Electrochemical recycling of lithium-ion batteries: Advancements and future directions
Abstract
Lithium-ion batteries (LIBs) are at the forefront of technological innovation in the current global energy-transition paradigm, driving surging demand for electric vehicles and renewable energy-storage solutions. Despite their widespread use and superior energy densities, the environmental footprint and resource scarcity associated with LIBs necessitate sustainable recycling strategies. This comprehensive review critically examines the existing landscape of battery recycling methodologies, including pyrometallurgical, hydrometallurgical, and direct recycling techniques, along with emerging approaches such as bioleaching and electrochemical separation. Our analysis not only underscores the environmental and efficiency challenges posed by conventional recycling methods but also highlights the promising potential of electrochemical techniques for enhancing selectivity, reducing energy consumption, and mitigating secondary waste production. By delving into recent advancements and juxtaposing various recycling methodologies, we pinpoint electrochemical recycling as a pivotal technology for efficiently recovering valuable metals, such as Li, Ni, Co, and Mn, from spent LIBs in an environmentally benign manner. Our discussion extends to the scalability, economic viability, and future directions of electrochemical recycling, and advocates for their integration into global battery-recycling infrastructure to address the dual challenges of resource depletion and environmental sustainability.
1 INTRODUCTION
Since their introduction into the market, lithium-ion batteries (LIBs) have transformed the battery industry owing to their impressive storage capacities, steady performance, high energy and power densities, high output voltages, and long cycling lives.1, 2 There is a growing need for LIBs to power electric vehicles and portable devices as the world transitions to renewable energy, which highlights the importance of developing recycling techniques, which is crucial for preventing the depletion of rare resources, such as lithium, nickel, cobalt, and manganese, while meeting current electrochemical requirements. This topic is also supported by politically set targets and standards; for example, the European unit, stipulates that 45% of portable batteries must be collected by 2023, 63% by 2027, and 73% by 2030. Updated regulations have set minimum thresholds for reusing recovered materials from manufacturing and consumer waste, with new batteries mandated to incorporate recovered cobalt (16%), lead (85%), lithium (6%), and nickel (6%).3, 4 Such requirements create high societal pressure to test and implement various industrial recycling methods that are environmentally friendly and as versatile as possible for different cell types and cell chemistries in the coming years.
A conventional LIB comprises a cell housing predominantly fabricated from stainless steel, which serves as a structural support for interconnecting components, batteries, and internal control elements. Irrespective of cell shape, the active portion comprises an anode material affixed to a copper current collector and a cathode material coated onto an aluminum current collector. The separator, which is commonly composed of microporous polymers, acts as an electrically insulating and ionically conductive film that prevents direct contact between the negative and positive electrodes,5-7 which protects against potential short circuits within the electrochemical cell. The electrolyte plays a chemical role in facilitating ionic conductivity between the electrodes to maintain electroneutrality during charging and discharging.8-10 Electrolytes in LIBs are usually liquids containing dissolved ions, which are characterized as lithium-containing salt solutions in organic solvents.11, 12 More innovative electrolyte solutions exist, such as solid, quasi-solid, and polymer electrolyte configurations. Unlike liquid electrolytes, solid electrolytes consist of solid materials such as lithium phosphate (Li3PO4), lithium silicate (Li2SiO3), or polymers.13, 14 They offer potential benefits, as they are safer, less volatile, and operate over a wide range of temperatures. Quasi-solid electrolytes combine the properties and advantages of both liquid and solid electrolytes.15 They typically consist of liquid electrolytes embedded in solid matrices that acts synergistically as a single material, which facilitates flexibility and ease of handling while improving safety and reducing the risk of leaks. A gel electrolyte is an example of a quasi-solid electrolyte in which a liquid electrolyte is embedded in a gel–matrix material.16
As commercial battery cells reach the end of their cycling lives, researchers have focused on methods for recycling specific components. At this point, a battery module is meticulously diagnosed and assessed; it is earmarked for recycling if it no longer functions satisfactorily or fails to deliver the required performance. Following collection, the module is prepared for disassembly by removing the external housing components, wiring, and other attachments to facilitate access to individual battery cells. The battery module is then entirely dismantled and the individual components are separated, with materials, such as metals, plastics, and electrolytes separated after the remaining components of the battery cells have been shredded.17 Separation processes typically involve melting, screening, grinding, magnetic separation, flotation, electrostatic, and chemical methods.18-22 In the end, a black mass containing a mixture of electrochemically active components and other inseparable leftover materials is obtained, which is crucial for elemental recycling. While the LIB anode predominantly features graphite, the cathode often contains a significant proportion of crucial and limited metals, such as manganese (Mn), lithium (Li), cobalt (Co), and nickel (Ni). Consequently, the cathode constitutes a more significant percentage of the battery in terms of cost and mass; hence, recycling the elements within the cathode is of heightened significance. In addition to the metallic compounds used in LIBs and their associated expenses, considering the availability of graphite and alternative commercial anode materials for LIB use is crucial because a lithium-ion cell contains significantly more (at least 11-times more) graphite than lithium, depending battery type and application.23-26 In most cases, the electrode materials are closely attached to a current collector; consequently, the black mass may lead to additional elements in the leaching solution for recycling. The are several comprehensive reviews on electrochemical recycling methods for batteries; however, there systematic reviews that focus on comparing and developing different methods for the specific recycling of spent LIBs are lacking. The unique aspects of this review are its in-depth analysis of recent advancements, detailed comparisons of methodologies, and a focus on the latest innovations in the field, which includes more-closely examining the practical applications of these methods and their industrial scalabilities, thereby providing a targeted and updated perspective.
Several battery-recycling methods have been developed and are currently being implemented in industrial facilities; these methods are compared in Figure 1. Pyrometallurgical and hydrometallurgical recycling, particularly the former, have been widely studied. These methods have garnered significant research attention owing to their abilities to effectively process large volumes of material, as evidenced by their widespread adoption, particularly in regions such as China.
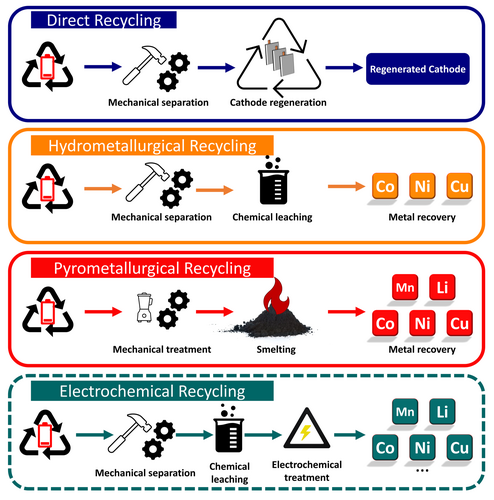
Pyrometallurgical LIB recycling involves the use of thermal treatment at high temperatures. During this process, battery components, such as the cathode and anode materials, are melted and separated to recover valuable metals. This method typically involves melting, refining, and separating the metallic battery components.20, 27-30 Pyrometallurgical recycling methods can be further subdivided into roasting and smelting. The active cathode material is heated in the presence of a reducing agent, such as carbon, charcoal, or coke, during roasting or calcination. This process generates carbon residues and a blend of alloys or intermediate compounds that predominantly consist of impure metals or oxides. These materials can subsequently undergo further refinement.31 With this in mind, Li et al. introduced an oxygen-free roasting process at 1000°C, followed by wet magnetic separation. Residues such as Co, lithium carbonate (Li2CO3), and graphite remained after the lithium cobalt oxide (LiCoO2) and graphite had reacted, and was separated through wet magnetic separation, resulting in recovery rates of 95.7% for Co, 98.9% for Li2CO3, and 91.1% for graphite.32 Liu et al. determined that the optimal temperature for lithium nickel manganese cobalt oxide (NMC) decomposition is 600–700°C, during which NMC thermally decomposes into Li2CO3, manganese oxide (MnO), nickel oxide (NiO), and Ni. Subsequent water leaching and evaporative crystallization led to leaching efficiencies of 93.7%, 93.3%, 98.1%, and 98.7%, for Li, Ni, Co, and Mn, respectively, after roasting.33
Hydrometallurgical battery recycling uses aqueous solutions or liquids to extract valuable metals from battery components.34, 35 This method typically involves dissolving the battery materials in a suitable solvent, such as an acid or base, to leach the desired metals. The leaching solution containing the dissolved metals is then processed using various separation and purification steps to recover the target metals in usable forms. Typical leaching components include hydrochloric acid, citric acid, nitric acid, phosphoric acid, sulfuric acid, oxalate, and ascorbic acid.36 For instance, in 2018 Vieceli et al. reported an active-material-rich fraction containing 95% of the original electrode, 2% of the original steel, and 22% plastics following battery disassembly and subsequent physical separation. Several reducing agents have been investigated, among which sodium metabisulfite has emerged as the most effective for enhancing metal dissolution during leaching with sulfuric acid. Approximately 90% of the metal can be extracted after just 30 min of leaching, with the current collector also a component of the solution containing the precious cathodic elements.37
Porvali et al. reported the direct leaching of LIB waste using HCl as the medium, which provided a means for recovering Li, a component typically lost to slag fractions in modern high-temperature processes.38 Initial screening and density separation were used to separate Al and Cu, as current-collector materials, from the battery elements. Leaching in 4 M HCl at various temperatures between 50 and 80°C led to 60–80% Li and 50–75% Co solubilisation. Using tailored precipitation processes, 99.6% iron (Fe), 81.3% copper (Cu), and 80.5% aluminum (Al) were subsequently separated, leaving 97.1% Ni, 97.2% Co, and 97.3% Mn in solution, which were partially separated using a saponified extractant in sulfonated kerosene. Li2CO3 was ultimately recovered with a purity of 95% and in 50% yield, necessitating further steps in the overall process.38 Figure 2 illustratively shows the actual performance of various recycling methods that consider different key points.
