Astrocyte reactivity in a mouse model of SCN8A epileptic encephalopathy
Abstract
Objective
SCN8A epileptic encephalopathy is caused predominantly by de novo gain-of-function mutations in the voltage-gated sodium channel Nav1.6. The disorder is characterized by early onset of seizures and developmental delay. Most patients with SCN8A epileptic encephalopathy are refractory to current anti-seizure medications. Previous studies determining the mechanisms of this disease have focused on neuronal dysfunction as Nav1.6 is expressed by neurons and plays a critical role in controlling neuronal excitability. However, glial dysfunction has been implicated in epilepsy and alterations in glial physiology could contribute to the pathology of SCN8A encephalopathy. In the current study, we examined alterations in astrocyte and microglia physiology in the development of seizures in a mouse model of SCN8A epileptic encephalopathy.
Methods
Using immunohistochemistry, we assessed microglia and astrocyte reactivity before and after the onset of spontaneous seizures. Expression of glutamine synthetase and Nav1.6, and Kir4.1 channel currents were assessed in astrocytes in wild-type (WT) mice and mice carrying the N1768D SCN8A mutation (D/+).
Results
Astrocytes in spontaneously seizing D/+ mice become reactive and increase expression of glial fibrillary acidic protein (GFAP), a marker of astrocyte reactivity. These same astrocytes exhibited reduced barium-sensitive Kir4.1 currents compared to age-matched WT mice and decreased expression of glutamine synthetase. These alterations were only observed in spontaneously seizing mice and not before the onset of seizures. In contrast, microglial morphology remained unchanged before and after the onset of seizures.
Significance
Astrocytes, but not microglia, become reactive only after the onset of spontaneous seizures in a mouse model of SCN8A encephalopathy. Reactive astrocytes have reduced Kir4.1-mediated currents, which would impair their ability to buffer potassium. Reduced expression of glutamine synthetase would modulate the availability of neurotransmitters to excitatory and inhibitory neurons. These deficits in potassium and glutamate handling by astrocytes could exacerbate seizures in SCN8A epileptic encephalopathy. Targeting astrocytes may provide a new therapeutic approach to seizure suppression.
Key Points
- Astrocytes, but not microglia, become reactive in spontaneously seizing mice expressing the human SCN8A mutation N1768D.
- Astrocyte reactivity is not observed at a time point before the onset of spontaneous seizures even though increases in neuronal excitability have been initiated and audiogenic seizures can be induced.
- Reactive astrocytes have reduced Kir4.1 channel currents suggesting an impairment in K+ ion uptake.
- Reactive astrocytes have reduced glutamine synthetase expression suggesting impairments in glutamate homeostasis.
- Astrocytes do not express Nav1.6 indicating that the physiological changes observed in astrocytes are in response to increases in neuronal excitability.
1 INTRODUCTION
De novo missense mutations of the gene SCN8A, which encodes the voltage-gated sodium channel Nav1.6 are associated with SCN8A epileptic encephalopathy.1 Clinical features include seizure onset between 4 and 18 months of age, developmental delay after seizure onset, epilepsy with multiple seizure types, and sudden unexpected death in epilepsy (SUDEP) in 10% of cases.2 The expression of Nav1.6 is predominantly neuronal, with high expression concentrated at the axon initial segments (AIS) and nodes of Ranvier where it promotes neuronal excitability.3-5
Most neurologic disorders, including epilepsy, involve not just neuronal dysfunction but also glial dysfunction. Astrocytes, in particular, play critical roles in maintaining ion and neurotransmitter homeostasis to ensure proper neuronal function.6 During instances of insult to the brain astrocytes undergo a process termed reactive astrogliosis.7 Reactive astrocytes display altered morphology, physiology, and gene expression profile compared with astrocytes in the healthy brain, contributing to disease pathology. Several studies have demonstrated that manipulations which produce reactive astrocytes can promote neuronal hyperexcitability8 or lead to the development of seizures.9 Astrocyte-specific deletion of either KCNJ10, encoding the inward rectifying potassium channel Kir4.1,10 or inhibition of the glutamate degradation enzyme glutamine synthetase (GS), facilitate the development of spontaneous seizures in mouse models.11 Reactive astrocytes commonly downregulate these critical genes, which could contribute to brain pathology.6 Moreover, reactive astrocytes are present in human resected tissue from patients suffering from mesial temporal lobe epilepsy (MTLE) and mutations in KCNJ10 are causative for EAST syndrome (epilepsy, ataxia, sensorineural deafness. and tubulopathy), characterized by epilepsy.12, 13
