Pseudohyperkalemia due to cryohydrocytosis in GLUT1 deficiency syndrome. A case report and literature review
IRCCS Istituto delle Scienze Neurologiche di Bologna: Reference Centre for Rare and Complex Epilepsies (EpiCARE).
This work has not been previously presented at meetings.
Abstract
Cryohydrocytosis is a form of stomatocytosis characterized by the leakage of sodium and potassium from red blood cells at low temperatures, characterized by pseudohyperkalemia. Stomatin-deficient cryohydrocytosis is an extremely rare variant that only recently has been related to pathogenic variants in the SLC2A1 gene, encoding the main glucose transporter of the blood–brain barrier and red blood cells, GLUT1. It follows that GLUT1 deficiency syndrome, a rare but significant cause of metabolic epilepsy, may present with stomatin-deficient cryohydrocytosis, although this correlation has only been reported in a few instances. We present the case of a patient carrying a novel de novo SLC2A1 pathogenic variant presenting with GLUT1 deficiency syndrome, pseudohyperkalemia, and splenomegaly consistent with cryohydrocytosis. We also review the previously reported cases of stomatin-deficient cryohydrocytosis in the literature. As highlighted by our case, elevated potassium levels are a cause of concern, and GLUT1 deficiency syndrome patients are thus at risk of being subjected to unnecessary examinations; pseudohyperkalemia may be underrecognized in clinical practice.
INTRODUCTION
The hereditary stomatocytoses are a series of dominantly inherited hemolytic anemias characterized by increased cation permeability of the erythrocyte membrane.1 Cryohydrocytosis is a rare variant of stomatocytosis characterized by the leakage of sodium and potassium from red blood cells at low temperatures (4–8°C).2 The low-temperature leaks cause pseudohyperkalemia, artifactually high plasma potassium readings in routine blood tests, as this cation leaks from the abnormal red cells when they are cooled down from the body to refrigerator temperatures after venepuncture.
Most cases of cryohydrocytosis result from mutations in the membrane domain of band 3 (anion exchanger 1, encoded by SLC4A1).3 More recently, an extremely rare variant of stomatin-deficient cryohydrocytosis has been described.4-8 This is caused by heterozygous pathogenic variants in SLC2A1, encoding GLUT1, the main glucose transporter in the blood–brain barrier (BBB), and red cell membrane. Patients present with the classical features of cryohydrocytosis associated with neurological manifestations and cataracts. Indeed, the term “GLUT1 deficiency syndrome” (GLUT1DS) encompasses a spectrum of neurological conditions sharing clinical worsening in states of low glucose availability, most commonly fasting. The “classical” GLUT1DS was described by De Vivo in 19919 and is characterized by developmental and epileptic encephalopathy. However, several other “non-classic” neurological manifestations due to GLUT1 deficiency have been subsequently described, including paroxysmal exercise-induced dyskinesia and epilepsy, paroxysmal choreoathetosis with spasticity, early onset absence epilepsy, and myoclonic atonic epilepsy.10
Very few cases with GLUT1 deficiency and stomatin-deficient cryohydrocytosis have been described thus far. Given its rarity and its “mild” hematologic phenotype, it is reasonable to hypothesize that cryohydrocytosis is at risk of being missed in patients with GLUT1DS. Moreover, while not dangerous for the patient, pseudohyperkalemia might represent a significant point of concern for the clinician if not recognized.
This was the case for a patient carrying a novel SLC2A1 variant, with an unusual phenotype of GLUT1DS previously described,11 for whom, however, the cause of pseudohyperkalemia has only now been identified as cryohydrocytosis. Herein, we will provide a comprehensive overview of stomatin-deficient cryohydrocytosis in GLUT1DS by describing our patient and reviewing previously reported cases.
1 CASE REPORT
A 21-year-old man came to our attention during the transition from pediatric to adult epilepsy care. His familial history was remarkable for psychiatric disorders from maternal lineage and epilepsy from paternal lineage.
The firstborn was delivered through cesarean section at 36 weeks of gestation due to preeclampsia.
During his neonatal period, he developed jaundice and congenital bilateral cataracts were detected. A slight delay was noticed during psychomotor development.
At 6 years of age, he developed almost daily seizures with a brief loss of awareness, occasionally associated with eyelid myoclonia. EEG demonstrated generalized epileptiform abnormalities (Figure 1). Seizures were more frequent in the morning and did not respond to antiseizure medications such as ethosuximide, levetiracetam, lamotrigine, and valproate.

At 9 years of age, he developed a slight coordination impairment, stable over time. He was admitted to the child Neuropsychiatry unit of our clinic to perform a full clinical assessment. While a definite diagnosis was not reached at the time, physical examination revealed a head circumference near the lower limit of normal and splenomegaly. Notably, serum potassium was noticed to be elevated at first (7.2 mmol/L), but following exams in the same laboratory during his hospital stay showed lower results (5.8 and 5.4 mmol/L), this was attributed to technical errors.