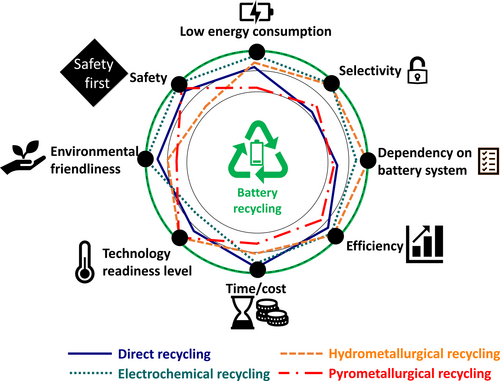
Key metrics, such as energy and chemical consumption, and carbon emissions can be used to better compare electrochemical battery recycling processes that use pyrometallurgical and hydrometallurgical methods.39 This approach highlights the importance and potential advantages of electrochemical recycling over traditional techniques. Energy consumption is a critical factor in the recycling process. Pyrometallurgical recycling involves high-temperature treatments that include roasting and smelting, which require substantial amounts of energy.30 Quantifying the energy required for these processes and the high temperatures necessary for metal extraction in pyro-based recycling has revealed that significant expenditure on energy is required. In contrast, hydrometallurgical recycling operates at relatively lower temperatures but still requires energy for its various leaching and separation stages.40 Electrochemical recycling, on the other hand, uses electricity to drive extraction and separation; this method consumes less overall energy, particularly when powered by renewable energy sources. Chemical consumption is another vital factor that must be considered. Pyrometallurgical recycling often requires fluxes and reducing agents that contribute to overall chemical usage. Hydrometallurgical recycling involves the use of acids, bases, and other reagents for leaching and purification,41 while electrochemical recycling aims to minimize chemical usage by primarily relying on electrical energy. Comparing chemicals consumed by electrochemical processes with those of pyrometallurgical and hydrometallurgical methods highlights the reductions achieved by the former and the necessary chemicals involved, consistent with a more environmentally friendly approach. Carbon emissions are a major concern for all industrial processes. High-temperature pyrometallurgical recycling processes produce substantial carbon emissions. While hydrometallurgical recycling may produce lower direct emissions, they still produce significant indirect emissions associated with the production of chemicals and waste management. Electrochemical recycling has the potential to significantly reduce carbon emissions, particularly when renewable energy sources are used; its environmental benefits are highlighted by analyzing the carbon emissions associated with electrochemical recycling, and factoring in the source of electricity and the overall efficiency of the process.
Alternative methods are urgently needed owing to aforementioned efficiency drawbacks associated with previously established recycling methods. Direct recycling, which involves regenerating and reusing battery components without breaking down their chemical structures, is a highly efficient and sustainable method. Bai et al. proposed a new solvent-separation method that uses ethylene glycol, which enables the electrode materials (anode and cathode) and current collectors in LIBs to be rapidly and efficiently separated. In addition to completely detaching them from the current collectors, the electrode materials retain their crystalline structures, morphologies, and electrochemical performance. In contrast, the current collectors (Al and Cu foil) were completely restored without any corrosion or residue. The recycling loop can be closed by recycling the solvent for further use, making this an environmentally friendly and cost-effective method that can lead to significant advancements in battery recycling.42
Further concepts, such as that reported by Xu et al., describe how the original composition, structure, and electrochemical performance can be restored, particularly through defect healing. Specifically, spent LiFePO4 (LFP) cathodes were successfully directly regenerated by combining low-temperature water leaching with citric acid, lithium hydroxide (LiOH), and rapid annealing. The electrochemical performance of the LFP electrodes subjected to direct recycling can exhibit comparable performance to that of pristine LFP electrodes across a range of degradation conditions.43 The drawbacks of this method include the need for expensive and partially environmentally questionable chemicals, such as LiOH and N-methyl-2-pyrrolidone (NMP). The environmental impact of NMP, coupled with its potential carcinogenicity, associated respiratory difficulties, and severe health complications, highlight the need for alternative electrode-preparation methods.44, 45 Other direct-recycling approaches have been reported, such as that reported by Gao et al. that focused on separating LiCoO2 from the aluminum foil in waste LIBs and investigated the influence of the firing temperature, lithium source, and Al2O3 coating on the recovery of recycled LiCoO2. The spent LiCoO2 electrode was treated with Li2CO3 (as the lithium source) at 800°C to form a layered structure devoid of impurities. Electrochemical performance was further improved by applying aluminum oxide (Al2O3) to the surface of the LiCoO2. This treatment facilitated the regeneration of LiCoO2 with properties that closely resemble those of commercially available LiCoO2.46 Therefore, direct recycling offers a more energy-efficient and potentially more cost-effective solution, which is particularly applicable to slightly degraded batteries. Electrochemical recycling is a promising method for recovering high-purity materials and is particularly suitable for large-scale industrial applications. Both methods have unique strengths and challenges, with the choice ultimately depending on factors such as the condition of the spent battery, the desired purities of the recovered materials, and resource availability.
In addition to direct recycling, other environmentally and energy-friendly recycling methods are required and various alternative approaches have been proposed, including bacterial bioleaching,47-49 in which bacteria indirectly extract valuable metals from battery components under acidophilic conditions. Specialized bacteria can degrade battery materials and release metals such as Li, Ni, Co, and Mn from electrodes. These bacteria typically produce organic acids or other compounds that aid metal dissolution, rendering them accessible for extraction. Bioleaching is an environmentally friendly and sustainable LIB-recycling method because it avoids the use of aggressive chemicals and minimizes waste generation. Moreover, bioleaching has the potential to recover metals from spent batteries more efficiently than traditional methods, making it an attractive approach for LIB recycling.47-49 In 2008, Mishra et al. suggested using the chemolithotrophic and acidophilic Acidithiobacillus ferrooxidans bacterium, which utilizes sulfur (S) and iron(II) ions as energy sources to produce metabolites, such as sulfuric acid and iron(III) ions that can dissolve metals from spent batteries. Using LiCoO2 electrodes as an example, Co was observed to dissolve significantly faster than Li using this bacterium.50 Another study explored the use of a biohydrometallurgical method that leveraged the fungal activity of Aspergillus niger to recover Cu, Li, Mn, Al, Co, and Ni from spent LIBs under diverse conditions.48 The highest recovery efficiencies of 100% Cu, 95% Li, 70% Mn, 65% Al, 45% Co, and 38% Ni were attained at a pulp density of 1% during bioleaching in the spent medium. In comparison to other identified organic acids, citric acid significantly influenced the bioleaching efficacy of A. niger, with maximum recovery efficiencies recorded at a pulp density of 1% during bioleaching in the spent medium.48
Electrochemically separating ions from spent battery materials is another promising approach.51-53 Electrochemical battery recycling involves reusing batteries in which electrochemical processes had been used to recover the elements. Its distinction from bioleaching lies in the use of electrochemical methods instead of biological (or purely chemical) processes. Typically, this entails disassembling the batteries and applying processes such as melting, electrolysis, or other chemical reactions to separate the battery components and reclaim valuable metals and materials. This method can be used to recover metals such as lithium, cobalt, and nickel from batteries, enabling their reuse in new batteries or other applications. Electrochemical battery recycling, which mostly uses hydrometallurgical leaching solutions, is often regarded as an environmentally friendly and efficient method because it contributes to resource conservation and reduces the need for new raw materials. However, the broad range of electrochemical recycling possibilities have not been systematically reviewed and comprehensively analyzed compared to their applications and performance improvements.
Herein, we provide a comprehensive review aimed at fulfilling the requirements of more-efficient and environmentally friendly lithium-ion-battery-recycling strategies. Therefore, we focus on electrochemical methods, commencing by introducing various technologies, state-of-the-art milestones, and future challenges, after which we explore different methodologies related to electrochemical recycling.
2 PRETREATMENT: PROCEDURES APPLIED TO BATTERIES PRIOR TO ACTUAL RECYCLING
Recycling spent LIBs involves several crucial steps to ensure efficient material recovery and environmental sustainability. The process begins with collecting and safely discharging the batteries to prevent any risk of short circuiting or chemical reactions during handling.17 Once discharged, the batteries are mechanically shredded into smaller pieces, which facilitates subsequent material separation and recovery.54 Following shredding, the various battery components are separated into fractions, such as metals, plastics, and electrolyte residues, using techniques such as magnetic separation, screening, and density-based methods.55 The cathode materials, which often contain valuable metals, are specifically recycled. Traditional methods include roasting (pyrometallurgy), in which materials are heated to high temperatures to convert them into recoverable forms, and leaching (hydrometallurgy), in which metals are dissolved in aqueous solutions using acids or bases to extract valuable components.56, 57 Additionally, bioleaching uses bacteria to enhance metal recovery, while electrochemical leaching uses electrochemical processes to extract and separate metals, potentially offering higher efficiencies and lower chemical usage.58 The final stage involves regenerating the recovered materials, which may include reconstructing electrodes or creating new battery components from recycled materials to restore performance. This structured approach ensures that valuable resources are efficiently recovered from spent LIBs, thereby minimizing environmental impact and promoting sustainability.
The extraction of metals and other valuable ions from leachates involves well-established traditional methods that are widely used in industrial and commercial applications. These include solvent extraction, in which metals are transferred from an aqueous phase to an organic phase by adding a suitable solvent that specifically reacts with the ions requiring extraction59, 60; this method is highly selective, scalable, and efficient. Other methods include ion-exchange, precipitation, and membrane separation. Ion exchange, in which a solution passes through a resin or another material that captures ions by exchanging them for other ions, is typically highly selective.61, 62 Precipitation, which is promoted by the addition of chemicals to the solution, effectively removes high concentrations of metals by causing the desired metals to precipitate as insoluble solids that are then recovered by filtration or sedimentation. Membrane processes, which are highly efficient, do not use chemical additives, and have smaller environmental footprints, include techniques such as reverse osmosis, nanofiltration, and electrodialysis. These methods separate ions using semipermeable membranes that retain specific molecules or ions.63, 64
These traditional methods are well established and have proven to be reliable in practice. However, there is a growing effort to develop new and more efficient methods that are environmentally friendly and consume less energy and chemicals, given the increasing importance of sustainability and waste reduction. Innovative approaches include the development of electrochemical recycling methods that use electricity instead of chemical reagents. These methods aim to reduce chemical consumption and minimize environmental impact, particularly when sustainable electricity is used. Current research is advancing these techniques by integrating solar and mechanical energy into electrochemical processes that enhance energy efficiency and sustainability.65
3 ELECTROCHEMICAL RECYCLING
In a similar manner to other recycling technologies, electrochemical recycling begins with an understanding of the various different battery components and their processing. The outer components of a typical commercial cell-pack battery system primarily consist of an aluminum casing along with the battery management system and cables. Figure 3A,B compares two typical commercial-cell/pack configurations and their compositions. Broadly, the data suggest that approximately 55 mass% of the battery system is ascribable to the battery cells, including the electrolyte (volatile component), separator (plastic), cell housing (Al), and electrodes.66 The anode comprises a graphite electrode coated on copper foil, whereas the primary component of the cathode is either lithium nickel cobalt manganese oxide (LiNi0.33Co0.33Mn0.33O2, NMC) or lithium iron phosphate (LiFePO4, LFP).67 On average, an electric vehicle NMC battery system contains 3.5 kg of Li, 10.9 kg of Co, 10.9 kg of Ni, and 9.8 kg of Mn.66, 68 These significant quantities, which are electrochemical-recycling targets, underscore the critical need for material recycling at the elemental level.
