Electrostatic charge injection for reusing face masks: Mechanisms, performance, and a household alternative
Abstract
The COVID-19 pandemic underscores the effectiveness of face masks in combating respiratory infectious diseases and the importance of adequate supply. However, the widespread use of disposable masks has led to severe environmental pollution. In this study, we propose a two-step strategy for mask reuse, aimed at both mitigating mask waste pollution and improving mask availability in future epidemic outbreaks. Our strategy involves disinfection and corona charging processes, enabling surgical masks to maintain a filtration efficiency of 88.7% even after five cycles of reuse. We highlight the crucial role of volume charges over surface charges in maintaining filtration performance stability and durability, and we visualize the underlying mechanisms using energy band diagrams and potential well models. Additionally, we introduce a simple household solution for simultaneously drying and charging, making it accessible for widespread use. Our research offers a viable strategy for promoting environmental sustainability and alleviating mask supply pressures during significant public health crises.
1 MAIN
Amid the global struggle with the lasting impacts of the COVID-19 pandemic, experts foresee the recurring global pandemic outbreaks,1-3 emphasizing the crucial requirement for ongoing preparedness. While face masks have unquestionably emerged as effective and economically viable protective measures, the widespread use of disposable masks, particularly those made from non-degradable materials, poses significant environmental hazards.4, 5 It is estimated that 129 billion face masks are consumed globally each month,6 producing a substantial amount of microplastics. Current strategies for managing mask waste encompass several methods, such as incineration, which, although reducing mask volume, raises concerns about air pollution.7, 8 Moreover, landfill disposal, while offering a short-term remedy, amplifies long-term environmental challenges due to the non-biodegradable nature of most masks.9, 10 Another avenue pursued is recycling and reprocessing. While recycling used masks to manufacture industrial products is a viable approach, it comes with inherent limitations and challenges, as it involves complex processes and may not always be cost-effective.11, 12
To address the environmental issues stemming from discarded masks, biodegradable materials have been proposed. Biodegradable materials include a range of options, such as petroleum-based polymers (e.g., polyvinyl alcohol, polyvinyl butyral, polybutylene succinate, and polybutylene adipate terephthalate) and bio-based polymers (e.g., cellulose, polylactic acid, and polyhydroxyalkanoates).13-15 Biodegradable materials can reduce the degradation time to less than 200 days;16 in comparison, polypropylene masks require centuries to break down into microplastics and never fully decompose.17 However, the market remains hesitate considering that the cost of biodegradable masks is an order of magnitude higher than polypropylene masks, and their production capacity is limited.
An economically viable solution is reusing face mask, a practice that not only mitigates environmental pollution but also helps address mask shortages during critical periods, such as pandemics or other public health emergencies. It is necessary to thoroughly remove pathogens from used masks through disinfection methods (e.g., the use of alcohol, heating, ultraviolet light, and others)18 to ensure their safe reuse. While existing disinfection methods though effectively kill pathogens, many also deplete or remove all static charges. Static charges create non-uniform electric fields within the mask. These electric fields induce a dielectrophoretic phenomenon, wherein aerosols are subjected to electrostatic forces, causing them to move toward the fibers and eventually be captured. This process is called electrostatic adsorption. In practice, electrostatic adsorption accounts for up to 80% of the overall filtration efficiency of electrostatic masks such as surgical masks or N95 respirators.19 This explains why these masks can maintain excellent breathability while achieving high filtration efficiency. The capture efficiency of electrostatic adsorption is proportional to the charge density of the filter medium.20 Therefore, the charge storage capacity determines the filtration performance, and replenishing the charge after disinfection is crucial for restoring efficient air filtration.
Various methods for charge replenishment are documented in the literature. For instance, Hossain et al.21 utilized a high-voltage power supply to recharge decontaminated KN95 masks. The procedure involved washing the masks at 40 °C with detergent in a washing machine, followed by thorough drying. Subsequently, a potential of 1 kV was applied to the masks for 60 min, leading to a notable recovery in filtration efficiency from 75% to 95%. In another study, Sugihara22 employed a van de Graaff generator to swiftly recharge N95 masks, resulting in an elevation in filtration efficiency from 72% to 94% within 3 min. The authors attributed this quick recovery to the use of a high voltage of 100 kV and emphasized the safety of this approach due to its extremely low current, with the metal electrode remaining electrically floating. Moreover, several studies have reported the rapid restoration of charge in decontaminated masks using high-voltage power supplies, with voltages ranging from 10 to 25 kV. These methods achieved effective recovery within minutes23-25 or even as quickly as 30 s.26 Furthermore, Wang et al.27 introduced a method that utilizes an ordinary hair dryer to restore charges to masks. This method involves immersing the masks in hot water, typically between 60 and 80 °C, for 30 min, followed by a 10-min drying process using a hair dryer. After this procedure, the static electricity of the surgical mask could be recovered to 90% of the level of a new mask, effectively restoring filtration efficiency to over 95%.
