95% CI) were estimated by the inverse variance method.
Results
Four trials which enrolled 2187 participants (1569 in the PER group and 618 in the placebo group) were included. Results showed that 8 or 12 mg per day had the best effect on all three outcomes, with no significant difference between 8 and 12 mg per day (≥50% reduction, 35.5% vs 36.1%, P = .84; ≥75% reduction, 17.8% vs 19.1%, P = .64; seizure-free, 3.5% vs 3.7%, P = .85). In addition, 12-mg PER compared to 8 mg had a higher proportion of trial withdrawal (8.7% vs 17.0%; P < .00001) and treatment-emergent adverse event (TEAE) resulting in dose reduction/discontinuation (18.5% vs 32.0%; P < .00001). The adverse events (AEs) significantly associated with adjunctive PER were dizziness, somnolence, fatigue, and irritability.
Significance
Adjunctive treatment of PER was associated with a more significant reduction in the frequency of seizures in patients with refractory epilepsy than placebo, but with a higher frequency of AEs. PER at a daily dose of 8 mg appears to have the best ratio between efficacy and tolerance in most study participants.
Key points
- Perampanel (PER) as a new adjunctive treatment for drug-resistant seizures is efficacy.
- Adjunctive treatment of PER was associated with a higher frequency of adverse effects.
- The optimal dose of Perampanel is 8 mg per day as a new adjunctive treatment for drug-resistant seizures between efficacy and tolerance.
1 INTRODUCTION
It is estimated that the incidence of epilepsy is about 80/100 000, and the prevalence is 5/1000, with about 70 million people suffering from epilepsy globally.1, 2 Treatment of epilepsy is symptom based. Although many patients with epilepsy can control their seizures, there are still more than one-third of the cases of seizures that cannot be controlled.3 Uncontrolled epileptic seizures are likely to be a risk factor of impaired quality of life, disability, and premature death with severe physical and psychological dysfunction.4 However, with the increasing appearance and use of anti-seizure medications (ASMs) over the past few years, the burden of treating drug-resistant epilepsy has not been reduced. New and effective treatment options are still needed.5 The safety and effectiveness of newer drugs also require periodic evaluation.
Perampanel (PER) is a selective, non-competitive, and orally active alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor antagonist. This receptor plays a vital role in mediating rapid excitatory synaptic transmission, generation, and transmission of epileptic activity.6 In 2014, the European Union and the United States approved of PER (for patients 18 years of age or older), and to date, more than 40 countries around the world have approved of this drug.7, 8 All anti-seizure medications (ASMs) were associated with the risk of treatment-emergent adverse events (TEAEs); Therefore, minimizing TEAEs is an important consideration when using ASMs. This meta-analysis assesses the efficacy and safety of adjunctive PER for the treatment of refractory focal-onset seizures in patients with epilepsy.
2 METHODS
2.1 Search strategy
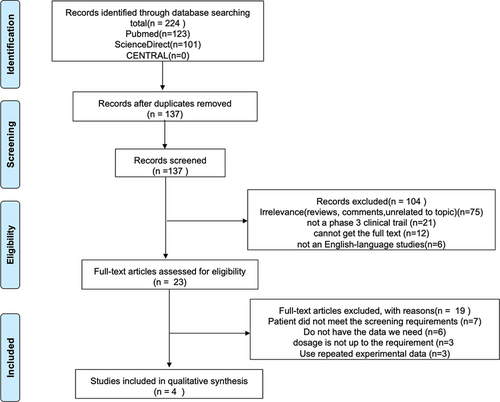
We (Yiming Li and Ya Zeng) independently searched PubMed, ScienceDirect, and Central Register of Controlled Trials (CENTRAL) from their earliest dates up to February 1, 2021. The final search string was “perampanel” [Mesh] or “3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one” AND “refractory partial-onset seizures” [Mesh]AND randomized controlled trial [ptyp]. No other filters were used. Studies were selected with the following entry criteria: Phase III, randomized, double-blind, placebo-controlled, parallel-group design with 6-week observational baseline and 19-week double-blind treatment phase (6-week titration period and 13-week maintenance period). Participants who meet the following criteria are included: age > 12, diagnosed with focal-onset seizures (partial-onset seizures) at least two ASMs failures in the previous 2 years, at least five focal seizures in the 6-week baseline phase and was taking stable doses of 1-4 approved concomitant ASMs. Exclusion criteria were as follows: not English language studies, RCT articles using the same experimental data, articles without recording 50% reduction in the frequency of seizures, and articles using indeterminate drug doses. No PROSPERO registration number for the moment. The specific screening process is shown in Figure 1.