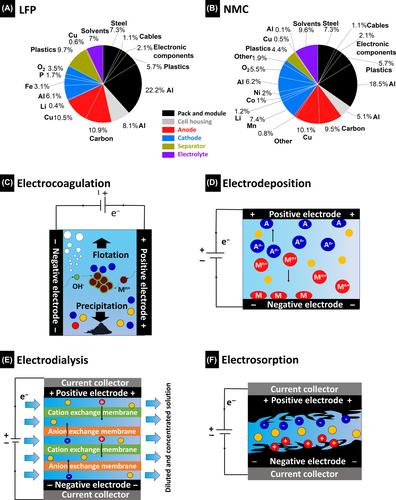
Electrochemical battery recycling uses electrochemical processes to recover valuable materials, particularly metals, from depleted batteries.69 This method involves disassembling the battery components and leveraging electrochemical reactions to segregate and recover the target materials.70 Owing to its efficiency and eco-friendliness, electrochemical battery recycling plays a pivotal role in resource conservation and mitigating the environmental footprint associated with the extraction of raw materials for fresh battery production. Several studies have systematically investigated the electrochemically selective recovery of critical elements from various liquid media, such as saltwater, mining water, brine, and geothermal water.71-78 The very low concentrations of lithium ions is often challenging here; however, they are much higher in spent-battery leaching solutions.79 Therefore, the evaluated technologies can be adapted to spent-battery solutions. Batteries exist as solid waste after reaching end-of-life. Most electrochemical recycling methods require the elements to be in solution; therefore, the black mass needs to be scraped from the current collector and dissolved in different media. Mostly, an acidic solution or harsh chemical environment is needed, which forms the basis of the hydrometallurgical process.80 Instead of using more energy in the form of heat or more chemicals for selective precipitation, electrochemical recycling methods offer a more sustainable option while avoiding the generation of secondary waste or high (alkaline) pH.81 The adaptable, modular, reversible, and scalable characteristics of electrochemical metal-recovery methods can significantly enhance operational efficiency and cost-effectiveness. The following section discusses recent advances in electrochemical methods for recovering specific elements from spent LIBs. With a special emphasis on selectivity, we highlight technologies engineered specifically for the recovery of critical elements, such as Li, Co, Ni, and Mn.
Selectively separating various ions from aqueous media is challenging because they often exhibit similar electrochemical properties82; hence, they undergoing similar reactions at the electrode surface, which makes them difficult to distinguish. Additionally, ion concentration, reaction kinetics, and electrolyte-solution composition can further complicate the separation process. Developing selective electrochemical separation methods requires a deep understanding of the specific reaction mechanisms and potential influencing factors, as well as the development of suitable electrode materials and operating conditions. Furthermore, certain combinations of ions are particularly challenging to separate, especially when they share very similar chemical and physical properties or when the concentrations of interfering ions are much higher than those of the recovered ions. In recent years, various electrochemical methods have been developed to selectively uptake specific ions.
The following section introduces various electrochemical methods for recovering rare elements from aqueous media containing numerous ions. Figure 3C–F display schematic representation of the underlying mechanisms, while Table 1 lists advantages and disadvantages, and Table 2 compares the performance parameters used in several reported studies that implement electrochemical-recovery methods. This followed by a discussion on the practical applications of these methods to the recovery of key elements from current battery solutions, focusing on the electrochemical recovery of Li, Ni, Co, and Mn (Table 3).
| Electrochemical method | Advantages | Disadvantages |
|---|---|---|
| Electrodeposition |
|
|
| Electrosorption |
|
|
| Electrodialysis |
|
|
| Electrocoagulation |
|
|
| Electrochemical regeneration |
|
|
- Note: Green indicates advanteages. Red indicates disadvantages.
| Study | Recycling methodology | Metal recovery | Recovery rate | Efficiency | Energy consumption | Reference |
|---|---|---|---|---|---|---|
| Kim et al. | Electrodeposition | Co, Ni | Co: 96.4 ± 3.1% Ni: 94.1 ± 2.3% |
N.A. | N.A. | 86 |
| Freitas et al. | Hydrochemical leaching | Co | N.A. | 96.9% | N.A. | 87 |
| Hoshino et al. | Electrosorption (CDI) | Li | 0.35 μmol g−1 of lithium per adsorbent | N.A. | N.A. | 100 |
| Nie et al. | Electrosorption | Li | 21 mg Li per electrode | N.A. | 3.0 ± 0.5 Wh molLi−1 | 101 |
| Wang et al. | Sorption/Desorption | Li | 20–25 mg Li g−1 | N.A. | 102 | |
| Zhang et al. | Electrocoagulation | Li | >95% | N.A. | 0.06 kWh gLi−1 | 106 |
| Zhang et al. | Electrochemical Regeneration | Li | N.A. | N.A. | N.A. | 107 |
| Zhang et al. | Electrochemical Regeneration | Li | N.A. | N.A. | N.A. | 109 |
| Kong et al. | Electrochemical Redox Method | Li Co |
99.0% Li+, 98.2% Co2+ 89.6% Al foil |
N.A. | N.A. | 110 |
| Study | Recycling methodology | Metal recovery | Recovery rate | Efficiency | Energy consumption |
|---|---|---|---|---|---|
| Song et al.120 | Electrodialysis | Li P |
Lithium concentration: 22.5 g L−1; P/Li ratio: 0.23 | 88.3% | N.A. |
| Xing et al.121 | Electrodialysis | Li | 99.4% recovery rate; 63.9% purity | N.A. | N.A. |
| Li et al.122 | Suspension Electrolysis | Li Fe |
99.9% lithium recovery; <0.2% iron yield | N.A. | N.A. |
| NEU Battery Materials125 | Electrochemical Separation | Li | N.A. | N.A. | N.A. |
| Yang et al.126 | Direct Electrochemical Oxidation | Li | Nearly 100% | N.A. | N.A. |
| Lupi et al.133 | Hydrometallurgical Method with Electrolysis | Co Ni |
Co: 100% recovery Ni: High purity with less than 100 ppm contamination |
Co: 96% current efficiency; Ni: 87% current efficiency | 2.8 kWh kg−1 for Co; 3.0 kWh kg−1 for Ni |
| Long et al.136 | Electrochemical Separation | Co | N.A. | N.A. | N.A. |
| Wang et al.137 | Deep Eutectic Solvent Electrochemistry | Co | Co: 54 ± 3% 4.1 ± 0.1 mg h−1 m−2 |
N.A. | |
| Freitas et al.138 | Galvanic Deposition | Co Cu |
N.A. | N.A. | N.A. |
| Kim et al.86 | Molecularly Selective Electrodeposition | Co Ni |
Co: 96.4 ± 3.1%; Ni: 94.1 ± 2.3% purity | N.A. | N.A. |
| Santos et al.142 | Chemical and Electrochemical Recycling | Mn | N.A. | Charge efficiency of 83.7% | N.A. |
| Roriz et al.143 | Chemical and Electrochemical Recycling | Mn | 97.4% | N.A. | N.A. |
3.1 State-of-the-art methods for recovering elements from aqueous solutions
3.1.1 Electrodeposition
Metals are deposited onto and electrode from solution during electrodeposition and electroplating. Electroplating is widely used in industry to apply a thin metal layer onto a metal object with the aim of tuning its surface properties.83, 84 Electrodeposition is achieved by applying an electric current, which reduces dissolved metal cations from a solution, which are ions carrying a net positive charge, resulting in the formation of a thin, cohesive metal coating on an object that serves as the negative electrode (anode). The potential bias achieved in this manner determines the elements that can be electrodeposited,84, 85 thereby enabling the efficient recovery of metals that can then be reused to produce new batteries or in other applications. Because electrodeposition is a very efficient and selective method, it can also be used to extract metals such as lithium, cobalt, nickel, and other valuable materials from recycled battery components.
In 2021, Kim et al. reported that selective metal deposition is pivotal for the sustainable recycling of LIB electrodes.86 However, large-scale applications face challenges because metals with similar reduction potentials interfere with this precise process. Using Co and Ni as examples, the proposed approach involves synergistically adjusting parameters to optimize metal selectivity during electrochemical deposition by controlling speciation through electrolyte engineering in conjunction with surface functionalization with charged polymers. Concentrated chloride solutions offer precise control through the formation of complexes with opposing charges. Specifically, cobalt forms anionic complexes ([CoCl4]2−), while nickel retains its cationic form ([Ni(H2O)5Cl]+). Furthermore, functionalizing electrodes with positively charged polyelectrolytes, such as poly(diallyldimethylammonium) chloride, modifies [CoCl4]2− mobility through electrostatic stabilization; this selectivity adjustment for Co depends on the polyelectrolyte loading. This approach was successfully demonstrated using a commercial lithium nickel manganese cobalt oxide electrode, which resulted in the recovery of pure Co and Ni in yields of 96.4 ± 3.1% and 94.1 ± 2.3%, respectively.86
Freitas et al. isolated a spent LiCoO2 electrode by disassembling a LIB and separating its components.87 Several washing steps were then used to isolate the cathode material from the current collector and remove any remaining traces of organic solvent from the electrolyte. Finally, the black mass obtained was dissolved in HCl and mixed with H2O2. Among other methods, the pH of the leaching solution was later adjusted using sodium hydroxide (NaOH), with Coulombic efficiency observed to depend on pH during electrochemical cobalt recycling. The highest Coulombic efficiency of 96.9% was obtained at pH 5.4.87
3.1.2 Electrosorption
Electrosorption is a process in which ions from a solution are adsorbed onto an electrode to remove or concentrate them from the solution.88 Electrosorption is achieved by applying an electric potential difference between two electrodes, which results in the ions in solution migrating toward the electrode and becoming adsorbed on its surface. Subsequent treatment detaches the adsorbed material from the electrode, thereby enabling efficient metal recovery. This process enhances the yield of valuable materials during battery recycling, rendering them suitable to produce new batteries or other applications.