Although these studies have provided essential insights and guidance for addressing the issue, several unanswered questions remain that require further exploration and clarification. Specifically, accurately characterizing the electrostatic charge on the filtration layer is crucial for understanding its relationship with filtration efficiency. Current methods for measuring electrical charge either concentrate on the entire mask, which does not accurately reflect the charge on the filtration layer, or they assess the charge specifically on the filtration layer, often neglecting the potential impact of contact electrification during the layer's extraction from the mask. Moreover, it is important to recognize that current tools for direct charge measurement primarily target surface charges, leaving the influence of volume charges on filtration performance under-investigated. This influence of surface and volume electrostatic charges on filtration efficiency remains largely unexplored.
In this research, we unveil the potential for reusing disposable surgical masks through a dual-step strategy combining disinfection with 75% alcohol and subsequent performance restoration via corona charging. By accurately monitoring the surface charge level on the filtration layer and investigating its correlation with filtration performance, we find that not only the readily measurable surface charges, but also the more elusive volume charges—particularly those deeply embedded within the forbidden band introduced by a high bias—play a pivotal role in ensuring the stability and durability of filtration performance. Leveraging energy band diagrams and potential well theories, we shed light on the underlying mechanisms of this phenomenon. Applying a high bias voltage facilitates the migration of charges into deep potential wells, where their release is significantly hindered by the elevated energy barriers, thereby enhancing charge retention and, by extension, the stability and durability of the mask's filtration capabilities. By optimizing the corona charging parameters, we demonstrate that the mask's filtration efficiency can be maintained at 88.7% even after five cycles of reuse. Furthermore, we propose a simple yet effective method that can be easily implemented at home, allowing for the simultaneous execution of the drying and charging processes. Our findings lay a foundation for the sustainable reuse of face masks, offering a viable solution to reduce environmental impact and ease the demand for masks during epidemics.
2 RESULTS
2.1 Surgical mask reuse via decontamination and charge re-injection
- Surface charges. The ionization of air molecules, primarily CO3− ions in negative corona charging,28 results in the deposition of a substantial number of negative ions on the filter medium. Serving as energy carriers, these ions convey the energy from free electrons at the needle electrode to the fiber surface. Upon charging the fiber surface, they revert to molecules and are subsequently dispersed. These surface charges typically reside in surface traps located within approximately 0.5 μm from the surface.29
- Shallow trap charges. Under elevated corona voltage conditions, neutral excited species propelled by the corona wind transfer charges from surface traps into the material, where they are captured by shallow traps.29, 30 These shallow traps are inherently linked to the physical disorder that arises from the various configurations of PP molecular chains.31 They represent localized states within the forbidden band (band width ~ 8.3 eV),32 situated within a narrow energy range of 10−2–1 eV31 from the extended states (i.e., conduction and valence bands), rendering them accessible to carriers. Shallow trap charges possess a certain degree of mobility and can migrate through thermal activation or hopping.31, 33, 34
- Deep trap charges. When the bias voltage is increased further, some charges can penetrate deep traps, leading to the emergence of an additional category of volume charge, referred to as deep trap charges. These deep traps originate from chemical disorders caused by impurities, which introduce extra energy levels within the forbidden band.35 Typically, these additional energy levels are situated deeper within the forbidden band.31 As a result, charges confined in deep traps demand more energy to be released, indicating their enhanced stability. These volume traps, including both deep and shallow traps, are located within a few 100 mm beneath the surface.36 Given that the PP fiber used in this study has a diameter ranging from several to 10 of micrometers (Supplementary Figure 1c), this implies that volume charges are capable of permeating the entire sample.