2.2 Outcome measures
The primary outcome was the responder rate, defined as the percentage of patients who experienced a ≥50% reduction in seizure frequency in the maintenance period relative to baseline in the European Union. Responder rate is a single point in the entirety of response and not a continuous variable. Thus, the 50% responder rate provides less information from all possible responses; it may be less sensitive. Increasing the gauge of responder rate to 75% showed more significant improvements compared with placebo. So, we defined a ≥75% reduction in seizure frequency and seizure free as the secondary outcome.
Safety outcomes were as follows: 1. the proportions of patients who drop out of the treatment for any reason; 2. all TEAEs related to PER (ie, dizziness, somnolence, headache, fatigue, upper respiratory tract infection, nasopharyngitis, gait disturbance, or irritability).
2.3 Data and assessment of the risk of bias
To ensure the consistency of the data collection of each study, we conform to the conditions of the study of the following information into a structured Excel data table: research characteristics (such as sample size, titration time, and maintenance time), the patient's characteristics (such as age, gender, etiology, duration of disease, and complications), and treatment regimen, concomitant ASMs, and clinical outcomes (evaluation forms from the Cochrane Manual were used to assess the quality of selection, performance, detection, attrition, and reporting biases for each qualifying study [2011]).9
2.4 Statistical analysis
To develop the meta-analysis, we used Cochrane Collaboration's Review Manager software (RevMan 5.4) to derive pooled effect estimates, such as odd ratios (OR) and associated 95% confidence intervals (CI). Statistical significance was assessed at a nominal α level of .05. The heterogeneity index I2 determined the choice of fixed-effect model or random-effect model.
3 RESULTS
3.1 Study characteristics
Two hundred and twenty-four records were initially identified by searching the database and trial register. Four randomized controlled trials (RCTs) were retrieved for detailed evaluation.10-13 The essential characteristics of the studies are provided in Table 1.
| Article | Inclusion criteria | Race |
Female gender, n (%) |
Mean age (years) | Intervention group | ||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo |
PER 2 mg |
PER 4 mg |
PER 8 mg |
PER 12 mg |
|||||
| Jacqueline A. French 201312 | Diagnosis of simple or complex focal seizures permitted only one inducer ASM and must have been on a stable dose of any concomitant benzodiazepines |
White (322) Asian (42) Others (22) |
65 (47.8)/64 (49.6)/71 (58.7) | 34.4 (13.6)/36.7 (14.4)/ 35.5 (14.1) | n = 136 | n = 129 | n = 121 | ||
| Jacqueline A. French 201214 | Diagnosed with focal-onset seizures, with stable doses of 2-3 approved ASMs |
White (334) Asian (4) Others (50) |
67 (55.4)/68 (51.1)/65 (48.5) | 35.6 (14.7)/35.8 (14.2)/36.7 (14.6) | n = 121 | n = 133 | n = 134 | ||
| T. Nishida 201713 | / | 90 (48.6)/95 (52.8)/84 (48.8)/92 (54.4) | 33.4 (12.6)/33.8 (13.6)/33.6 (12.2)/34.6 (12.8) | n = 185 | n = 180 | n = 172 | n = 169 | ||
| GL Krauss 201215 | Diagnosed with simple or complex focal-onset seizures, with stable doses of 2-3 approved ASMs |
White (459) Asian (244) Others (3) |
89 (50.9)/94 (54.0)/84 (48.0)/93 (51.7) | 34.5 (13.2)/33.1 (13.2)/33.6 (14.1)/32.3 (12.3) | n = 175 | n = 174 | n = 175 | n = 180 | |
3.2 Risk of bias assessment
Four studies were multicenter, randomized, double-blinded, placebo-controlled, parallel-group trials, which resulted in a low risk of bias (Table 2).