Electrochemical capacitive deionization (CDI) is a common electrosorption method. CDI relies on immobilizing ions at the liquid–solid interface of nanoporous carbon through their reversible electrosorption.88 Two nanoporous carbon electrodes are arranged with a separator in a conventional CDI cell; this separator, which is often an open channel or porous dielectric material, prevents short-circuiting between the electrodes. An unvarying electrical voltage or current is then applied across the cell to generate a potential gradient that caused the salt ions in the feed water to migrate into the electrical double layer of the electrode material, thereby extracting salt from the effluent water stream via ionic electrosorption. The electroadsorbed ions are released in the subsequent discharge phase, which enables recovery of the previously invested charge. Over the years, various CDI systems have been optimized, including alloying- or conversion-type systems that enhance uptake capacity, membrane integration to improve selectivity, and the introduction of bi-electrolyte systems capable of providing higher desalination capacities owing to electrochemical potential windows with extended stabilities.89-94 Today, diverse arrays of optimized exchange membranes and electrochemical electrode materials are available for use. Numerous materials have been extensively studied for the selective uptake of lithium and sodium owing to their use in alkali-ion-battery applications.77, 95
The selective recovery of Li from aqueous solutions containing various cations (Li+, Na+, K+, Ca2+, and Mg2+) has previously been investigated.96 This system comprised a modified capacitive membrane desalination setup consisting of a lithium manganese oxide electrode to capture lithium ions, and a carbon electrode to capture anions. The influence of various cations was studies, which revealed the following selectivity order: Li+ ≫ Mg2+ > Ca2+ > K+ > Na+, revealing that the ionic radius and oxidation state influence selectivity. Around 0.35 μmol g−1 of lithium was regained per adsorbent, which is sevenfold greater than that attained through physisorption under identical experimental conditions.96
Another study using a typical battery material, specifically LiFePO4LFP, as the lithium-collecting electrode material, investigated the impact of various cationic additions.97 Including Ca2+ in the solution along with dissolved oxygen was found to enhance LiFePO4 capacity degradation, whereas Na+ and Mg2+ did not significantly influence LiFePO4 stability.97 LiFePO4 stability was notably improved by employing a process involving continuously purging the electrolyte with nitrogen and the use of a carbon coating of the electrode material. LiFePO4/C exhibited a lithium-extraction capacity of 21 mgLi per electrode accompanied by an energy consumption of 3.0 ± 0.5 W h molLi−1, resulting in a capacity retention of 82% over 10 cycles when subjecting a 5 mM LiCl + 50 mM NaCl solution to a cell voltage in the −0.5 V to +0.5 V range.
In 2014, Lemaire et al. established a sorption/desorption method for recovering lithium from aqueous solutions, such as leachates from used LIBs obtained during hydrometallurgical recycling.98 Preliminary investigations revealed that activated carbon and hexagonal mesostructured MCM 41 aluminosilicate adsorbed lithium inefficiently, whereas 13× molecular sieves and Amberlite IR 120 showed similar uptake capacities; both materials exhibited fast sorption and desorption kinetics, which were identified as ion-exchange processes that successfully was fitted to the developed model. This study suggested that lithium can possibly be recovered using concentration effects (maximum 20–25 mgLi g−1), although the final lithium concentration may not be sufficient to precipitate a typical lithium salt. However, the precipitation of less-soluble Li3PO4 may offer a lithium-recovery solution. Future experiments are expected to include selectivity testing and dynamic scale-up, with plans to investigate lithium separation from real LIB leachates containing other metallic elements, and evaluate the economic feasibility of the process.98
3.1.3 Electrodialysis
Electrodialysis separates ions in an electrolyte solution using a membrane and electric field. A typical electrodialysis cell comprises a series of anion- and cation-exchange membranes arranged alternately between the anode and cathode.99 Ions in the electrolyte solution are immobilized by an electric field and pass through selective membranes during electrodialysis; these membranes allow certain ions to pass while retaining others. Cations effortlessly cross negatively charged cation-exchange membranes, whereas positively charged anion-exchange membranes effectively retain them. Likewise, negatively charged anions pass through anion-exchange membranes, whereas they are impeded when attempting to traverse a cation-exchange membrane. This process leads to elevated ion concentrations in the alternating compartments (concentrates), while the other compartments become concurrently depleted. This process enables metal ions to be separated and concentrated from other charge carriers, thereby facilitating efficient recovery. Electrodialysis is a crucial battery-recycling technology because it helps maximize the yield of valuable materials and enhances recycling efficiency.
In 2013, Hoshino reported a method for extracting lithium from seawater using electrodialysis100; this process entails selectively permeating lithium ions from the anode to the cathode via an organic membrane saturated with an ionic liquid (PP13-TFSI). The ion concentration on the cathode side was analyzed over varying dialysis durations, which delivered a lithium concentration of 5.9% after 2 h at an applied voltage of 2 V. Notably, other ions present in seawater did not penetrate the membrane. Both ends of the impregnated membrane were subsequently coated with Nafion 324, which effectively prevented leakage of the ionic liquid and resulted in a substantial increase in the lithium concentration (to 22.2%).100
Competing ions can hinder the selective electrochemical uptake of ions, particularly at very high concentrations, thereby necessitating the consideration of complex ion combinations. For example, Nie et al. successful recovered lithium from salt lakes in China and the Dead Sea (which are characterized by high Mg/Li ratios) using monovalent selective ion-exchange membranes and electrodialysis.101 These results demonstrate that electrodialysis with monovalent selective ion-exchange membranes exhibits a significant capacity to separated Li+ from Mg2+. In contrast, Mg2+ was adsorbed and fixed by the charged groups of the cation-exchange membrane, thereby enabling lithium-ion penetration. The Mg/Li mass ratio of the product stream was reduced to 8.0 (which is 19-times less than the Mg/Li ratio of 150 in the feedstock material) by employing optimized electrodialysis parameters, which concurrently afforded a lithium-recovery rate of 95.3%.101
Electrodialysis using Li-selective ceramic membranes is an efficient method for separating lithium from seawater, albeit at a notable energy expense. Conversely, reversible electrochemical processes, such as those associated with redox flow batteries, offer potential solutions for mitigating the energy drawbacks of electrodialysis-based systems. In 2022, Wang et al. introduced a continuous electrochemical lithium-extraction battery that employed flow redox electrolytes and LISICON membranes to recover lithium from aqueous solutions.102 The seawater-extracted ions contained 93.5% lithium and the battery operated cleanly using the Fe[CN]63−/Fe[CN]64− redox pair, with no toxic byproducts, safety concerns, and minimal energy consumption (2.5 Wh gLi−1), thereby contributing to lower CO2 emissions.102 Furthermore, the versatility of the LE-RFB system feedwater, which contains diverse chemical compositions that require processing, highlights its potential for extracting lithium from acidic leaching solutions obtained from spent LIBs using hydrometallurgical methods.
3.1.4 Electrocoagulation/electroflocculation
Electrocoagulation or electroflocculation is a conventional physicochemical treatment method in which an electric current destabilizes and precipitates metal ions dissolved in solution.103 During this process, metal ions are adsorbed onto the electrode to form aggregates known as floccules that are subsequently separated. The coagulation mechanism has been continually scrutinized, with the prevailing understanding suggesting that coagulation primarily occurs by reducing net surface charges, thereby enabling colloidal particles that were previously electrostatically stabilized to approach each other and aggregate through van der Waals forces. The reduction in surface charge is ascribable to a decrease in the repulsive potential of the electrical double layer caused by the presence of an oppositely charged electrolyte. During electrocoagulation, the coagulant is formed in situ through the electrolytic oxidation of a suitable anode material. Typical sacrificial anodes such as iron or aluminum are produced via the electrolysis of metal cations, while the cathode electrolyzes water to form H2 (g) and OH−.104 Metal cations and OH− migrate within the solution and combine in an electric field, and undergo hydrolysis to yield a hydroxide floc. These floccules subsequently aggregate and give rise to structures characterized by high specific surface areas and abundant surface hydroxyl groups. Ultimately, these structures float to the surface of the solution on air, thereby facilitating efficient solid–liquid separation.105 This technique is commonly used to treat wastewater and industrially processed water; it effectively eliminates charged ionic species, including metals, from wastewater by facilitating their reactions with either oppositely charged ions carrying or floccules composed of metallic hydroxides formed within the effluent. This process facilitates the efficient removal of metals from solution, thereby contributing to the purification and separation of battery materials.
Zhang et al. investigated recovering Li+ from artificial brine through electrocoagulation using aluminum electrodes. Various factors that affect electrocoagulation performance, particularly how they impact efficiency and energy consumption, were analyzed, which revealed the pivotal role played by current density, as it significantly influences electrocoagulation performance in terms of recovery efficiency and energy consumption. An efficient and economical treatment protocol was established at a current density of approximately 77 mA cm−2 and pH 6.5 by considering both recovery efficiency and energy consumption. More than 95% of the lithium ions (1000 mg L−1) were recovered under the optimized operating conditions at a relatively low energy consumption of 0.06 kWh gLi−1.106
3.1.5 Electrochemical regeneration
Electrochemical regeneration is a recycling process in which spent battery materials are restored or regenerated via electrochemical reactions,107, 108 which, unlike the electrochemical recycling methods mentioned above, does not necessarily involve the dissolution of battery components. In contrast, electrochemical recycling methods directly recycle electrode materials. This process can encompass various approaches, including restoring the electrochemical properties of the electrode materials or removing deposits or impurities accumulated during battery usage. Typically, electrochemical regeneration involves subjecting the battery or materials intended to be regenerated to an electric current to facilitate the desired electrochemical transformations. This process can extend the lifespans of battery materials and improve their performance, leading to more efficient utilization and enhanced recycling.