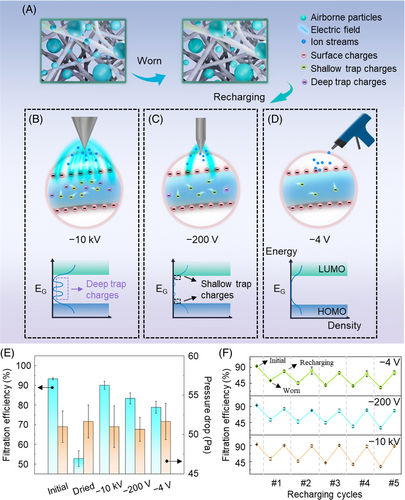
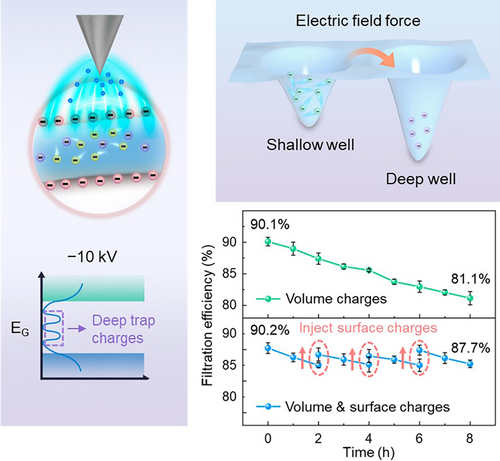
The strong electric field generated by the high-voltage power source, capable of delivering voltages exceeding −10 kV, facilitates the injection of a substantial quantity of volume charges, including a notable amount of deep trap charges (Figure 1B). In contrast, the negative ion generator (providing a bias voltage of −200 V) mainly introduce shallow trap charges due to its comparatively weaker electric field (Figure 1C), and the anti-static gun (approx. −4 V) primarily introduces surface charges as a result of the deposition of the ion stream (Figure 1D). It is crucial to note that the terms “deep” and “shallow” in relation to volume charges do not refer to their spatial dimensions; instead, they indicate the depth of the potential wells where charges reside, representing the amount of energy required for the charges to detrap31, 37, 38 (see energy band diagrams in Figure 1B–D).
As a result, a charging bias of −10 kV can effectively increase the capture efficiency of a decontaminated and dried mask from 52.8% to 90.1%, closely approximating the efficiency of a new mask at 93.3%. It is worth noting that this method is highly versatile, proving to be equally effective for masks across various brands. (as seen in Supplementary Figure 3). On the other hand, masks recharged with lower biases (−200 V for the negative ion generator and −4 V for the anti-static gun) display marginally lower efficiency levels, which are nonetheless suitable for environments with relatively low pollution levels (Figure 1E). None of the charging methods significantly affect the breathability of the masks (Figure 1E and Supplementary Figure 3). Importantly, the process of replenishing charge to restore filtration performance is repeatable. Periodic tests indicate that filtration efficiency can be effectively restored even after five decontamination-charging cycles, especially when using high bias voltage of −10 kV, with the efficiency remaining around 88.7% (Figure 1F). Throughout the process, the mask's appearance and microscopic morphology of the filtration layer remain unchanged (Supplementary Figure 4). This emphasizes that as long as the fiber structure remains intact, this method can be reliably repeated with careful cleaning and charge reinjection.
2.2 The correlation between static charge levels and filtration efficiency
To accurately monitor the charge level on the filtration layer and assess its impact on filtration efficiency, we conducted focused studies specifically on this layer. The filtration layer plays a significant role in determining the filtration efficiency of the mask, considering that it contributes nearly 80% to the overall filtration efficiency (Supplementary Figure 5). As depicted in Figure 2A, there is a noticeable reduction in surface charge levels after 10 h of wear, resulting in a decrease in filtration efficiency from 92.5% to 84.5%. After decontaminating the used mask and allowing it to dry, the charges are almost entirely depleted, leading to a 97.7% reduction in electric potential compared to a new mask (from −2.62 to −0.06 kV). As a result, the filtration efficiency also drops to a mere 52.8%. Subsequently, we reintroduced charges to the filtration layer and observed a positive correlation between electric potential and filtration efficiency (Figure 2B). The corona charging at −10 kV proved to be the most effective, achieving an electric potential of −4.76 kV and the highest filtration efficiency of 90.7%.

The stability of filtration performance is critical in determining the shelf life of a mask. As depicted in Figure 2C, the filtration layer treated at −10 kV demonstrates only a minor decrease in removal efficiency, from 90.3% to 87.1%, after a 15-day storage period in an indoor exposure environment. In contrast, the filtration layer treated at −4 V exhibits the poorest stability, with a decrease from 76.2% to 61.8%. This is because low bias-introduced charges are predominantly surface charges, which are prone to dissipation due to compensation effects from polar groups in water molecules and ions with opposite charges in the atmosphere. It is noteworthy that the rate of charge dissipation is significantly influenced by storage conditions. When the mask is stored in an environment with low or no airflow (as indicated by the black and red lines in Supplementary Figure 6), charge degradation occurs at a relatively slow pace. If the mask is stored in a sealed environment, similar to commercially available masks sealed in plastic packaging, charge degradation occurs at a negligible rate. Conversely, when exposed to an environment with a continuous influx of aerosol particles, charge degradation accelerates as charges are transferred to the particles (as indicated by the blue line in Supplementary Figure 6).