In the following years, several investigations were undertaken: brain MRI was negative for developmental abnormalities, karyotype analysis was normal, and neuropsychological tests at 12 years of age demonstrated mild intellectual disability.
At 15 years of age, based on the clinical picture, sequencing of SLC2A1 was performed, allowing the identification of a novel pathogenic variant (c.1336_1338del, p.Ile4436del) and, consequently, the diagnosis of GLUT1DS. Segregation analysis disclosed that the variant arose de novo.
Following diagnosis, a 4:1 ketogenic diet (KD) was then started, with a rapid, significant overall improvement in seizures, EEG, and quality of life (Figure 2).
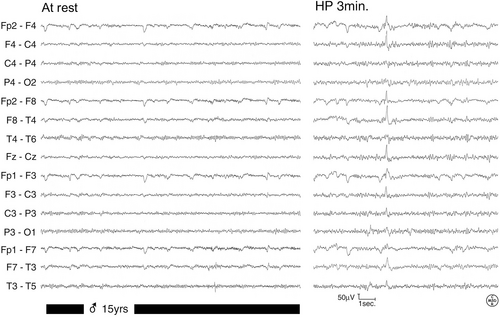
Unfortunately, periods of clinical worsening related to poor KD compliance persisted during adolescence. However, upon reaching adulthood the patient attained acceptable control of seizures, which have become weekly with lamotrigine and valproate.
Concerning blood potassium levels, the patient was repeatedly tested, giving inconstant, higher results than normal over the years: 6 mmol/L at 10 years of age, 6.4 mmol/L at 15 years of age, and 6 mmol/L at 16 years of age. Repeated electrocardiography tests always resulted unremarkable and, notably, electrolyte level assessment through arterial blood gas analysis showed normal potassium levels; nephrological evaluations excluded renal and suprarenal causes of hyperkalemia, in particular chronic kidney disease, reflux nephropathy, or obstructive uropathy. Following a review of the literature, it was concluded that GLUT1DS-related pseudohyperkalemia is the most fitting diagnosis.
On a last note, to appreciate the justified concerns of health professionals detecting hyperkalemia in such a context, a concerned laboratory doctor, unaware of our patient's condition, contacted the police force upon discovering his high potassium level to find and help the patient, who was meanwhile at home and in normal conditions.
2 LITERATURE REVIEW
A table with a summary of cases described in the literature, as well as ours, is provided below (Table 1).
| Authors | Description | SLC2A1 mutation |
|---|---|---|
| Lande et al. (1982)4 | First two cases of stomatin-deficient cryohydrocytosis |
Ile435 or Ile436del in one patient Not known in the other |
| Fricke et al. (2004)5 |
First description of neurological dysfunction in stomatin-deficient cryohydrocytosis: Two cases described, one from Ref. [4] |
Ile435 or Ile436del in the patient from Ref. [4] Gly286Asp |
| Weber et al. (2008)6 | Correlation between nonclassical GLUT1DS and abnormalities of red blood cell membrane (echinocytosis) | Gln282_Ser285del |
| Flatt et al. (2011)7 | Confirmation that the two patients of Ref. [5] suffered from GLUT1DS | Same as Ref. [5] |
| Bawazir et al. (2012)8 | Pseudohyperkalemia in a case of GLUT1DS with cryohydrocytosis | Ile435 or Ile436del |
| Our case (2014, 2022)11 | A new SLC2A1 mutation causing pseudohyperkalemia | Ile446del |
In 1982, Lande et al.4 described a patient with congenital hemolytic anemia and cryohydrocytosis, in which erythrocytes lacked a band 7 membrane protein.
In 2004, Fricke et al. presented four patients suffering from hereditary stomatocytosis, of which one was Lande's original cryohydrocytosis patient, here clinically characterized.5 All these cases lacked the band 7 erythrocyte protein, that is, stomatin.
Along with Lande's patient, another person in this series was affected by cryohydrocytosis: the most striking feature in these two patients was the presence of neurological dysfunction, absent in the other two cases with stomatocytosis.
The former patient presented hyperbilirubinemia and significant hepatosplenomegaly. Additionally, since the first year of life, he suffered from cerebellar ataxia and seizures, as well as communicant hydrocephalus, for which ventriculoperitoneal shunting was placed. Most notably, during this procedure, the patient underwent a hemolytic crisis. Other significant features were mild intellectual disability, macrocephaly, brachydactyly, and congenital cataracts. Hyperkalemia was noted but no ECG abnormalities were found.
The latter patient had a similar phenotype consisting of hepatosplenomegaly, cataract, seizures, and intellectual disability.