Zhang et al. investigated electrochemically regenerating lithium cobalt oxide materials from used battery electrodes,107 which demonstrated that Li ions can be successfully introduced into the end-of-life LixCoO2 electrode using an electrochemical re-lithiation method. This process is accelerated by higher concentrations of Li2SO4 or higher cathodic current density. X-ray diffractometry (XRD) confirmed that the peak positions of the re-lithiated products are consistent with those of the LiCoO2 standard, and that annealing successfully restored the crystal structure of the re-lithiated product. The activation energy for electrochemical re-lithiation was determined to be 22 kJ mol−1, with a corresponding equilibrium constant (k0) of 1.35·10−6 cm s−1. The electrode fabricated from the regenerated LiCoO2 exhibited a charge capacity of 136 mAh g−1, which is close to that of the commercial LiCoO2 electrode (140 mAh g−1).107
In another study, Zhang et al. recovered LFP as the cathode material from used LIBs after 1500 cycles and mixed it with carbon black and polyvinylidene fluoride (PVdF) binder to prepare regenerated cathodes.109 The recovered material was regenerated by intercalating Li ions through charge/discharge cycling (LixFePO4/C (0 < x < 1)) in half cells against elemental lithium. The regenerated LiFePO4/C delivered discharge capacities of 150 mAh g−1 and 50 mAh g−1 at 0.1 and 15 C, respectively. The regenerated LiFePO4/C cathode and a fresh LixFePO4/C cathode were compared on through full-cell testing with a graphite electrode as the anode. The regenerated cathode exhibited an initial discharge capacity of 133 mAh g−1 at 0.1 C, which is higher than that (114 mAh g−1) recorded for the fresh cathode.109
3.1.6 Further electrochemical methods
Recycling methods are highly diverse owing to the variety of electrochemical methods available for recovering electrode materials and individual elements, with most of them categorizable as either electrosorption, electrodeposition, electrodialysis, or electrocoagulation methods. Here, we introduce additional electrochemical recycling methods. First, Kong et al. developed a pioneering electrochemical redox method that enabled the simultaneous recovery of spent LIB anodes and cathodes.110 This study efficiently leached Li+ and Co2+ (99.0% Li+, 98.2% Co2+) and also recovered aluminum foil (89.6% Al foil) and graphite through electrochemical leaching under mild conditions (−0.15 V, 0.5 M H2SO4, 29.9°C) with a low Ecell value (<1 V) using a “sandwich-type” electrode structure. The electrode directly contacts the cathode and anode materials of the spent LIB, which streamlines the process. Anodic oxidation of the Cu foil hinders the oxygen evolution reaction (OER) and reduces the Ecell value. Electrochemical analyses confirmed the irreversible dissolution of the cathode active materials within the H2SO4 system. The corrosion kinetics further revealed rapid crystal dissolution in the H2SO4 system, with a higher H+ concentration leading to faster leaching. Furthermore, this method was extendable to LIBs that feature LiNixCoyMnzO2 as the cathode material. Life-cycle and economic assessments confirmed the benefits of the developed recycling route for reducing environmental impact and enhancing profitability.110
Significant advancements in LIB recycling have been achieved through the development of a novel process that integrates reductive thermal treatment and electrochemical leaching. Traditional acid leaching methods, which rely heavily on sulfuric acid and reducing agents, have proven inefficient and costly owing to the consumption of large amounts of these chemicals.111-113 The study of Lei et al. introduced a combined approach in which the LiNi1/3Co1/3Mn1/3O2 cathode material undergoes reductive thermal treatment at 1600°C for 120 min, transforming it into a mixture of Li2CO3, NiO, Co3O4, Mn2O3, and MnO2.114 This thermal reduction process decomposes the layered crystal structure and enhances the leachability of the material by liberating oxygen from the framework. Electrochemical leaching under optimized conditions (a 20 mL g−1 liquid-to-solid ratio, 1.5 M H2SO4, 0.8 A, and 150 min at room temperature) subsequently yielded impressive recovery rates of 90.59% for Ni, 90.53% for Co, 66.40% for Mn, and 100% for Li.114 Compared to traditional methods, this integrated approach is more efficient and economically beneficial because it reduces reliance on hazardous chemicals, minimizes acid consumption, and maximizes the use of spent materials. This innovative method represents a significant step toward a more sustainable and environmentally friendly process for recycling valuable metals in spent LIBs.
Traditionally, anode and cathode materials are recycled separately, which heightens environmental impact and operational complexity. Gu et al. introduced a groundbreaking approach by integrating targeted electroredox processes that enable the efficient and comprehensive recycling of both cathode and anode materials.115 This method led to Li+ and Co2+ recoveries of 98% of 99%, respectively, from the cathodes, and 99% of the Cu2+ and graphite from the anodes, and generated electricity during the leaching process. The targeted oxidation of copper and aluminum effectively liberated the active materials from the anodes while minimizing the OER, resulting in low energy consumption and a low global warming potential.115 Preliminary pilot-scale experiments validated the effectiveness and practicality of the second-generation ECL technique. This innovative approach highlights the potential of simultaneously recycling both the cathodes and anodes efficiently by simplifying the pretreatment processes to achieve high recovery rates. The ability of this method to generate electrical energy during recycling combined with its low environmental impact supports the broader goals of carbon peaking and carbon neutrality, thereby marking a significant advancement in sustainable LIB-recycling technology.
3.2 Challenges for electrochemical battery recycling methods
While electrochemical recycling methods for LIBs provide several promising advantages, they also face significant shortcomings that need to be addressed for broader adoption and efficiency. Electrochemical recycling consumes large amounts of energy and is a primary issue associated with these processes. These methods often require substantial energy inputs, making them costly and potentially environmentally damaging if nonrenewable energy is used. To address this issue, future developments should focus on enhancing the energy efficiencies of electrochemical methods and integrating renewable energy sources, such as solar or wind power, to reduce their environmental impact. The complexity of the electrochemical recycling processes is another critical shortcoming. Methods such as electrowinning and electrochemical separation involve intricate procedures that require precisely controlling various parameters and pose operational challenges. Simplifying these processes through automation and advanced control systems is expected to improve operational efficiency and reduce the necessity for highly specialized knowledge, leading to methods that are more accessible and more practical. The high initial costs associated with establishing electrochemical recycling facilities also pose barriers, particularly for smaller recycling firms; the equipment and infrastructure required can be prohibitive expensively. Future strategies should focus on developing cost-effective technologies and providing financial incentives or subsidies that lower the initial investment burden, thereby encouraging wider adoption. Although electrochemical methods can deliver highly pure recovered materials, they may show lower recovery rates for certain elements than other recycling methods. Research should be directed toward optimizing the recovery rates for all valuable elements, thereby ensuring comprehensive recycling and maximizing the material value of spent LIBs. Scaling these processes to the industrial level provides further challenges owing to the need for extensive infrastructure and a consistent energy supply. Developing modular and scalable systems that can be incrementally expanded to meet growing recycling demands may address this issue without requiring massive upfront investment. Effective waste management is crucial. The byproducts and residual waste generated by electrochemical processes must be managed responsibly to avoid secondary pollution. Establishing robust waste-management protocols and developing methods for minimizing and recycling waste are essential for environmentally sustainable electrochemical recycling. Economic viability is another concern because electrochemical-recycling profitability is influenced by fluctuating market prices for recovered materials and operational costs. Enhancing economic models by improving process efficiencies and integrating market-responsive strategies ensure sustainable profitability. In addition, the handling and disposal of chemicals used in electrochemical processes pose environmental and safety risks. Prioritizing environmentally benign chemicals and implementing strict safety protocols will protect both workers and the environment.
Several key issues need to be addressed in order to advance electrochemical recycling methods. Reducing the carbon footprint and operational costs associated with energy consumption is vital and can be achieved by integrating renewable energy sources. Research, development, and process optimization are expected to refine electrochemical techniques and enhance the efficiencies, selectivities, and recovery rates for all valuable elements within LIBs. Reducing costs through innovative technologies and materials, along with financial support, can reduce entry barriers. The implementation of advanced automation and control systems will help manage the complexities of electrochemical recycling processes and ensure consistent and reliable operations. The development of modular and scalable recycling systems will facilitate flexible expansion as demand grows. Furthermore, with the aim of minimizing environmental impact, comprehensive waste-management systems should be established to handle byproducts and residues responsibly. Creating adaptable economic models that respond to market conditions will ensure the financial sustainability of electrochemical recycling. Finally, upholding stringent safety and environmental standards when designing and operating recycling facilities is crucial in order to protect human health and the environment. By addressing these shortcomings and focusing on future developmental paths, electrochemical recycling is expected to become a more viable and sustainable method for recycling LIBs, thereby contributing significantly to the circular economy and environmental conservation efforts, and paving the way for a more sustainable future.
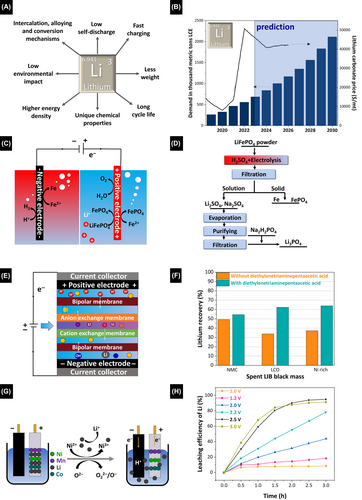
3.3 Lithium recycling
Lithium is a light alkali metal found in various mineral forms, including lithium brine, lithium pegmatite, and lithium clay.116, 117 Its unique chemical properties make it particularly suitable for batteries, given its high electrochemical voltage and energy density that contributes to efficient energy storage and rapid charging and discharging (Figure 4A). The demand for lithium has surged in recent years due to the growing electric-vehicle and renewable-energy market (Figure 4B). Mining primarily extracts lithium from mineral deposits, although lithium can also be extracted in regions with saline lakes and groundwater resources. Given its strategic energy-transition and electromobility importance, lithium has become a major focus of raw-material policies and the global economy. However, concerns persist regarding the environmental and societal impacts of Li mining, which has prompted the search for alternative, more efficient, and sustainable extraction and recycling methods. Recycling Li from old batteries is crucial for conserving resources, protecting the environment, recovering valuable materials, improving energy efficiency, and reducing waste. Lithium is a finite resource, and its increasing demand for use in batteries, particularly for electric vehicles, requires efficient resource utilization and a reduction in our dependence on primary lithium sources. Improper disposal of spent batteries can also lead to lithium leaking into the environment, which may cause ecological damage that can be mitigated by recycling. Moreover, LIBs will continue to dominate the market for approximately one more decade and remain a key component of electronics manufacturing as research into various battery chemistries continues.118 Furthermore, recycling facilitates the recovery of valuable and scarce materials, including lithium, cobalt, nickel, and other metals, from cathode materials, promoting resource conservation and reducing the need for primary raw materials.