Building upon the filtration layer recharged at −10 kV, we further investigated its durability. Our observations revealed a reduction in removal efficiency from 90.1% to 81.1% after an 8-h continuous test (Figure 2D). Note worthily, the 81.1% efficiency still significantly outperforms that of samples where charges were completely depleted, as demonstrated by the decontaminated and dried sample shown in Figure 2B. This finding suggests that volume charges can consistently contribute to particle removal, even when surface charges are neutralized by particles (as indicated in Supplementary Figure 6), thereby ensuring a minimum standard of protection. Furthermore, we found that timely supplementation of surface charges resulted in a swift recovery of the capture efficiency. With three supplements of surface charges, we were able to maintain a filtration efficiency of 87.7%, even after the period of durability testing (Figure 2D). This highlights the effectiveness of the high-bias corona charging treatment when used alongside surface charge replenishment. The efficacy stems from the stable volume charges, which ensure stable and durable filtration performance, while the timely replenishment of surface charges facilitates a rapid restoration of filtration efficiency. This elucidates why the approach of pre-injecting volume charges, coupled with the use of contact electrification to supplement surface charges, as reported in our prior work,39 can sustain prolonged protective efficacy.
2.3 The impact of charge types on filtration efficiency: mechanistic insights and experimental validation
To elucidate the underlying mechanism contributing to the observed enhancement in stability and durability of the filtration layer treated with high-bias corona discharge, we propose a hypothesis that the application of high bias promotes the migration of charges toward deep trap sites. The detrapping of these deep trap charges requires a higher energy input, which inherently increases their stability (Figure 3A). This implies that although surface charges gradually diminish during use, the volume charges within the deep trap sites remain unaffected. Consequently, this ensures a minimum standard of filtration performance. Furthermore, these stable volume charges could potentially stimulate the reinduction of surface charges (Figure 3B).
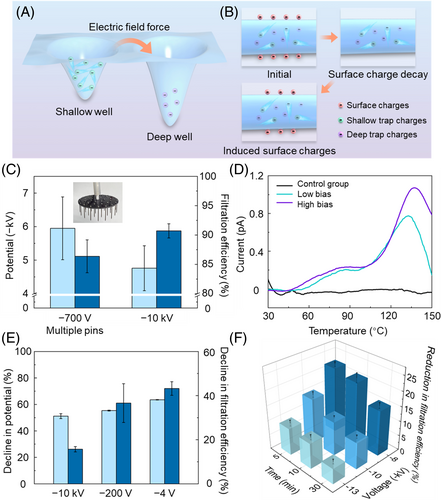
To validate our hypothesis, we utilized another negative ion generator equipped with a multi-pin electrode, which allowed for the injection of a larger quantity of charges under a relatively low voltage. Despite the voltage on the pin being −700 V, it could impart a higher surface potential to the sample compared to that charged at −10 kV (Figure 3C). However, the filtration efficiency remained lower than that charged at −10 kV. This discrepancy is attributed to the presence of deep trap charges. At present, no direct methodology exists for characterizing volume charges, including deep and shallow trap charges. The electrostatic tester used in this study can measure only surface charges, a limitation shared by other conventional methods referenced in the literature, such as Faraday cups and voltmeters. As a result, these methods may underestimate the level of electrostatic charge in the samples, particularly in those treated with high voltage, as high voltage induces a significant number of volume charges. Here, we utilized the thermally stimulated discharge (TSD) as an indirect evaluation technique for volume charges. According to the results obtained from the differential scanning calorimetry (DSC), the PP nonwoven material exhibited an endothermic peak at 159.25 °C (Supplementary Figure 7). Therefore, the temperature range for the TSD tests was set between 30 and 150 °C. The sample with charges completely removed did not exhibit any significant discharge characteristics throughout the test period (indicated by the black line in Figure 3D). In contrast, a consistent low-temperature peak appeared at approximately 85 °C under both high and low bias conditions, suggesting the existence of approximate shallow trap charges. For the high-temperature discharge current peak, however, there was a notable rightward shift under a high bias scenario (−10 kV, indicated by the purple line in Figure 3D) when compared to the conditions of low bias (−700 V, indicated by the green line in Figure 3D). This shift indicates that charge detrapping requires more energy, and the increased peak current signifies a larger quantity of deep trap charges being captured. This explains why samples with high surface potential exhibit lower filtration efficiency compared to those with low surface potential. This is because the terms “high” and “low” here only represent surface charge levels and do not reflect the fact that samples subjected to high bias possess a significant amount of volume charge.