Microglia are also thought to be involved in epilepsy. In several models of temporal lobe epilepsy (TLE), microglia become reactive and contribute to seizures by the release of proinflammatory cytokines (IL-1β and TNF) which are proconvulsant and microglial specific knockout of the TSC1 gene results in microglial reactivity and the development of spontaneous seizures.14 Microglia may also have roles in regulating the ectopic neurogenesis and synaptic pruning that occurs in epilepsy.14
In this study, we investigated whether microglia and astrocyte reactivity were associated with seizures in the N1768D mouse model of SCN8A encephalopathy. This model bears the first described patient point mutation, p.Asn1768Asp, and recapitulates key aspects of the disease, including the development of spontaneous seizures and an increased risk of sudden death.1, 5 We show that astrocytes become reactive in the cortex and hippocampus of spontaneously seizing mutant mice and display reduced Kir4.1 currents, indicating an impairment in their ability to regulate extracellular K+. Interestingly, prior to the onset of seizures, astrocytes remained quiescent and displayed unaltered Kir4.1 currents. Astrocytes in spontaneously seizing mutant mice also exhibited reduced glutamine synthetase expression, the enzyme necessary for the conversion of glutamate to glutamine, indicating an impairment in glutamate processing. Surprisingly, microglial morphology was unchanged prior to and after the onset of spontaneous seizures. These deficits in the homeostatic functions of astrocytes, but not microglia, could contribute to the development of seizures in SCN8A encephalopathy, implicating a role for astrocytes in the pathology of this disease.
2 MATERIALS AND METHODS
2.1 Animals
All experiments were performed using C57BL/6J WT mice (JAX:000664), transgenic Tg(Aldh1l1-EGFP,-DTA)D8Rth/J mice (JAX:026033), and Scn8aN1768D knock-in mice, referred to as D/+.5, 15 All experiments performed on mice were conducted in compliance with the guidelines established by the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by the University of Virginia's Institute of Animal Care and Use Committee. Both male and female mice were used in experiments. D/+ mice were genotyped as previously described.3 To identify astrocytes Aldh1l1-EGFP and D/+ mice were crossed to create Aldh1l1-EGFP:D/+ mice. All mice were maintained on a 12-hour light/dark cycle and allowed access to food and water ad libitum.
2.2 Immunohistochemistry
Brain tissue for immunohistochemistry was processed as follows. Mice were anesthetized and transcardially perfused with 10 mL ice-cold 1× Dulbecco's phosphate-buffered saline (DPBS) followed by 10 mL ice-cold 4% paraformaldehyde (PFA). Sagittal brain sections were immersed in 4% PFA for 2 hours at 4°C then stored in 1× DPBS with 0.1% sodium azide. Brains were embedded in 2% agarose and 40 µm sagittal sections were obtained using a vibratome (Leica VT1200). The following antibodies were used: rabbit polyclonal to GFAP at 1:400 (Abcam, 7260), chicken polyclonal to GFAP at 1:1000 (Abcam, 4674), goat polyclonal to IBA1 at 1:500 (Abcam, 5076), chicken polyclonal to GFP at 1:1000 (Abcam, ab13970), rabbit polyclonal to glutamine synthetase at 1:400 (Abcam, ab176562), and rabbit polyclonal to Nav1.6 at 1:100 (Alomone, ASC-009). Primary antibodies were diluted in 2% goat or donkey serum depending on the secondary antibody (Jackson Immunoresearch) and 0.1% Triton-X (Sigma-Aldrich). Sections (40 µm) were stained free-floating in primary antibody on a shaker at 4°C overnight. Sections were washed twice with 1× DPBS and 0.1% Triton-X. Secondary antibodies were used at 1:1000 and diluted in 2% goat or donkey serum and 0.1% Triton-X. Sections were incubated in secondary antibodies for 1 hour at room temperature on a shaker. All secondary antibodies were obtained from Invitrogen and used according to the primary antibody being detected. Tissues were counterstained with NucBlue™ Fixed Cell ReadyProbes™ Reagent (DAPI) (Thermo Fisher, catalog #R37606) included in the secondary antibody solution. Tissues were mounted on slides using AquaMount (Polysciences, Inc).