Additionally, extracting lithium from primary sources requires significant energy, making lithium-battery recycling potentially more energy-efficient by reusing existing materials instead of mining and processing new ones. Waste management, which is crucial for all products, is another important aspect, as batteries pose potential environmental pollution risks if not properly discarded. Recycling removes batteries from the waste stream, leading to a lower environmental impact. Overall, recycling lithium batteries contributes to improving the sustainability of battery production and minimizing negative environmental impacts. Lithium, as one of the most crucial elements in high-performance devices, can be recycled from spent batteries. The knowledge gained from lithium-recovery studies can be applied to various natural aqueous reservoirs, including seawater, hydrothermal water, brine, and mining water, to enhance the recycling process.119
Most large-scale lithium-recycling methods are yet to be optimized. Electrochemical electrodialysis is an ion-selective lithium-ion recovery method, at least on the laboratory scale. Song et al. reported a method for recovering lithium from solutions with low lithium contents and high concentrations of other salts. An artificial battery solution containing Li+, Al3+, Ca2+, Co2+/3+, Cu2+, Fe3+, Mg2+, Mn5+, Ni2+, Zn2+, Cl−, SO42−, and Na+ was initially thoroughly cleaned to remove impurities and establish an optimal starting point for subsequent processing steps.120 Lithium was then precipitated by adding sodium phosphate, and various operating conditions were carefully examined to understand how they affect precipitation behavior (Figure 4C,D), which revealed that temperature plays a significant role in the precipitation process and that its influence is even more crucial than that of seed crystals or flocculants. The precipitated Li3PO4 was subsequently dissolved using an acidic anolyte. Electrodialysis using cation-exchange membranes was used to investigate the separation performance of lithium and phosphorus, which revealed that this electrochemical method effectively separates lithium from phosphorus, thereby reducing the P/Li mass ratio in the catholyte to 0.23. This 6.5-factor reduction compared to the initial ratio highlights the separation effectiveness of electrodialysis and underscores its potential role in recovering valuable resources.
Another crucial method involves effectively separating lithium and phosphorus in the catholyte by increasing the pH. This modification facilitates lithium segregation, leading to the formation of precipitates alongside the permeated phosphorus, which enhances purity. As a result, the purified catholyte exhibited a lithium concentration of 22.5 g L−1, which provides an excellent basis for producing lithium carbonate. In addition, the rate of Li2CO3 precipitation was examined under various conditions, with particular attention paid to temperature and the CO32−/Li+molar ratio, which delivered a precipitation rate of 88.3% at 80°C under the optimal conditions, indicative of the efficiency and practicality of this process. Overall, this study offered a promising approach to an efficient and environmentally friendly cyclic process for recovering lithium from spent LIBs.120
Xing et al., recovered lithium from leaching solutions using electrodialysis.121 Unlike many other studies that preferred to use simplified synthetic LIB solutions in recovery experiments owing to the various challenges posed by the original leaching solution, this study rigorously focused on the challenges posed by competing ions. Lithium-ion separation was achieved using an electrodialyzer with a bipolar membrane module (Figure 4E). Common chelating agents, including diethylenetriaminepentaacetic acid (DTPA), ethylenediaminetetraacetic acid (EDTA), hydroxyethylethylenediaminetriacetic acid (HEDTA), and l-glutamic acid (GLDA), were rigorously used to recover lithium by forming complexes with other metal ions in the leaching solution (Figure 4F). How the chelating agents, their respective dosages, and fluid dynamics impact the lithium recovery rate was meticulously examined. This methodological approach convincingly demonstrated an ability to recover lithium from various industrial LIB leachates, including lithium cobalt (LCO), nickel-rich leachates, and NMC. The highest Li-recovery rate of 99.4% was achieved under the optimal conditions, along with a purity of 63.9%.121
Li et al. implemented a suspension-electrolysis method, which involved adding another layer of complexity to the array of electrochemical techniques employed.122 With relentless advancements in LIB technology and the drive to minimize reliance on environmentally critical and costly materials, LFP batteries have emerged as cost-effective and stable options. An environmentally sustainable and efficient LFP-recycling method for is becoming increasingly apparent with the projected peak and subsequent decommissioning of the first wave of LFP batteries. Li et al. reported an acid-assisted electrochemical-extraction method to retrieve lithium ions from LFP powder in their research study. A portion of the LFP powder was subjected to direct acid leaching, and the resulting ferrous ions were separated by oxidation-induced precipitation and electroplating. The residual LFP powder was then directly oxidized by the anode to liberate lithium ions. A lithium-ion extraction efficiency of 99.9% was achieved, whereas the yield of iron ions was maintained at an exceedingly low value of less than 0.2%.122 This process efficiently and selectively separated lithium ions during leaching. The lithium ions are reclaimed following evaporation and concentrated into a highly pure lithium phosphate product. Compared with alternative recycling methods, the developed recovery approach requires fewer reagents and generates minimal waste, which introduced an environmentally friendly recycling pathway for the selective leaching and recovery of spent LFP batteries.
LFP batteries have garnered the attentions of electric vehicle (EV) manufacturers because they are cost-effective and exhibit low toxicity123, 124; they contain essential minerals, such as lithium and graphite, and are used in various electronic devices. Therefore, NEU Battery Materials developed an electrochemical-separation process to extract high-quality lithium from spent LFP batteries. In contrast to conventional recycling techniques that depend on chemical solutions and heat, the NEU method offers a notable reduction in energy usage, pollutants, and expenses. Using a proprietary technology stemming from redox studies at the National University of Singapore, the NEU method efficiently separates lithium from other battery constituents. The NEU methods were applied to the black mass byproduct of crushed LFP batteries, with electricity facilitating the electrochemical separation of lithium ions to produce lithium and hydrogen gas.125
Recovering lithium from spent ternary LIBs is essential for the circular use of energy materials on the path toward sustainability. Existing methods mainly focus on chemical leaching processes, which pose environmental hazards and produce low-purity lithium. In 2013, Yang et al. introduced a direct electrochemical-oxidation method for leaching lithium from spent ternary LIBs (Figure 4G).126 This method delivered a high lithium-leaching efficiency of almost 100% and high recovery purity without the need for additional metal leaching or other agents (Figure 4H), with voltage optimization supporting lithium leaching. Ni and O remain electroneutral, whereas Co and Mn retain their valence states. This method overcomes the issue of secondary pollution and is cost-effective and environmentally friendly compared to traditional chemical methods. Electrochemically recovering lithium from spent LIBs significantly contributes to sustainable energy development and environmental protection. Future research that investigates the electron-transfer process between NiOx and Li is crucial for electric lithium recovery.
3.4 Cobalt/nickel recycling
Cobalt is a relatively rare element that is primarily extracted as a byproduct during copper and nickel mining. Cobalt is distributed globally in a variety of geological formations, with approximately 60% of the world's cobalt produced in the Democratic Republic of the Congo. Other significant cobalt producers include Russia, Australia, Canada, and Cuba, whereas smaller deposits are located in the Philippines, Madagascar, and Finland (Figure 5A).127-129 Cobalt is often used in the cathode material in a LIB owing to its ability to deliver high energy densities and improve battery stability.130, 131 Nickel is adjacent to cobalt in the periodic table, and shares some of its physical and chemical properties. Nickel is a relatively abundant element found primarily in sulfide ores such as pentlandite and nickeliferous limonite. Major nickel producers include Indonesia, the Philippines, Russia, and Canada. Like cobalt, nickel is frequently used in LIB cathodes to enhance energy density and extend battery life. The positive effects of Ni and Co on the energy density of a LIB and its superior structural stability during charging and discharging outweigh the economic challenges posed by their high costs.
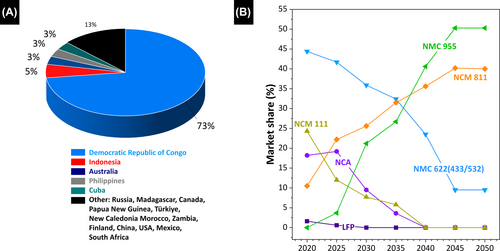
Experts anticipate a significant shift toward Ni-rich cathode materials in the coming years. This projection is driven by the superior energy densities and thermal stabilities of nickel-rich materials, coupled with their cost-effectiveness and widespread availability compared with cobalt (Figure 5B).132 However, the diverse array of materials and components in a LIB poses recycling challenges. Extracting Co and Ni from cathode materials requires complex processes that are not always economically or operationally efficient. Furthermore, technologies for recycling cobalt and nickel are still under development, with current methods often expensive and requiring specialized facilities and equipment. Resource dependency is a growing concern as the global demand for LIBs increases, which increases reliance on new cobalt and nickel sources, and emphasizes the need for recycling to mitigate the environmental impacts of mining. Overall, cobalt and nickel play crucial roles in battery technology, but recovering them from spent batteries remains a challenge that necessitates ongoing research and development.
So far, the selective separation of nickel and cobalt remains a significant challenge. Additionally, the electrochemical separation of cobalt and nickel ions poses difficulties due to their similar electrochemical properties and frequent coexistence in similar environments. Cobalt and nickel exhibit comparable electrochemical characteristics, making them behave similarly in an electrochemical cell and thus challenging to separate. One challenge is associated with their presence in complex mixtures with other ions, which complicates selective separation. Furthermore, they can exist in various oxidation states, which further complicates electrochemical separation. Furthermore, developing electrodes and electrolytes capable of selectively reacting with Co or Ni to efficiently separate them is another challenge. Given the complex electrochemical behavior of ion mixtures, separation requires specific chemicals and/or materials to achieve the desired selectivity. In the next section, we discuss the challenges and requirements of new selective separation methods.