According to the TSD results, 105 °C is sufficient for the elimination of shallow trap charges. To exclusively examine the role of deep trap charges, the charged filtration layers were subjected to a heating process (110 °C, 12 h) for the thermal cleaning of shallow trap charges. Consequently, all samples exhibited a decrease in their surface potential by 51% to 64%. The retention of surface potential can likely be attributed to the remaining deep trap charges inducing surface charges. Notably, the filtration efficiency of the high-bias-treated sample showed the smallest reduction of 15.7%, while the other samples experienced significant decreases of approximately 40% (Figure 3E). This observation provides additional evidence supporting the stability of the deep trap charges. Under the same treatment condition (heat at 110 °C for 12 h), it was observed that increasing both the voltage and duration of corona charging contributed to enhanced performance stability at high temperatures. After subjecting the filtration layer to −13 kV for 30 min, the fiber structure remained intact (Supplementary Figure 8) and there was only a 5.3% reduction in filtration efficiency. This suggests that more charges entered the deep capture traps, and these charges remained stable and did not dissipate even at high temperatures (Figure 3F). Although some literature reports saturation of charges within a couple of minutes of charge injection using corona discharge,40-42 our results indicate that longer treatment times provide charges ample time to migrate to deep capture traps, thereby enhancing performance stability. However, increasing the corona voltage further may not contribute to enhancing performance stability, due to the presence of back corona effects in the charged fabric.43
2.4 Filtration performance of recharged surgical masks
In the preceding sections, we delved into the impact of charge levels and types on the filtration performance of individual filtration layers. Now, we shift our focus back to the mask as a whole. As illustrated in Figure 1E, applying high-bias corona charging effectively restores the filtration efficiency of masks to levels comparable to those of new masks. However, questions regarding the stability and durability of these recharged masks arise, as these factors are pivotal in determining their longevity and consistent protective performance. Stability ensures that masks retain their protective efficacy over their intended storage period. Our results demonstrate that, after sealing −10 kV-treated masks in self-sealing bags for 30 days, the filtration efficiency remains nearly unchanged. Masks subjected to −200 and −4 V treatments experience minimal filtration efficiency declines of 1.5%. This outcome indicates that, under proper storage conditions, the filtration performance of recharged masks can be preserved for extended periods (Figure 4A). Durability, on the other hand, assesses the mask's ability to provide sustained protection. The durability of a mask is significantly influenced by environmental conditions, such as particulate matter (PM) concentration, airflow rate, and humidity. To evaluate the performance of recharged masks in challenging environments, we conducted an 8-h durability test in conditions with a PM2.5 index of 100 μg/m3. Similarly, the mask treated at −10 kV exhibits the least degradation in filtration efficiency, with only an 8.3% reduction, showcasing exceptional durability for prolonged use. Conversely, masks treated at −200 and −4 V show more significant reductions of 12.5% and 15.7%, respectively. Meanwhile, the mask treated at −10 kV also experiences the highest increase in pressure drop (12%). This increase is attributed to the mask's enhanced electrostatic adsorption capabilities, which lead to a higher adhesion of particles on the fibers (Figure 4B).