2.3 Quantification of immunohistochemistry
Imaging was performed on a Zeiss LSM 710 confocal microscope with a 20× or 63× objective. We used 3, 4, or 6 slices per mouse (field of view: FOV) to assess the percent area covered of respective immunoreactivity. During image acquisition microscope settings remained constant for WT and D/+ mice. Quantification of percent area covered was performed using ImageJ. The same threshold was applied to images from WT and D/+ mice. For Sholl analysis, brain slices were stained with the microglia/macrophage marker IBA1 and 20× images of cortical regions were acquired with the same settings for WT and D/+ mice. Fifty microglia were analyzed per mouse with 3 mice per group (WT and D/+).
2.4 Brain slice preparation for electrophysiology
Preparation of acute brain slices for electrophysiology experiments was modified from standard protocols previously described.16 Mice were anesthetized with isoflurane and decapitated. The brains were rapidly removed and kept in chilled ACSF (0°C) containing (in mmol/L): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 0.5 L-ascorbic acid, 10 glucose, 25 NaHCO3, and 2 pyruvate oxygenated with 95% O2 and 5% CO2. Horizontal brain slices (300 µm) of WT and D/+ mice were obtained using a vibratome (Leica VT1200) and were constantly oxygenated with 95% O2 and 5% CO2 throughout the preparation. Once sectioning was complete, sulforhodamine-101 (SR101) was added to the aCSF to a final dilution of 1 µmol/L.17 Slices were incubated in SR101 for 20 minutes at 34°C. Slices were then transferred to aCSF without SR101 for 10 minutes at 34°C to allow removal of excess SR101, before being stored at room temperature in oxygenated aCSF.
2.5 Astrocyte patch clamp recording
During recording, slices were held in a chamber perfused with heated (32°C), oxygenated aCSF. Astrocytes were identified based on uptake of SR101 using epifluorescent microscopy (Hamamatsu) and morphology using a Zeiss Axioskop 2 FS Plus microscope. Borosilicate glass pipettes (OD 1.0 mm, ID 0.58 mm, WPI) were pulled using a Brown-Flaming puller (Model P97; Sutter Instruments). Pipettes had resistances between 4 and 6 MΩ when filled with an internal solution of (in mmol/L): 130 KCl, 2 MgCl2, 10 HEPES, 5 EGTA, 2 Na2ATP, 0.5 CaCl2 with pH set to 7.3.18 Measurements were made in whole-cell patch clamp configuration using an Axopatch 700B amplifier (Molecular Devices, pCLAMP 10.6 software) and a Digidata 1322A (Molecular Devices). To analyze potassium (K+) currents, whole-cell patched astrocytes were voltage-clamped at −80 mV and stepped from −160 to 100 mV in 20 mV increments, first in aCSF without barium chloride (BaCl2), then with the addition of 100 µmol/L BaCl2 to the bath. Barium-sensitive currents were determined by subtraction of current traces in the presence of BaCl2 from baseline recordings in the absence of BaCl2.
2.6 Noninvasive seizure monitoring by video/EEG
To monitor spontaneous seizures, D/+ mice underwent stereotaxic (Kopf, Inc) surgery to implant EEG headsets (Plastics1, Inc). Surgeries were performed in accordance with the Animal Care and Use Committee guidelines at the University of Virginia, and as previously described.19 Briefly, anesthesia was induced with 5% and maintained with 0.5%-3% isoflurane. A midline skin incision was made over the skull for electrode placement over the left and right parietal cortices. Implantation of electrodes into the brain can result in both astrocytic and microglial activation, so we used EEG leads that were attached to the outer surface of the skull and did not penetrate the brain. These leads and the EEG headset were secured on top of the skull with dental cement. Mice were allowed to recover for a minimum of 2 days and were then housed in custom chambers with food and water ad libitum. EEG headsets were connected via custom cables to swivels above the chambers, allowing unrestrained movement. EEG signals were amplified at 2000× and bandpass filtered between 0.3 and 100 Hz, with an analogue amplifier (Neurodata Model 12, Grass Instruments Co.). Biosignals were digitized with a Powerlab 16/35 and recorded using LabChart 7 software (AD Instruments, Inc) at 1 kS/s. Video acquisition was performed by multiplexing four miniature night vision-enabled cameras and then digitizing the video feed with a Dazzle Video Capture Device (Corel, Inc) and recorded at 30 frames per second with LabChart 7 software in tandem with biosignals. Seizures were identified by cortical spike wave discharges and commensurate seizure behaviors that were all considered at least stage 5 on the modified Racine Scale.20 A subset of mice did not receive surface EEG implantation, but were only video monitored for the presence or absence of seizure behaviors in the same way. Only mice that had on average 2-3 seizures per day for 2-4 consecutive days were used in this study.