In 2008, Weber et al.6 described a family in which paroxysmal exercise-induced dyskinesia was present across three generations. The two children of the index case also presented with epilepsy, which worsened in the morning before breakfast and improved after meals. CSF analysis showed borderline to low glucose levels, which prompted the authors to consider GLUT1DS, confirmed by the detection of an inframe deletion in SLC2A1 (c.1022_1033del, p.Gln282_ser285del), located in a transmembrane segment of GLUT1. A key feature in all the family members was hemolytic anemia and deformed, “spiky” erythrocytes (echinocytosis). It was hypothesized that the GLUT1 deficiency led red blood cells to loose ions through the transporter. This was the first instance in which a GLUT1 deficiency/ SLC2A1 mutation was found to be correlated to cation leakage by erythrocytes.
In 2011, Flatt et al.7 identified two different SLC2A1 mutations in the two patients reported by Lande and Fricke: the missense variant p.Gly286Asp and the inframe deletion p.Ile435del (or p.Ile436del).
In 2012, Bawazir et al.8 described a pediatric patient with the same inframe deletion p.Ile435del (or p.Ile436del). During her first hours of life, the child developed hyperbilirubinemia, elevated liver function tests, hyperkalemia, and splenomegaly. Anemia persisted through her perinatal period of life, later remitting; echinocytes and stomatocytes were found in a peripheral blood smear. Neurologically, she presented with periventricular calcifications as shown by brain CT, diffuse hypertonia, nystagmus, and epilepsy progressing to status epilepticus in one instance. She was also found to suffer from bilateral cataracts. Hyperkalemia was later found to be caused by low temperatures. A ketogenic diet improved control over seizures and the overall clinical picture.
3 DISCUSSION
We reported a patient with a novel SLC2A1 variant causing GLUT1DS characterized by drug-resistant absence epilepsy responding to KD, associated with pseudohyperkalemia and cataracts. This phenotype was similar to previously reported cases with GLUT1DS and pseudohyperkalemia due to stomatin-deficient cryohydrocytosis, thus expanding the genotypes associated with this peculiar condition.
Our report aims to highlight the importance of recognizing this rare association, which may have relevant clinical implications. Indeed, hyperkalemia is a laboratory finding that must be readily addressed, especially when severe, as it may lead to potentially fatal cardiac arrhythmia. Moreover, incorrectly treating pseudohyperkalemia might also be deleterious. GLUT1DS, although uncommon, is a condition that is steadily increasing in recognition and diagnosis, meaning that pseudohyperkalemia might be more common than currently thought. Nonetheless, stomatin-deficient cryohydrocytosis still appears to be a remarkably rare entity compared with the incidence of GLUT1DS, meaning that only specific SLC2A1 mutations can induce temperature-dependent cation loss and erythrocyte deformities.
The mutation discovered in our patient (p.Ile446del) affects a transmembrane portion of the GLUT1 receptor located near the previously reported mutation in Ile 435–436, described in 2011.7 The closeness of this mutation could point to a similar pathophysiological mechanism, meaning that mutations of the transmembrane portions of GLUT1 lead to both glucose transport impairment and low-temperature cation leakage.
Stomatin is an integral membrane, lipid raft-associated protein which has been found to interact with various other proteins, modulating their work.12, 13 As for the reasons of stomatin deficiency in this form of cryohydrocytosis, the main proposed hypothesis stems from the knowledge that stomatin binds GLUT1, inhibiting glucose uptake and triggering dehydroascorbic acid uptake.14 Therefore, stomatin downregulation may be viewed as a way of enhancing alternative energy pathways in absence of viable glucose transportation, although such an idea has been debated.12, 15, 16 Moreover, stomatin may play other key roles, especially in the maturing reticulocyte.15
4 CONCLUSIONS
The GLUT1DS spectrum encompasses a wide range of entities showing significant clinical heterogeneity. While stomatin-deficient cryohydrocytosis has only been described in very few individuals across the world, we expect that our report will facilitate both neurologists and hematologists to diagnose this underrecognized condition in further patients with GLUT1DS, avoiding unnecessary clinical concern and investigations due to pseudohyperkalemia.
AUTHOR CONTRIBUTIONS
A.F. wrote the first draft. L.M., M.S., L.L., and F.B. revised the manuscript providing corrections. All the authors approved the final version.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose.
REFERENCES
Test yourself
-
Which is the gene encoding for the Glucose Transporter 1 (GLUT1)?
- SLC2A1
- SLC4A1
- SLC2A4
- CACNA1A
- KCNAB1
-
In cryohydrocytosis, red blood cells are affected by:
- Cold temperatures (4–8°C)
- Hot temperatures (36–40°C)
- Moderate temperatures (20–24°C)
- B and C
- A and B
-
Pseudohyperkalemia is:
- A harmless finding
- A medical urgency
- Potentially harmful in the long run
- B and C
- None of the above
Answers may be found in the supporting information.