In 2005, Lupi et al. introduced a multistage approach for recovering nickel and cobalt from spent batteries.133 Hydrometallurgical methods for recycling lithium-ion and lithium–polymer batteries containing LiCoO2 and LiCoxNi(1-x)O2 cathode materials involve several steps, including cathodic paste leaching, nickel–cobalt separation via solvent extraction, and subsequent Co and Ni metal recovery through electrolysis. Electrolytic cobalt recovery at a current density of 250 A m−2 was found to result in efficient Co deposition, with a current efficiency of approximately 96% and a specific energy consumption of approximately 2.8 kWh kg−1 achieved.133 Additionally, electrolysis at a constant potential was found to be highly effective for cobalt recycling, with 100% recovery reported, with the electrochemical filter producing a solution containing less than 1 ppm Co. Furthermore, electrolytic nickel recovery at the same current density yielded an attractive Ni deposit, with a current efficiency of approximately 87% and a specific energy consumption of approximately 3.0 kWh kg−1.133 Electrolysis of a partially depleted Ni solution at constant potential resulted in a very pure powder with less than 100 ppm nickel remaining in solution. These results underscore the feasibility and efficiency of the developed hydrometallurgical method for recycling Co and Ni from LIBs and lithium–polymer batteries.
The lithium cobalt nickel oxide (LiCoₓNi1-xO₂, 0 < x < 1) cathode material is widely applicable to commercial LIBs. Direct electrochemical nickel recovery from the leachate obtained by dissolving this cathode is not feasible owing to anomalous cobalt deposition.134 Nickel was recovered using galvanostatic and potentiostatic electrolytic methods following its separation from cobalt by solvent extraction. The operating conditions, such as temperature, pH, Ni concentration, bath agitation, and current density, were investigated, with galvanostatic conditions enabling efficient Ni-metal deposition. Conversely, potentiostatic conditions led to almost complete removal of the nickel from the electrolyte.
Hydrometallurgical processes for recycling lithium-ion and polymer batteries that feature LiCoₓNi1-xO₂ cathodes have been widely explored.30, 135 This process encompasses several key steps, including cathodic paste leaching, cobalt–nickel separation via solvent extraction, and the subsequent recovery of nickel metal through electrolysis at constant current density. In addition, Ni powder was recovered via electrolysis at constant potential.134 Here, solvent extraction used modified Cyanex® 272 in kerosene to separate Co and Ni, with the resulting solution, which contained minimal Co, becoming a suitable electrolyte for Ni production following the removal of activated carbon. Electrolytic Ni recovery under specific operating conditions delivered good Ni deposition in high yield and with low energy consumption. Electrolysis of a Ni solution at constant potential generated almost 100% pure powder within a short period of time, with only small amounts of Ni remaining in solution.134
Long et al. examined the use of copper metal hexacyanoferrate (CuHCF) nanoparticle films to electrochemically separate Co2+ from aqueous solutions (Figure 6C).136 Various conditions, included ones with coexisting ions and various initial concentrations, pH values, potentials, and contact times, were considered, with higher removal efficiencies observed at reduction potentials rather than oxidation potentials, with pH 8.0 found to be optimal for Co2+ adsorption (Figure 6D). This study also investigated how different LiNO3 ionic strengths impact adsorption, which revealed that the presence of Li+ has little influence on Co2+ removal and highlights the cobalt-recovery potential from spent LIBs containing LiCoO2. Adsorption behavior followed the Redlich–Peterson isotherm model, while the adsorption kinetics were explained using the Elovich model, suggestive of chemical adsorption.136 These findings suggest that CuHCF nanoparticles may play promising cobalt-recovery roles, with sorption capacity successfully regenerated.
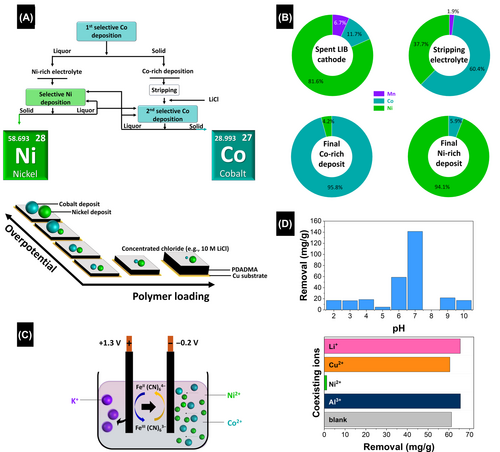
The electrochemical recovery of cobalt from deep eutectic solvents is promising for LIB recycling. This study investigated the effects of applied potential, operating temperature, and substrate on cobalt recovery from a solution containing choline chloride and urea,137 which revealed that the choice of substrate significantly influences the recovery mode, with stainless-steel mesh found to be optimal owing to its high selectivity and rapid recovery rate. In addition to a high (54 ± 3%) Faradaic efficiency for Co, stainless-steel mesh exhibited a high specific cobalt recovery rate of 4.1 ± 0.1 mg h−1 cm−2 and facile cobalt collection and substrate regeneration. A moderate cathodic potential and a temperature in the 94–104°C range were found to be crucial for efficient and selective cobalt recovery.137 These findings contribute to a better understanding of metal recovery from deep eutectic solvents and offer new avenues for controlling metal growth by adjusting the composition and crystal structure of the substrate.
Freitas et al. investigated the use of spent LIBs to create cobalt and copper multilayers via galvanic deposition.138 This study explored the influence of pH on nucleation, growth mechanisms, morphology, and crystal structure. Nucleation occurred immediately at pH 2.7 and the deposition of cobalt on various substrates progressed to pH 5.4. Copper deposition on cobalt followed a similar trend. Galvanic deposition at pH 5.4 yielded more-porous structures than those formed at pH 2.7. XRD revealed the presence of CuO, Cu2O, and cubic-centered Cu and Co structures at both pH values. Electrochemical impedance spectroscopy showed a characteristic Co–Cu multilayer circuit with irregular electrolyte deposition and cobalt dissolution. Nucleation models indicated immediate nucleation at pH 2.7 and progressive nucleation at pH 5.4.138 Morphological analysis showed that the Co and Cu multilayers were more porous at pH 5.4, suggestive of a predominant progressive nucleation mechanism. Conversely, multilayers deposited at pH 2.7 were less porous due to immediate nucleation. The XRD patterns showed specific reflections for Cu and Co multilayers at various pH values and charge densities. The equivalent circuit constructed for the Cu–Co multilayers based on the EIS data indicated irregularities attributable to a constant-phase element.
Garcia et al. used an electrochemical quartz-crystal microbalance to investigate the cobalt-electrodeposition mechanism under various pH conditions.139 Co electrodeposition proceeded via direct reduction at pH 5.40, with a mass-to-charge (M/z) ratio of approximately 33.00 g mol−1 under both potentiodynamic and potentiostatic conditions. In contrast, potentiodynamic deposition involved adsorbed hydrogen at pH 2.70 to yield a lower M/z value of 13.00 g mol−1.139 Direct reduction and the formation of adsorbed hydrogen occur concurrently under potentiostatic conditions at this pH, with higher M/z ratios observed at more-negative potentials. Cobalt electrodissolution occurs directly to form Co2+ at pH 2.70, whereas intermediate Co+ species are involved at pH 5.40. These observations are crucial for optimizing electrochemical recycling processes for recovering cobalt from spent batteries.
Barbieri et al. synthesized Co(OH)2 and Co3O4 films from the leached cathodes of spent LIBs.140 Co(OH)2 was electrodeposited onto conductive glass at −0.85 V with a charge density of 20 C cm−2 and an efficiency of 66.67%. Heating the Co(OH)2 film at 450°C for 3 h in air converted it into Co3O4 with an efficiency of 64.29%. Cyclic voltammetry in 1.0 mol L−1 KOH revealed three anodic peaks for Co(OH)2, indicative of the steps involved in oxidizing Co(OH)2 to CoO2. The absence of cathodic peaks indicates that this oxidation process is irreversible. Co3O4 exhibited a redox pair consistent with reversible oxidation between Co3O4 and CoO2. The Co3O4 electrode exhibited a charging efficiency of 77% over the first 10 cycles and specific capacitances of 31.2 F g−1 at 1.0 mV s−1 and 10.5 F g−1 at 10 mV s−1, highlighting its potential as a pseudocapacitor.140 Structural analysis confirmed the porous nature of the Co(OH)2 electrode, with than 10 μm in size. Heat treatment produced fissures and larger Co3O4 clusters that enhanced electrolyte diffusion and charge transfer, and supported their use in electrochemical-device applications. This study demonstrated the feasibility of recycling Co(OH)2 and Co3O4 from spent LIBs, thereby adding value to materials that may otherwise harm the environment; it also provided crucial insight for the development of advanced electrocatalysis and energy-storage technologies.
Kim et al. discussed the importance of molecularly selective metal deposition for sustainable LIB-electrode recycling, focusing on metals such as cobalt and nickel that are challenging owing to their similar reduction potentials.86 The authors demonstrated a method for achieving molecular selectivity for Ni and Co during potential-dependent electrodeposition, which involved combining interfacial design with electrolyte control (Figure 6A). Specifically, the use of concentrated chloride helps to control speciation by forming anionic cobalt chloride complexes ([CoCl4]2−) while retaining nickel in a cationic ([Ni(H2O)5Cl]+) form. Additionally, functionalizing the electrode with a positively charged polyelectrolyte enables cobalt selectivity to be adjusted based on polyelectrolyte loading. This strategy was used to recover multi-component metals from commercial lithium nickel manganese cobalt oxide electrodes, with overall purities of 96.4 ± 3.1% and 94.1 ± 2.3% reported for cobalt and nickel, respectively (Figure 6B).86 However, techno-economic analysis was used to identify the cost constraints associated with the background electrolyte. Overall, this study suggested that selective electrodeposition is a promising efficient separation method for battery recycling that facilitates the direct recovery of cobalt and nickel from used NMC cathodes, as well as potential future material-processing applications through morphological control and structuring.