| Outcome or subgroup | Number of studies | Participants | I2, % | Odds ratio (95% CI) | P |
|---|---|---|---|---|---|
| 1.1 50% reduction in the seizure frequency | 4 | 2187 | 37% | 1.96 [1.56, 2.45] | <.00001 |
| 1.1.1 PER 2 mg/d vs placebo | 1 | 364 | / | 1.18 [0.70, 2.00] | .53 |
| 1.1.2 PER 4 mg/d vs placebo | 2 | 751 | 0 | 1.45 [1.02, 2.08] | .04 |
| 1.1.3 PER 8 mg/d vs placebo | 4 | 1222 | 0 | 2.12 [1.63, 2.75] | <.00001 |
| 1.1.4 PER 12 mg/d vs placebo | 3 | 866 | 41 | 2.53 [1.87, 3.44] | <.00001 |
| 1.2 75% reduction in the seizure frequency | 4 | 2178 | 0% | 2.74 [1.93, 3.89] | <.00001 |
| 1.2.1 PER 2 mg/d vs placebo | 1 | 265 | / | 1.94 [0.87, 4.34] | .1 |
| 1.2.2 PER 4 mg/d vs placebo | 2 | 709 | 0% | 1.73 [1.04, 2.88] | P = .03 |
| 1.2.3 PER 8 mg/d vs placebo | 4 | 1224 | 0% | 3.01 [2.04, 4.43] | P < .00001 |
| 1.2.4 PER 12 mg/d vs placebo | 3 | 868 | 0% | 3.29 [2.10, 5.15] | P < .00001 |
| 1.3 Seizure freedom during the treatment | 4 | 2178 | 0% | 3.24 [1.42, 7.83] | .005 |
| 1.3.1 PER 2 mg/d vs placebo | 1 | 265 | / | 1.55 [0.26, 9.39] | P = .63 |
| 1.2.2 PER 4 mg/d vs placebo | 2 | 709 | 0% | 3.20 [1.02, 10.00] | P = .05 |
| 1.2.3 PER 8 mg/d vs placebo | 4 | 1224 | 0% | 3.51 [1.45, 8.51] | P = .005 |
| 1.2.4 PER 12 mg/d vs placebo | 3 | 868 | 0% | 3.88 [1.35, 11.14] | P = .01 |
3.3 Efficacy
We compared four dosage types of PER (2, 4, 8, or 12 mg) with placebo; pooled data from the four RCTs showed that PER 4, 8, 12-mg groups all had a superior response compared with the placebo group, and the PER doses of 8 and 12 mg appeared to be more effective than 4-mg dose of PER (8 mg: ≥50% reduction, 25.6% vs 35.5%, P = .002; ≥75% reduction, 12.4% vs 16.8%, P = .07; seizure-free, 3.5% vs 3.6%, P = .99; 12 mg: ≥50% reduction, 25.6% vs 36.0%, P = .002; ≥75% reduction, 12.4% vs 19.1%, P = .01; seizure-free, 3.5% vs 3.7%, P = .86). We compared the PER doses of 8 mg with 12 mg in three efficacy outcomes (≥50% reduction, 35.5% vs 36.1%, P = .84; ≥75% reduction, 17.8% vs 19.1%, P = .64; seizure-free, 3.5% vs 3.7%, P = .85), the data showed that there is no significant difference between the doses of 8 and 12 mg. The details of 50% reduction in seizure can be found in Figure 2. To sum up, the efficacy of different doses of PER is in the following order: 8 = 12 mg > 4 mg, the minimum effective dose of PER may be 4 mg/d as no statistically significant difference in the 2 mg/d dose compared with placebo.

3.4 Treatment withdrawal and adverse events
In all trials, 152 (9.52%) and 27 (4.3%) patients withdrew from the study due to drug-related TEAEs in the PER-added and placebo groups, respectively (OR 2.50, 95% CI 1.64-3.82; I2 = 0%; P < .0001). The incidences of patients' withdrawal from the study due to drug-related TEAEs were higher in PER supplementation with 2, 8, and 12 mg compared with the placebo group (2 mg, 6.7% vs 3.8%, P < .02; 8 mg, 8.4% vs 4.4%, P < .004; 12 mg, 17.0% vs 4.6%, P < .00001). PER supplementation with 4 mg showed no significant difference (3.7% vs 3.6%, P < .92) (Table 3).