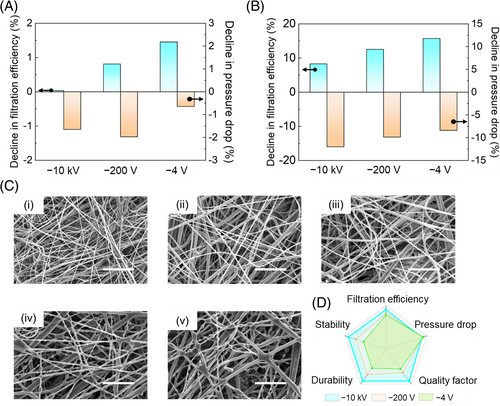
Examining the microscopic changes in fiber appearance during the particle capture process (Figure 4C) provides valuable insights into the effectiveness of particle capture by masks treated with high bias. Initially, the microfiber surfaces are pristine and smooth (i). After a continuous exposure to particles for 2 h, a small number of spindle-like particles begin to accumulate on the fiber surfaces (ii). These particles result from the drying of oily droplets generated from incense burning.44 As the exposure extends to four to 6 h, the quantity of spindle-like contaminants increases, and they start to merge with adjacent ones (iii and iv). After 8 h, these contaminants have visibly coalesced and expanded in size. These findings validate the ability of the recharged masks to effectively suppress particle penetration (v). For a comprehensive and intuitive comparison of the three recharging methods, we present a radar chart that compares five-dimensional parameters (Figure 4D). This chart illustrates that the recharging techniques do not noticeably impact breathing resistance (). Among the methods, high bias treatment using a high-voltage power source achieves the highest filtration efficiency (), resulting in the highest quality factor (). is an indicator of air filter performance, and it can be calculated using the formula . Additionally, owing to the outstanding stability of the deep trap charges, the high bias-treated samples exhibit superior stability and durability, as demonstrated in Figure 4A,B.
2.5 A household alternative for surgical mask reuse
Although the methods introduced above have proven to be feasible and effective, they all require specialized equipment. Is there a convenient, low-cost method that people can use at home? To answer this question, we propose a household solution utilizing commercially available negative ion hairdryers to infuse used masks with charges (Figure 5A). These hairdryers are equipped with a negative ion generation module, capable of emitting a significant quantity of negative ions, which can effectively rejuvenate the electrostatic charge of the mask fibers. Simultaneously, the airflow significantly accelerates the drying process. As demonstrated in Figure 5B, naturally drying a mask under ambient conditions with a relative humidity (RH) of 55% takes over 4 h. In contrast, drying time is reduced to approximately 3 h in an environment with 20% RH. Most notably, using the hairdryer can drastically reduce the drying time to about 5 min.

Before use, the microscopic structure of the filtration layer shows a pristine fiber surface (Supplementary Figure 9). After being worn for 10 h, a small number of particles are observed adhering to the fibers, highlighted by the red circles in Figure 5C. The minimal particle adhesion is likely due to the favorable air quality conditions in Hong Kong during the test period. Subsequent cleaning with 75% alcohol not only effectively removes these particles (as shown in Figure 5D) but also disinfects and cleans the mask. This dual functionality is made possible due to the mask material's high wettability to alcohol (Supplementary Figure 10). While soaking masks in hot or even boiling water can kill microorganisms, the mask's limited wettability in water hinders the complete removal of charges, especially volume charges, and poses challenges in thoroughly cleaning foreign particles from the mask's surface.
Figure 5E illustrates the dynamic changes in mask performance over time. Initially, as the mask is worn, a gradual decline in filtration efficiency is observed alongside an uptick in breathing resistance. This deterioration is a direct result of the mask absorbing moisture from the wearer's breath and collecting environmental contaminants. The subsequent decontamination and drying process effectively eliminates the accumulated moisture and pollutants, resetting the mask's breathing resistance to that of a brand-new one. However, this process also results in a substantial drop in the mask's filtration efficiency, as it strips away the electrostatic charges essential for trapping particles. Surprisingly, employing a negative ion hairdryer for just 10 min revitalizes the mask, boosting its filtration efficiency back to 85%, which is as effective as a fresh mask's 92% filtration capability. Impressively, this recharging maintains its efficacy even after five cycles, with the filtration efficiency slightly reduced to 83.7%. This at-home recharging solution stands out not only for both its practicality and accessibility, offering a lifeline for mask rejuvenation during critical times.
3 DISCUSSION
This study presented an effective strategy for the reuse of face masks by combining disinfection with charge re-injection techniques. We proposed a testing approach to precisely monitor surface charge on the filtration layer and accurately evaluate mask filtration performance. Our findings uncovered a notable discrepancy between surface charge levels and filtration efficiency, emphasizing the critical influence of volume charges, particularly deep trap charges, on filtration performance, especially in terms of stability and durability. The pivotal role of volume charges was demonstrated through energy band diagrams and potential well models, and validated through a series of direct and indirect experimental assessments. The recharged masks displayed a filtration efficiency of 90.1%, with a slight reduction to 88.7% after five reuse cycles. Furthermore, by applying a combined approach of high-bias corona charging and timely surface charge replenishment, we observed a minimal decline in filtration efficiency of only 2.5% over an 8-h test period. Additionally, we introduced a convenient household solution for simultaneously performing the drying and charging processes using a negative ion hairdryer. All the recharged masks exhibited excellent cellular biocompatibility (Supplementary Figures 11 and 12), indicating that the injected electrostatic charges only help restore filtration efficiency without introducing cytotoxicity. In summary, the approach we present, which combines disinfection with charge re-injection techniques, offers a versatile strategy for mask reuse. This method is highly adaptable, not only suitable for commercially available PP filter media but also applicable to electrostatic filters made using other processes. Furthermore, by incorporating specific materials, additional functionalities can be imparted to the filters. For instance, the photothermal properties of gold nanorods can be harnessed for pathogen eradication,45 while the nanosized copper46 or silver47 particles can be introduced to induce DNA single-strand breaks in cells, thereby providing antimicrobial effects.