2.7 Data analysis
Clampfit software version 10.7 was used to analyze the electrophysiological recordings. Sholl analysis was performed in ImageJ using the Sholl analysis plugin.21 Data represent means ± standard deviation (SD). Statistical significance was determined using Student's t test or a standard one-way or two-way ANOVA followed by Sidak multiple comparisons (GraphPad Prism 8).
3 RESULTS
3.1 Astrocytes become reactive in spontaneously seizing SCN8A mutant mice
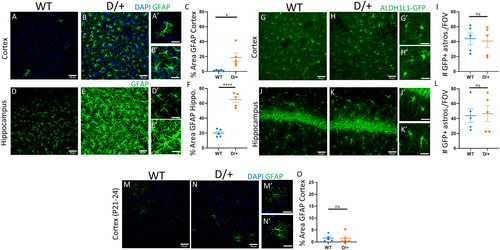
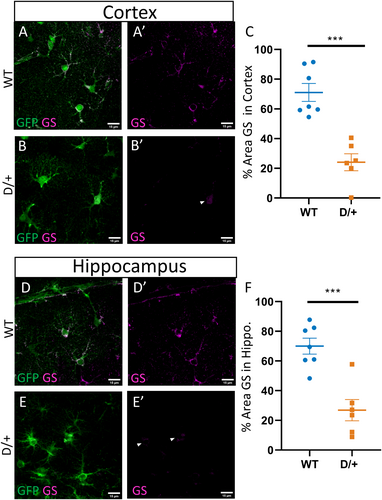
Increases in the expression of the cytoskeletal protein glial fibrillary acidic protein (GFAP) is indicative of astrocyte reactivity. We therefore assessed the percent area stained for GFAP in adult WT and D/+ mice that were confirmed to be spontaneously seizing. In WT mice, few cortical astrocytes expressed GFAP (Figure 1A,A’; n = 5 mice). In contrast, in spontaneously seizing adult D/+ mice, there was widespread GFAP expression in cortical astrocytes, indicative of reactivity (Figure 1B,B’,C; Table 1; n = 5 mice). In addition to the cortex, there was also a significant increase in GFAP expression in hippocampal astrocytes from spontaneously seizing D/+ mice (Figure 1E,E’,F: n = 5 mice) compared to WT (Figure 1D,D’; Table 1: n = 5 mice). The increase in GFAP in cortical and hippocampal astrocytes from seizing D/+ mice could be explained by either an increase in individual astrocyte GFAP expression or by an increase in the number of cortical and hippocampal astrocytes. Since GFAP is not uniformly expressed by all astrocytes and is only found in the astrocyte primary processes and labels astrocyte somas poorly, using GFAP to accurately assess astrocyte cell numbers is not ideal. To overcome this, we used mice which express GFP under the ALDH1L1 promoter22 (Aldh1l1-EGFP:D/+) to selectively label astrocytes in the brain and address whether the increase in GFAP expression was due to an increase in astrocyte numbers. We observed no increase in the number of GFP-labeled astrocytes per FOV (6 per mouse) in either the cortex (Figure 1G-I: n = 5 mice) or hippocampus (Figure 1J-L: n = 5 mice) of spontaneously seizing D/+ mice when compared to WT age-matched controls, suggesting that there was no change in the total number of astrocytes present (Table 1).