Electrochemical recycling technologies for spent LIBs have shown significant promise in terms of addressing the dual challenges of resource depletion and environmental sustainability. By leveraging techniques such as electrodeposition, highly pure valuable metals, such as Co, Ni, and Mn, can be selectively recovered in a highly efficient manner. Studies have shown that optimized electrochemical processes can enhance the performance and applicability of recovered materials in various electrochemical devices, including pseudocapacitors and electrocatalysts.
3.5 Manganese recycling
Manganese is widely distributed in various geological formations worldwide. The largest Mn deposits are found in South Africa, Australia, China, Brazil, Gabon, and India. Manganese is primarily extracted by mining other metals, such as iron, nickel, and copper. Manganese is commonly used in batteries owing to its various beneficial properties; in particular, it has been used as a cathode material in LIBs because it offers a high energy density, good conductivity, and structural stability, thereby contributing to battery performance and longevity.
Manganese is more cost-effective and more readily available than other cathode materials, making it an attractive option for battery manufacturing. However, recycling Mn from batteries is challenging. LIBs contain various materials and components; hence, extracting Mn from cathode materials requires complex processes. Furthermore, current recycling technologies for manganese are not as advanced as those for other materials, such as lithium and cobalt. Developing cost-effective and environmentally friendly recycling methods for manganese remains an important task for the battery-recycling industry. Manganese is primarily recycled through mechanical, hydrometallurgical, or pyrometallurgical processes, which are not always economical or environmentally friendly. However, electrochemical approaches for Mn recycling have also been proposed and require further investigation.
Manganese is primarily found in lithium manganese oxide (LiMn2O4) batteries141 and comprises approximately one-third of the electrode mass. Manganese is also commonly used as a dopant in lithium cobalt oxide (LiCoO2) and LiFePO4 electrodes, which enhances the electron conductivity and improves the lithium-ion diffusion rate, thereby boosting charging and discharging efficiencies and the overall performance of the battery. Moreover, manganese maintains the structural integrity of the electrode, resulting in an extended battery lifespan.
Manganese resources are also present in different battery types, including nickel–metal-hydride (Ni–MH) batteries that require reliable and efficient recycling. In 2012, Santos et al. reported chemical and electrochemical methods for recycling Mn, Co, Ni, and Zn from the positive electrodes of spent Ni-MH batteries (Figure 7C).142 The chemically precipitated materials consisted of Mn3O4, Co(OH)2, β-Ni(OH)2, Zn(OH)2, carbonate, nitrate, and sulfate anions effectively extracted from the mother solution along with adsorbed water. Cyclic voltammetry demonstrated low current densities at scan rates above 10 mV s−1, which is ascribable to the formation of hydroxide films. Inductively coupled plasma optical emission spectroscopy (ICP-OES) revealed the presence of Ni2+, Co2+, Zn2+, and Mn2+, which highlights the anomalous behavior of the electroplating process (Figure 7B). The quantity of deposited nickel ions correlated with the composition of the sulfamate bath, whereas the presence of manganese in the electrolytic deposits is ascribable to Mn(OH)2 precipitation, while Zn(OH)42− remained unreduced within the examined potential range. The galvanically deposited material contained Mn3O4, Co, CoO, Co(OH)2, and Ni, with charge efficiency of 83.7% at −1.1 V versus Ag/AgCl at a charge density of −90 C cm−2.142 The dissolution of electrolytic deposition depends on the applied potential. The chemically precipitated materials comprise Mn3O4, Co(OH)2, β-Ni(OH)2, and Zn(OH)2, with incorporated manganese and zinc as additives to enhance electrode capacity. The infrared spectrum of the recovered materials show a band at 1384 cm−1 that is attributable to the carbonate and nitrate ions adsorbed by the precipitates from the mother solutions. Thermogravimetric analysis revealed mass losses due to the removal of adsorbed water, nitrate, and carbonate, while the oxides were observed to be thermodynamically stable to 1200°C.
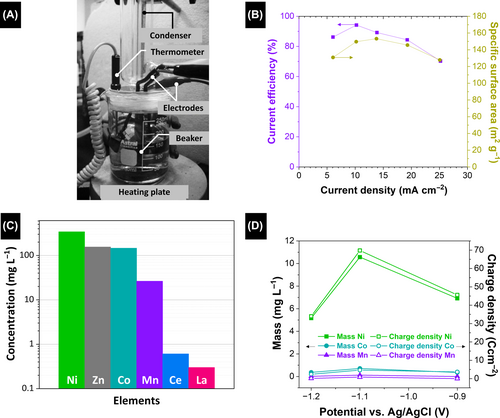
Roriz et al. used electrolytic processes to examine the use of spent batteries as sources of manganese dioxide (MnO2) (Figure 7A).143 An electrolyte solution containing various metal ions was also used. Electrolytic manganese dioxide was produced by electrolysis at various current densities and temperatures. The analyzed materials exhibited optimal purities, current efficiencies, and specific surface areas at current densities between 0.0102 and 0.0139 A dm−2. The ε-MnO2 variant was observed in all experiments, with samples containing more than 90% of this variant at current densities in the 0.61–2.51 A dm−2 range (Figure 7B). A MnO2 yield of 97.4% was recorded for the sample produced at a current density of 1.93 A dm−2.143 Changing the current density did not affect the structure of the resulting manganese dioxide, with ε-MnO2 predominating in all samples.
4 OUTLOOK, PERSPECTIVE, AND CONCLUSIONS
As part of the global energy transition, LIBs are pivotal for driving technological advancements, particularly for electric vehicles and renewable energy-storage systems. The widespread adoption of LIBs has prompted concerns regarding their environmental impact and the scarcity of critical resources, necessitating the exploration of sustainable recycling strategies. This review critically evaluates various battery-recycling methodologies that range from traditional pyrometallurgical and hydrometallurgical techniques to emerging approaches, such as bioleaching and electrochemical separation. While conventional recycling methods present environmental and efficiency challenges, electrochemical techniques show considerable promise in terms of enhancing selectivity, reducing energy consumption, and minimizing the production of secondary waste. Electrochemical recycling, particularly after leaching, has emerged as a pivotal solution for recovering valuable metals, including Li, Co, Ni, and Mn, from spent LIBs in an efficient and environmentally friendly manner. By examining recent advancements and comparing various recycling methodologies, this review highlights the potential of electrochemical recycling in addressing the dual challenges of resource depletion and environmental sustainability. Furthermore, the discussion extends to considerations surrounding scalability, economic viability, and future directions for electrochemical recycling, and advocates for its seamless integration into global battery-recycling infrastructure. Advanced electrochemical recycling methodologies show promise for resource conservation and exhibit significant potentials for bolstering environmental stewardship within the battery-recycling industry.
In particular, lithium recycling is important for preserving resources and reducing waste, with electrochemical processes showing encouraging outcomes. Similar cobalt- and nickel-recycling progress has been made using hydrometallurgical and electrochemical means. Moreover, efficient manganese recovery can be achieved through electrochemical processes, despite not having been demonstrated over a wide range. Ongoing research and innovation are imperative for promoting a circular economy and diminishing the environmental footprint associated with battery production and disposal. Advancements and possible integration of electrochemical-recycling methods into the global battery recycling infrastructure are crucial for addressing resource depletion and environmental sustainability concerns, which necessitates concerted efforts to develop and implement large-scale electrochemical recycling technologies, thereby heralding a more-sustainable future.
Advancements in electrochemical recycling have helped to solve the pressing environmental challenges associated with LIBs while promoting a shift toward a more sustainable and resource-efficient future. The capacities of electrochemical techniques to selectively extract valuable metals from spent LIBs and their potentials to minimize energy consumption and reduce secondary waste production are significantly promising for transforming the battery-recycling landscape. Continuing progress this field, driven by ongoing research and innovation, has led to growing optimism regarding the potential of electrochemical recycling to drive substantial positive changes. Progress to date and the impending adoption of electrochemical recycling methods within the global battery-recycling infrastructure have set the stage for a future in which concerns regarding resource depletion are addressed, environmental sustainability is prioritized, and a circular economy becomes increasingly attainable.
AUTHOR CONTRIBUTIONS
Stefanie Arnold: Investigation; data curation; visualization; writing—original draft; writing—review and editing. Jean Gustavo de Andrade Ruthes: Visualization; writing—review and editing. Choonsoo Kim: Writing—review and editing. Volker Presser: Supervision; funding acquisition; writing—review and editing.
ACKNOWLEDGMENTS
We acknowledge the support of the European Union's eLiRec project from the European Regional Development Fund (EFRE) and the State of Saarland, Germany. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies

Stefanie Arnold received her B.Sc. and M.Sc. from the Karlsruhe Institute of Technology (KIT) and her Ph.D. from Saarland University for her work on next-generation batteries and electrochemical water desalination. Her expertise lies beyond lithium-ion battery chemistries. As a postdoc at the INM—Leibniz Institute for New Materials, she is currently exploring environmentally friendly battery recycling technologies.

Jean G. A. Ruthes received his B.Sc. and M.Sc. in Chemistry from the Universidade Federal do Paraná in Curitiba, Brazil. At present, he is a Ph.D. student at the INM – Leibniz Institute for New Materials, working on advanced 2D heterostructures and gels for electrochemical energy storage.

Choonsoo Kim has been a professor at Kongju National University since 2018. He earned his B.S. in 2007 and his Ph.D. in 2015 from Seoul National University. His interdisciplinary research addresses environmental and sustainability challenges by integrating environmental engineering, electrochemistry, and materials science, with a particular emphasis on water applications.

Volker Presser is a full professor at Saarland University and Research Department Leader at the INM—Leibniz Institute for New Materials (Saarbrücken, Germany). He obtained his Ph.D. from Eberhard-Karls University (Germany) and was a Humboldt Fellow at Drexel University (USA) in the Group of Yury Gogotsi. Since 2021, he has been Managing Co-Director of the Saarland Center for Energy Materials and Sustainability (saarene) and Editor-in-Chief of Energy Advances (Royal Society of Chemistry).