4 METHODS
4.1 Samples and decontamination
Surgical masks (CityUMask, complied with the ASTM F2100-11 Level 2 standard) were utilized in their original state. To validate the method's universality, two other commercially available surgical masks were also investigated. Unless otherwise specified, the experiments were conducted based on the CityUMask. The decontamination process involved immersing masks in 75% alcohol for 3 min, with gentle agitation to enhance cleaning effectiveness. Following this procedure, the masks were left to air-dry in preparation for the subsequent experiments. During the air-drying phase, each mask was suspended to prevent contact with surrounding objects and eliminate the potential impact of contact electrification. To track the surface potential of the filtration layer, it was isolated by cutting it out and subjected to the same disinfection procedure as the entire mask for consistency. We also employed other disinfection methods for comparison (Supplementary Figure 13). While 75% alcohol significantly reduced electrostatic charges and consequently lowered filtration efficiency, it ensured effective sterilization and aided in removing adsorbed pollutants. This was attributed to the superior ability of ethanol to permeate mask materials. When a drop of 75% ethanol was applied, the mask material rapidly absorbed it. In contrast, the mask exhibited poor wettability with water, with a water contact angle of 152.5° (Supplementary Figure 10). Although this could help in retaining charges, it resulted in suboptimal disinfection and cleaning efficacy, regardless of the temperature used. Heat treatment at 100 °C was effective in eliminating pathogens, but it did not contribute to the cleaning process.
4.2 Charge injection methods
The corona charging process was conducted using a variety of devices. The primary device was a high-voltage power source (JMDC-P30-1 mA, JEMAN), which was coupled with a needle electrode (20-gage needle used for electrospinning, 38 mm-long, 0.6 mm-inner diameter, and 0.9 mm-outer diameter). Additionally, two distinct models of negative ion generators were employed: the JP-A2241, featuring a carbon-brush electrode, and the W660, which equipped a multi-pin electrode with each pin being 16.5 mm long and 0.5 mm in diameter, and a tip curvature of approximately 0.1 mm. An anti-static gun (Milty Zerostat 3) was also utilized, which had a needle electrode at the nozzle with a similar tip curvature. The high-voltage power source was capable of delivering an output voltage of up to −30 kV, which was used to supply high bias voltage for corona charging. Unless otherwise specified, a voltage of −10 kV was applied for 5 min to perform the charging. The negative ion generators operated at lower voltages, with JP-A2241 generating approximately −200 V, and W660 producing around −700 V at the pin tip. These devices also followed a 5-min charging process. The anti-static gun utilized the mechanical collision of two piezoelectric plates to generate a potential difference, which was then discharged through the tip of the gun nozzle to emit ion streams. The voltage at the tip was around −4 V. Pressing and releasing the trigger respectively emitted positive and negative ion streams. For each charge injection procedure, the trigger was pressed and released 30 times. To guarantee the exclusive injection of negative charges, the gun nozzle was directed at the sample only during the release of the trigger and directed away from the sample while the trigger was being pressed. In all three methods, the samples were placed atop a grounded electrode (aluminum foil) and oriented vertically 3 cm beneath the electrode. It is important to note that the different geometrical dimensions and shapes of the tips preclude a direct comparison between the three devices. In addition, the ambient humidity influences the types and ratios of ions produced during corona charging, which in turn impacts the efficacy of the charging process.28 For this study, the humidity level was 60 ± 5% and the temperature was controlled at 22 ± 0.5 °C.
4.3 Surface potential measurement
A non-contact electrostatic tester (JH-TEST) was utilized to measure the surface potential. The samples were suspended in isolation during testing to minimize potential interference from surrounding static-generating materials or devices. The probe of the electrostatic tester was positioned 2.5 cm away from the test point. This distance was ensured by the precise alignment of two light spots emitted by the electrostatic tester. The displayed number on the tester represented the real-time surface potential. Five locations were tested for each measurement (Supplementary Figure 14), and the average and standard deviation were calculated.