| Staining | Brain region | WT | D/+ | P value/test |
|---|---|---|---|---|
| GFAP | Cortex | 1.48 ± 0.39% (n = 5) | 18.71 ± 6.03% (n = 5) |
P = .0215 Students t test |
| Hippocampus | 20.05 ± 2.25% (n = 5) | 64.93 ± 4.08% (n = 5) |
P < .0001 Students t test |
|
| Cortex P21-P24 | 1.60 ± 0.63% (n = 5) | 1.60 ± 0.96% (n = 5) |
P = .5476 Students t test |
|
| GFP | Cortex | 43.93 ± 7.73 (n = 5) | 40.80 ± 8.57 (n = 5) |
P = .7935 Students t test |
| Hippocampus | 43.87 ± 9.01 (n = 5) | 46.67 ± 10.71 (n = 5) |
P = .8464 Students t test |
|
| Glutamine synthetase | Cortex | 75.75 ± 7.55% (n = 7) | 20.15 ± 7.33% (n = 6) |
P = .0002 Students t test |
| Hippocampus | 74.45 ± 5.69% (n = 7) | 27.64 ± 11.24% (n = 6) |
P = .0005 Students t test |
Previous studies have shown that D/+ mice are susceptible to audiogenic seizures and have hyper-excitable neurons at time points before the onset of spontaneous seizures which occurs between 8 and 16 weeks postnatal.3, 23 To explore whether astrocyte reactivity occurred before spontaneous seizure onset, we examined the percent area covered by GFAP expression in young WT (n = 5) and D/+ (n = 5) mice (P21-24; 3 FOV per mouse). In young, nonseizing D/+ mice, there was no difference in cortical (Figure 1M-O) or hippocampal (data not shown) GFAP expression indicating a lack of astrocyte reactivity prior to seizure onset (Table 1).
3.2 Microglial reactivity is not associated with SCN8A epileptic encephalopathy
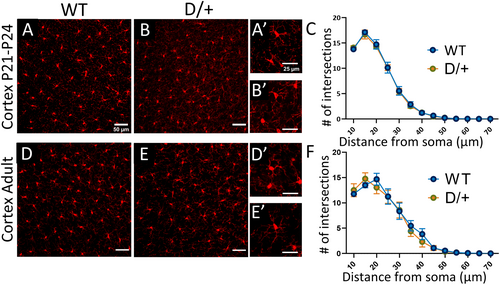
Microglia are the resident immune cells of the brain, as such, they are uniquely poised to respond to insults or alterations of brain homeostasis by becoming reactive. Reactive microglia are characterized by a change in morphology including reduced arbor complexity and have been detected in mouse models of seizures and in epileptic human resected tissue.24, 25 Reactive microglia exhibit a reduction in process complexity with distance from the soma which can be assessed using Sholl analysis. Staining of microglia with IBA1 showed no change in microglia morphology in preseizing age D/+ mice (Figure 2B) or in spontaneously seizing adult D/+ mice (Figure 2E) compared to age-matched WT mice (Figure 2A,D) (n = 3 WT and 3 D/+ mice, 50 cells per mouse; 3 FOV per mouse, P-value = .8156 for young mice and P-value = .7013 for seizing mice, two-way ANOVA, with Sidak multiple comparisons). No changes in process complexity with distance from soma were detected in either preseizing D/+ mice (Figure 2C) or spontaneously seizing D/+ mice (Figure 2F) compared to age-matched WT controls.
3.3 Astrocytes in spontaneously seizing SCN8A mice display reduced Kir4.1 currents
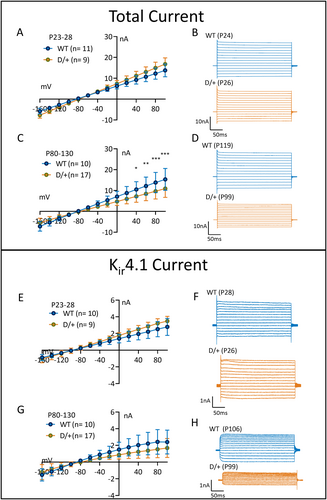
Astrocyte reactivity is associated with the downregulation of genes/proteins involved in the homeostatic functions of astrocytes such as the inward rectifying Kir4.1 channel. The Kir4.1 channel mediates astrocytic uptake of extracellular K+ that is released following neuronal repolarization after action potentials. Due to the importance of astrocytic Kir4.1 channels to buffer K+ and its potential to be downregulated in reactive astrocytes, we explored Kir4.1 currents in astrocytes from young preseizing, as well as adult spontaneously seizing D/+ mice. In preseizing mice (P23-P28), there were no significant differences in the total or Kir4.1 currents between WT and D/+ mice (Figure 3A,B,E,F) (WT; n = 11, 3 mice and D/+; n = 9, 3 mice; P-value = .2162 for total current and P-value = .0722 for BaCl2-sensitive current, two-way ANOVA with Sidak multiple comparisons). In contrast, in spontaneously seizing adult D/+ mice, the total current (Figure 3C,D) and Kir4.1 current (Figure 3G,H) amplitudes in reactive astrocytes were significantly reduced by 30% and 36%, respectively (WT; n = 10, 3 mice and D/+; n = 17, 3 mice; P-value = .0085 for total current and P-value = .0245 for BaCl2-sensitive current, two-way ANOVA with Sidak multiple comparisons). These data are suggestive of a disruption in the astrocytic K+ buffering capacity in seizing D/+ mice. Interestingly, a trend for an increase in the total and Kir4.1 current was observed in young, preseizing D/+ mice.