| Outcome or subgroup | Studies | Events/participants (%) | I2 (%) | Odd ratio (95% CI) | P | |
|---|---|---|---|---|---|---|
| PER | Placebo | |||||
| Treatment dropout | ||||||
| PER any dose | 4 | 152/1569 (9.5%) | 27/618 (4.4%) | 0 | 2.50 [1.64, 3.82] | <.001 |
| PER 2 mg/d | 1 | 12/180 (6.7%) | 7/185 (3.8%) | 0 | 1.82 [0.70, 4.72] | .02 |
| PER 4 mg/d | 2 | 13/384 (3.7%) | 13/361 (3.6%) | 0 | 1.04 [0.47, 2.27] | .92 |
| PER 8 mg/d | 4 | 53/633 (8.4%) | 27/617 (4.4%) | 15 | 2.00 [1.24, 3.22] | .004 |
| PER 12 mg/d | 3 | 74/435 (17.0%) | 20/433 (4.6%) | 0 | 4.23 [2.53, 7.08] | <.001 |
| Any TEAE leading to dose reduction/interruption | ||||||
| PER any dose | 4 | 286/1569 (18.2%) | 24/618 (3.9%) | |||
| PER 2 mg/d | 1 | 3/180 (1.7%) | 6/185 (3.2%) | 0.51 [0.12, 2.05] | .34 | |
| PER 4 mg/d | 2 | 32/348 (9.2%) | 13/361 (3.6%) | 0 | 2.13 [1.20, 3.79] | .01 |
| PER 8 mg/d | 4 | 112/606 (18.5%) | 24/618 (3.9%) | 0 | 5.41 [3.57, 8.21] | <.001 |
| PER 12 mg/d | 3 | 139/435 (32.0%) | 18/433 (4.2%) | 0 | 9.87 [6.24, 15.62] | <.001 |
| Any TEAE | ||||||
| PER any dose | 4 | 1026/1569 (75.6%) | 411/618 (66.5%) | 0.98 [0.92, 1.05] | .62 | |
| PER 2 mg/d | 1 | 111/180 (61.7%) | 101/185 (54.6%) | 1.13 [0.95, 1.35] | .17 | |
| PER 4 mg/d | 2 | 232/348 (66.7%) | 218/361 (60.4%) | 27% | 1.10 [0.99, 1.23] | .09 |
| PER 8 mg/d | 4 | 479/570 (84.0%) | 411/682 (60.3%) | 91% | 1.25 [1.02, 1.55] | .03 |
| PER 12 mg/d | 3 | 383/435 (88.0%) | 310/433 (71.6%) | 61% | 1.21 [1.09, 1.35] | .0004 |
| Any treatment-related TEAE | ||||||
| PER any dose | 4 | 936/1569 (59.7%) | 234/618 (37.9%) | 62 | 2.72 [2.23, 3.31] | .00001 |
| PER 2 mg/d | 1 | 67/180 (37.2%) | 59/185 (31.9%) | 1.17 [0.88, 1.55] | .29 | |
| PER 4 mg/d | 2 | 158/348 (45.4%) | 111/361 (30.7%) | 0 | 1.48 [1.22, 1.79] | .0001 |
| PER 8 mg/d | 3 | 292/477 (61.2%) | 169/485 (34.8%) | 0 | 1.70 [1.49, 1.95] | <.00001 |
| PER 12 mg/d | 2 | 236/314 (75.2%) | 110/297 (37.0%) | 80% | 2.00 [1.40, 2.86] | .0002 |
Note
- Risk ratios are from a fixed-effects model.
In addition, 12-mg PER compared to 8 mg had a higher proportion of trial withdrawal (8.7% vs 17.0%; P < .00001) and TEAEs resulting in dose reduction/discontinuation (18.5% vs 32.0%; P < .00001) without a significant increase in efficacy. To sum up, the safety of different doses of PER is in the following order: 4 > 8 > 12 mg. Two milligrams was not included in this comparison because 2 mg had no efficacy; thus, we considered the safety of 2 mg to be meaningless.