4.4 Household method
We also introduced a household method that utilizes negative ion hairdryers (Mi Ionic Hair Dryer H300) to execute both drying and charge injection simultaneously. The decontamination process aligned with the aforementioned method, followed by blowing with the hairdryer. The hairdryer provided an airflow velocity of 20 m/s and released an anion concentration of 50 million/cm3 at its outlet. During this process, the sample was positioned approximately 5 cm away from the hairdryer.
4.5 Filtration performance measurement
The filtration performance was evaluated using a custom-made testing platform utilized in our previous research,39 which assessed filtration efficiency, pressure drop, stability, and durability. PMs were generated by burning incense and delivered through compressed air, with an additional compressed air stream used to adjust the PM concentration. By modulating the flow rates of these two streams, the PM2.5 index could be adjusted within the range of 0–2000 μg/m3. Unless specifically stated otherwise, the PM2.5 index was maintained at 300 μg/m3, with a constant flow rate of 18 L/min throughout the study. Two A4-CG laser sensors were used to detect the number concentration of PMs, which represents the number of PMs in 0.1 L of air. Filtration efficiency was assessed by comparing the reduction percentage of the PM number concentration before and after passing through the tested sample, specifically for particles with an aerodynamic equivalent diameter between 0.3 and 0.5 μm. The pressure drop across the tested sample was gaged using a Testo 510 differential pressure gage. Mean values and standard deviations were calculated from measurements obtained from three samples. Durability tests were performed at a lower PM2.5 index of 100 μg/m3.
4.6 Cytotoxicity test
The biocompatibility of the mask filtration layer materials, sourced from the initial mask, masks recharged with a high-voltage power supply (−10 kV), and masks recharged with a negative ion hairdryer, was assessed using a live/dead assay with L929 cells. The filtration layer materials were cut into 5 mm × 5 mm to serve as samples, which were then incubated in the culture medium overnight. L929 cells were seeded onto a culture plate and co-cultured with the samples. After incubation periods of 24, 48, and 72 h, the cells were stained using the live/dead kit assay (Beyotime Biotechnology, Beijing, China) and subsequently imaged using a fluorescence microscope (TS100, Nikon, Tokyo, Japan). Furthermore, cell proliferation was evaluated using the cell counting kit-8 (CCK-8) assay (Beyotime Biotechnology, Beijing, China). At time intervals of 24, 48, and 72 h, a 1:10 (v/v) dilution of CCK-8 solution relative to the culture medium was introduced into each well, followed by absorbance measurement at 450 nm using a microplate reader (SpectraMax M5e, MD, USA).
4.7 Characterization
The morphology of the samples was examined using a scanning electron microscope (EVO MA10, ZEISS) operating at an acceleration voltage of 15 kV. The crystalline phase was analyzed via X-ray diffraction using a PANalytical X'pert3 diffractometer, which was equipped with Cu Kα radiation and operated at 40 kV and 40 mA. Differential scanning calorimetry (DSC 3, METTLER TOLEDO) was carried out in an argon atmosphere at a scanning rate of 10 °C/min to ascertain the melting characteristics of the PP melt-blown nonwoven fabric. The TSD current spectra measurement was carried out in a programmable temperature-controlled oven (EC1A, Sun Electronic Systems). The tests were performed in a short-circuit mode, with a 12-mm diameter copper plate serving as the electrode, which was directly pressed onto the sample surface. The temperature range for the measurements was set from 30 to 150 °C, with a heating rate of 3 °C/min. The current induced through the electrode was recorded using a Keithley 6517B, which was interfaced with a computer for data acquisition and analysis.
AUTHOR CONTRIBUTIONS
Zhengbao Yang conceived the idea and supervised the research. Zehua Peng proposed the idea, designed the research, performed the experiments, and drafted the paper. Zhiyuan Li, Xingcan Huang, and Xinge Yu contribute to the biocompatibility experiments and analysis. Michael K.H. Leung, Zuankai Wang, and Zhengbao Yang reviewed and revised the manuscript.
ACKNOWLEDGMENTS
The work described in this paper was supported by Innovation and Technology Fund (Project No. ITS/065/20) from Innovation and Technology Commission of Hong Kong Special Administrative Region, and General Research Grant (Project No. 11212021, No. 11210822) from the Research Grants Council of the Hong Kong Special Administrative Region.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings within this study are available from the authors upon reasonable request.