3.4 Expression of glutamine synthetase is reduced in spontaneously seizing SCN8A mice
Another important function of astrocytes is the uptake of glutamate and its conversion to glutamine, a precursor for both glutamate and GABA synthesis.6 This conversion is carried out by the enzyme glutamine synthetase (GS), which is necessary to supply neurons with neurotransmitter precursors and to prevent the reversal of astrocyte glutamate transporters and the release of glutamate from astrocytes. Staining of GS, as defined as percent area covered, was reduced in cortical (Figure 4A-C) and hippocampal (Figure 4D-F) astrocytes from spontaneously seizing D/+ (n = 6) mice compared to WT (n = 7) mice (Table 1; 4 FOVs per mouse). These results indicate that glutamate handling is likely impaired in spontaneously seizing D/+ mice and could contribute to pathology in this model.
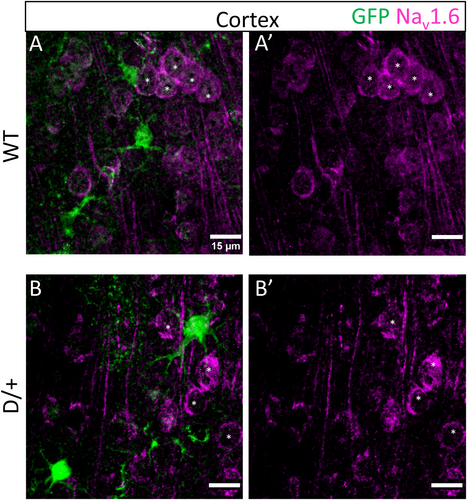
3.5 Astrocytes do not express the voltage-gated sodium channel, Nav1.6
Previous studies have suggested that astrocytes express Nav1.6.26-28 Since SCN8A encephalopathy is caused by mutations in SCN8A, we investigated whether astrocytes in spontaneously seizing D/+ mice express Nav1.6 using immunolabeling techniques. As expected, Nav1.6 labeled neuronal somata (asterisks) in WT (Figure 5A: n = 3 mice) and D/+ (Figure 5B: n = 3 mice) mice. However, Nav1.6 did not colocalize with the astrocyte reporter GFP fluorescence in the cortex or hippocampus (data not shown). Despite SCN8A encephalopathy being caused by mutations in SCN8A, our data suggest that astrocytes do not express Nav1.6 and their dysfunction in this disease is likely secondary to aberrant neuronal activity caused by neuronal expression of the mutated Nav1.6.
4 DISCUSSION
SCN8A encephalopathy is caused primarily by gain-of-function mutations in the neuronal sodium channel Nav1.6 and causes severe, early-onset seizures and SUDEP.29 Due to the neuronal expression of Nav1.6 mechanistic studies into SCN8A encephalopathy have focused on the impact of mutations on the excitability of excitatory and inhibitory neurons. However, there is growing appreciation for the involvement of other cell types, principally astrocytes and microglia, in epilepsy pathology.6, 14 In the current study, we sought to investigate alterations in microglia and astrocyte morphology before and after the onset of spontaneous seizures in a knock-in mouse model carrying the human SCN8A mutation N1768D. We found that (1) astrocytes, but not microglia, become reactive in spontaneously seizing mice, (2) astrocyte reactivity is not observed at a time point before the onset of spontaneous seizures even though increases in neuronal excitability at this earlier time point have been reported,3 (3) reactive astrocytes have reduced Kir4.1 channel currents and reduced GS expression suggesting an impairment in K+ ion uptake and glutamate homeostasis, and (4) astrocytes do not express Nav1.6, suggesting that changes in astrocyte physiology are in response to proepileptic alterations in surrounding neuronal networks. This study provides support for a previously unappreciated role for astrocytes in SCN8A encephalopathy.