The incidence of treatment-related TEAEs was higher in the PER-added group (59.6% vs 37.9%; P < .00001) (Table 3), which was also related to dosage. TEAEs were higher in PER supplementation with 4, 8, and 12 mg compared with the placebo group (4 mg, 45.4% vs 30.7%, P = .0001; 8 mg, 61.2% vs 34.8%, P < .00001; 12 mg, 75.2% vs 37.0%, P = .0002). There was no significant difference in PER supplementation with 2 mg (37.2% vs 31.9%, P < .29) (Table 3).
There was no statistical difference in severe TEAEs (5.1% vs 5.1%; P < .88). The most common TEAE was dizziness and there is a significant difference between the PER-added group and the placebo group (29.1% vs 8.1%; P < .00001). In addition, the incidence of major TEAEs in the PER-added group and the placebo group was as follows: somnolence (16.0% vs 7.2%; P < .001), headache (8.6% vs 10.0%; P = .94), fatigue (7.2% vs 4.4%; P = .03), upper respiratory tract infection (5.0% vs 3.6%; P = .28), nasopharyngitis (8.4% vs 8.0%; P = .86), gait disturbance (3.8% vs 2.2%; 0.06), irritability (7.1% vs 2.3%; 0.003), rash (2.4% vs 1.1%; P = .31), nausea (3.3% vs 2.8%; 0.72), and falls (11.2% vs 6.6%; P = .12) (Table 4).
| Outcome | Studies | Events/participants (%) | I2 (%) | Odd Ratio (95% CI) | P | |
|---|---|---|---|---|---|---|
| PER | Placebo | |||||
| All TEAEs | 4 | 936/1569 (59.7%) | 234/618 (37.9%) | 62 | 2.72 [2.23, 3.31] | <.001 |
| Dizziness | 4 | 458/1569 (29.2%) | 50/618 (8.1%) | 80 | 4.83 [3.55, 6.58] | <.001 |
| Somnolence | 4 | 251/1569 (16.0%) | 45/618 (7.3%) | 64 | 2.45 [1.75, 3.41] | <.001 |
| Headache | 4 | 135/1569 (8.6%) | 62/618 (10.0%) | 0 | 1.01 [0.74, 1.39] | .94 |
| Fatigue | 3 | 86/1188 (7.2%) | 22/497 (4.4%) | 0 | 1.74 [1.07, 2.83] | .03 |
| Upper respiratory tract infection | 3 | 53/1052 (5.0%) | 13/361 (3.6%) | 0 | 1.41 [0.76, 2.62] | .28 |
| Nasopharyngitis | 2 | 89/1052 (8.5%) | 29/361 (8.0%) | 0 | 0.96 [0.62, 1.48] | .86 |
| Gait disturbance | 2 | 51/1319 (3.9%) | 11/482 (2.3%) | 0 | 1.88 [0.97, 3.66] | .06 |
| Irritability | 2 | 56/788 (7.1%) | 7/297 (2.4%) | 41 | 3.48 [1.54, 7.83] | .003 |
| Rash | 1 | 13/531 (2.4%) | 2/176 (1.1%) | 2.18 [0.49, 9.77] | .31 | |
| Nausea | 1 | 18/531 (3.4%) | 5/176 (2.8%) | 1.20 [0.44, 3.28] | .72 | |
| Fall | 1 | 30/267 (11.2%) | 8/121 (6.6%) | 1.79 [0.79, 4.02] | .16 | |
4 DISCUSSION
Epilepsy remains uncontrolled in one-third of patients despite appropriate medical therapy. PER has been marketed as third-generation ASMs and adjunctive treatments for focal-onset seizures in China in 2019. Guideline of American Academy of Neurology and the American Epilepsy Society 2018 recommended PER for treatment-resistant adult focal epilepsy (level A).14 Oral PER has superficial pharmacological characteristics: it can be rapidly absorbed from the gastrointestinal tract; Steady-state plasma concentrations can be reached within 14 days of oral administration, with a terminal half-life of approximately 70-120 hours.15 PER does not affect plasma concentrations of ASM taken simultaneously.16
Our meta-analysis results indicate that adjunctive treatment of PER at a daily dose of 4, 8, or 12 mg significantly reduced the number of seizures in patients with refractory focal seizures that were rarely discontinued due to unacceptable TEAES. The minimum effective dosage of PER maybe 4 mg/d, because there is no statistically significant difference in the 2 mg/d dose compared with placebo. However, since 2 mg was included in only one experiment, its effectiveness needs to be further verified. The PER doses of 8 mg and 12 mg are more effective than 4 mg, and the PER doses of 8 and 12 mg had no significant difference in efficacy. In addition, a small number of patients experienced seizures, which may be a dose-dependent phenomenon; although the number was small, this trend was statistically significant.