Astrocytes are key cells that function to maintain the homeostasis of the brain through buffering ions, such as K+, removing neurotransmitters from the extracellular space, and supporting neurons metabolically, among numerous other roles.30 Following insults to the brain, astrocytes become reactive and exhibit stereotypic changes in morphology and upregulate cytoskeletal proteins, such as GFAP and vimentin.7 Despite these stereotypic changes, the term astrocyte “reactivity” refers to a highly heterogenous process that alters astrocyte physiology in a context-dependent manner.31 The astrocytic response to CNS insults can be either beneficial or detrimental depending on the type of astrocyte responding (ie, protoplasmic vs. fibrous, cortical vs hippocampal), the type of eliciting event (ie, seizure or infection), and the temporal course of the insult (ie, early- vs late-stage disease).32, 33 The Barres group demonstrated that astrocytes responding to either systemic LPS injection or middle cerebral artery occlusion (MCAO) become reactive and can be neurotoxic (A1) or neuroprotective (A2), respectively.31, 34 Subsequent work using single-cell RNAseq of isolated astrocytes from various disease models and human samples have revealed astrocyte disease-specific gene expression profiles in Huntingtin's disease (HD), Alzheimer's disease (AD), and multiple sclerosis that conform neither to the A1 nor the A2 profile.32, 35-37
While reactive astrocytes can be beneficial as in the case of spinal cord injury by forming a barrier between healthy and necrotic tissue or in Toxoplasma gondii infection by producing chemokines to recruit helpful immune cells, reactive astrocytes can also be detrimental.38, 39 A common feature of reactive astrocytes is the downregulation of genes involved in maintaining CNS homeostasis such as Kir4.1, aquaporin 4, and neurotransmitter transporters and degrading enzymes.40 Tong et al (2014) demonstrated that AAV-mediated restoration of reduced Kir4.1 expression in reactive striatal astrocytes ameliorated the uncoordinated gait in an HD mouse model. Further, the inability of reactive astrocytes to regulate GABA transmission worsens murine cognition in an AD mouse model.41 Loss of the homeostatic functions of astrocytes is the most prominent feature of reactive astrocytes in epilepsy.6, 9, 42
Astrocyte reactivity is often associated with seizures and epilepsy and is considered a “hallmark feature” of MTLE.6, 43 Surgical resection of the glial scar containing reactive astrocytes in epileptic patients alleviates the seizures.42, 44, 45 There is debate regarding whether astrogliosis is a cause or consequence of seizures, but growing evidence suggests that astrocyte reactivity alone can lead to seizures8, 9 and mutations in astrocyte-specific genes can lead to epilepsy, as in the case of Alexander's disease (mutations in GFAP) and EAST syndrome (mutations in KCNJ10).46, 47 Astrogliosis is often associated with the downregulation of genes necessary for the homeostatic functions of astrocytes such as KCNJ10, encoding Kir4.1, glutamate transporters, and glutamine synthetase.40, 48
The presence of reactive astrocytes in the D/+ model of SCN8A epileptic encephalopathy was previously reported using transcriptome analysis that detected >3-fold elevated expression of the pan-reactive astrocyte transcripts Gfap, Vimentin, and Serpin3a in forebrain of D/+ mice within 24 hours after the onset of seizures, as well as elevated GFAP staining in hippocampus.34, 49 The reactive astrocyte genes Gfap, Vimentin, and Serpin3a were the only astrocyte-specific transcripts detected by Sprissler et al (2017)31, 34, 49 from whole-forebrain homogenate and are shared by both A1 and A2 reactive astrocytes. Neurotoxic A1 reactive astrocytes are induced by TNF and IL-1β released from activated microglia.34 We assessed microglial reactivity in seizing D/+ mice using Sholl analysis and found no evidence for activated microglia. Therefore, the lack of microglial reactivity suggests that reactive astrocytes in seizing D/+ mice are not A1 neurotoxic astrocytes. Conversely, MCAO-induced A2 reactive astrocytes are characterized by cell cycle gene upregulation and the production of neurotrophic factors such as leukemia inhibitory factor (LIF).31 Our study did not directly address astrocyte proliferation, however, counts of Aldh1L1-eGFP+ cortical and hippocampal astrocytes from WT and seizing D/+ mice demonstrate no change in astrocyte numbers between these two groups. The neurotrophic factor LIF has been implicated in the pilocarpine model of seizures as a proinflammatory cytokine promoting microglia and astrocyte reactivity.50 LIF knockout mice have reduced microglial and astrocyte reactivity and are more likely to survive pilocarpine-induced seizures than their WT counterparts.50 In this context, an A2 neurotrophic factor could have a seizure-promoting effect. Our data indicate that reactive astrocytes in seizing D/+ mice do not conform to the A1 or A2 phenotype and future experiments utilizing RNAseq on isolated astrocytes from WT and seizing D/+ mice could clarify the consequences of astrocyte reactivity in SCN8A epileptic encephalopathy.
We extend the findings of Sprissler et al (2017) to show that in spontaneously seizing D/+ mice, cortical astrocytes are also reactive and that these reactive astrocytes have reduced Kir4.1 channel currents, with likely deficits in K+ buffering, and reduced glutamine synthetase expression. We also show that astrocyte reactivity or deficits in Kir4.1 function do not precede the onset of spontaneous seizures even though proexcitatory events have been initiated in medial entorhinal cortex neurons at this time point3 and mice are susceptible to audiogenic seizures.23 Metabolic stress has been shown to induce astrogliosis51 and it is tempting to speculate that astrocytes become reactive as a consequence of increased demand to buffer excess K+ and glutamate from hyperactive neurons after the onset of spontaneous seizures. Astrocyte reactivity may represent the tipping point in the manifestation of seizures in SCN8A encephalopathy, leading to the increase in seizure frequency and eventual death typical of this mouse model.52
Astrocyte K+ buffering through the Kir4.1 channel is essential for proper brain function. Animal models of astrocyte-specific deletion of KCNJ10 consistently show early mortality with seizures.10, 53-55 Ablation of Kir4.1 impairs K+ buffering56 and elevated extracellular K+ promotes neuronal excitability.18 In humans, mutations in Kir4.1 cause EAST syndrome.47 Some of these mutations have been characterized as loss-of-function, thus impairing K+ buffering through Kir4.1.57 In addition to its contribution to K+ buffering, impairment of Kir4.1 function also reduced glutamate clearance.10, 54 This is likely due to the effect of Kir4.1 on the very negative resting membrane potential of astrocytes and the electrogenic nature of glutamate transporters which rely on the negative membrane potential to import glutamate.10, 30, 53, 58 Thus, perturbations in Kir4.1 function can contribute to seizures and epilepsy.
Our study also found that glutamine synthetase staining was reduced in spontaneously seizing D/+ mice. Glutamine synthetase is predominantly expressed by astrocytes and its function is critical for the continued uptake of glutamate by glutamate transporters and for the balance of excitatory and inhibitory tone.6, 8, 59 In resected tissue from patients suffering from MTLE with hippocampal sclerosis glutamine synthetase expression is greatly reduced60 and when an inhibitor of glutamine synthetase, methionine sulfoximine, is chronically administered to rats they develop recurrent seizures.61 Glutamine synthetase expression is also reduced in reactive astrocytes in a slice model of seizure-like activity corresponding to a decrease in inhibitory neurotransmission, a result of a smaller pool of transmitter availability in predominantly GABAergic interneurons.8
Lastly, we assessed whether astrocytes in spontaneously seizing D/+ mice express Nav1.6 since it has been reported that astrocytes can express Nav1.6 in pathological settings.27, 28 However, astrocytes in WT and spontaneously seizing D/+ mice did not express Nav1.6. The lack of Nav1.6 expression in astrocytes is not surprising and is likely due to the absence of Rbfox RNA-binding protein expression in astrocytes.62 Rbfox proteins are expressed in neurons and promote the expression of full-length Scn8a mRNA transcripts that contain alternative exon 18A. Cells lacking Rbfox proteins, including astrocytes, express alternative exon 18N with an in-frame stop codon that results in nonsense-mediated decay of the Scn8a transcript and lack of expression of Nav1.6 protein.62
In conclusion, the involvement of astrocytes in the pathology of SCN8A epileptic encephalopathy is supported by our findings, deepening our understanding of the mechanisms of this type of epilepsy. Our findings suggest that astrocyte functions become impaired in SCN8A encephalopathy, contributing to the manifestation of seizures and suggest new therapeutic directions for the treatment of this debilitating disease.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants R01 NS103090 and R01 NS120702 (MKP), NS034509 (WY) and Citizens United for Research in Epilepsy (ICW). We acknowledge the important contributions of Prof. Miriam H. Meisler.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. Experiments have been performed with all national and international guidelines. The principles outlined in the ARRIVE guidelines, Basel declaration, including the 3R concept, were considered when planning experiments. Our Animal Welfare Assurance Number is A3245-01. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.