Perampanel increased the incidence of TEAEs compared with placebo, which was higher in PER supplementation with 8 and 12 mg. The vast majority of TEAEs were mild/moderate at standard doses. Dizziness is the most common AE and may have a dose-response; however, only a small number of those patients (8.3%) discontinued treatment due to this symptom, and no safety issues have been identified.10 In addition, PER may increase the incidence of somnolence, fatigue, and irritability.
Patients experienced more TEAEs and had a higher proportion of trial withdrawal after taking the 12-mg dose, and the efficacy is less significant than that of 8 mg, so PER 8 mg/d may be the perfect option. However, there are still additional benefits for a significant number of patients to accept the 12 mg.17 The 12-mg dose may be an essential option to achieve the goal of a more significant reduction of seizures and free seizures in patients who can tolerate and do not achieve optimal response at an 8-mg dose.
In addition, although the maintenance period is twice if the titration period, the frequency of TEAEs during the maintenance period is lower than titration, indicating that they are transient, with no increase in the incidence of TEAE over time, and no potential tolerance.10, 18 The low or nonexistent incidence of the first occurrence of these TEAEs after 6 months to 1 year of treatment is further evidence that long-term treatment with PER is safe and well tolerated.18
Three patients died in these four RCTs; one died of sudden cardiac death in the placebo group, one died of an unknown cause in the PER 8-mg group and one died of convulsion during baseline. Because of the minor frequency of events, it remains unclear whether drugs caused the deaths. In addition, three patients from the placebo group, one from the PER 2-mg group, two from the PER 8-mg group, and two from PER 12 mg patients appeared with suicidal tendencies. Again, we do not know for sure whether the suicidal tendencies were related to the effects of the drugs. The data showed no statistically significant difference in severe TEAEs between the PER and the placebo groups. Overall, the incidence of psychotic serious TEAEs was higher in the 12-mg group than in the other dose or placebo groups. Although there was no statistically significant difference in severe adverse reactions between the placebo and PER groups, the proportion of TEAEs leading to discontinuation and dose reduction/interruption was higher in the PER group than in the placebo group. Dose reduction rather than PER withdrawal was used in most cases to deal with TEAEs.
5 CONCLUSIONS
Perampanel is an optional adjunctive method for refractory focal epilepsy. Adjunctive treatment of PER was associated with a more significant reduction in the frequency of seizures in patients with refractory epilepsy than placebo, but with a higher frequency of AEs. A daily dose of PER 8 mg is considered the best dosing option. To enhance patient tolerance, we suggest increasing and reducing the dose gradually when starting or discontinuing. More research will be forthcoming to explain further the true therapeutic potential and clinical significance of this latest ASM.
6 STRENGTHS AND LIMITATIONS OF THIS STUDY
- This is a meta-analysis of the efficacy and safety of different doses of adjunctive PER in patients with focal-onset seizures.
- In this meta-analysis, efficacy and safety analyses are mainly based on daily doses of PER estimates of seizure response during the maintenance phase, the most accurate phase to represent steady-state drug levels in the entire treatment period.
- This meta-analysis included only four RCTs. The literature did not rate "refractory" (for example, patients with focal epilepsy who had previously been resistant to more than three different drugs were significantly more resistant than patients who had been previously resistant to one drug).
- This meta-analysis inherited the general limitations of the four RCTs, such as the short duration of maintenance and the potential impact of concomitant drugs.
- This meta-analysis has no information about PER monotherapy's efficacy, tolerability, and safety PER during pregnancy and lactation.
CONFLICT OF INTEREST
Neither of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
Dong Zhou, Jie Mu, and Yiming Li proposed and designed the purpose; Yiming Li and Ya Zeng developed and revised the Searching strategy. All co-authors have contributed to the revision of the manuscript.