Review on Synthesis Methods of Carbon Nanotubes as Activated Carbon Composites Based on Biomass for Supercapacitors in Electric Vehicles
Abstract
Biomass can be converted into carbon through carbonization processes (pyrolysis and hydrothermal carbonization) and activation (physical and chemical). The resulting carbon has a high potential as a supercapacitor electrode material due to its porous structure, which supports rapid ion transport. Various methods have been developed to extract or transform biomass into porous carbon. One of the newly developed nanocarbon materials is carbon nanotubes (CNTs) because they have advantages in terms of mechanical, physical, chemical, and electrical properties. This review discusses various kinds of CNT synthesis as activated carbon composites for supercapacitors. The synthesis of these CNTs can be conducted through chemical and physical methods, including arc discharge, laser vaporization, and chemical vapor deposition (CVD). This work reviews various methods of CNT synthesis and analyzes the best methods to be used as composites for supercapacitors for electric vehicles. It is concluded that CVD is the best method for synthesizing CNTs. Its main advantage is that CNTs can be used directly without purification unless the catalyst particles need to be removed. However, further experimental studies are required to find the most optimal conditions for each composite from a type of mesoporous activated carbon and CNTs in terms of preparation and performance outcome.
1 Introduction
Energy is an important part of human needs. Human activities require energy sources in all fields. Humans need high amounts of energy for transportation,[1] electricity for household appliances,[2] industrialization,[3] and urbanization.[4] Energy sources are currently categorized into renewable or nondepleted energy and nonrenewable or depleted energy. Several renewable energy sources, such as wood energy, biomass, and biogas, are used as alternative fuels. Alternative fuels are needed because of the availability of reserves sourced from fossils, which have long been predicted to run out in the next few years.[5] The total world energy demand from fossil fuels is estimated at 13.731 billion tons of oil equivalent (BTOE) in 2012 and is expected to approach 18.30 BTOE in 2035.[6] The depletion of natural resources and unequal distribution, impacting the world economy (e.g., price fluctuations and unbalanced supply), have resulted in problems in various sectors such as energy generation and storage, industrialization, and transportation.[7-11] In addition, energy derived from fossil fuels cannot be fully utilized because it causes an increase in global warming.[12] Therefore, many efforts have been made to save energy using different energies, such as solar energy, wind energy, the kinetic energy of flowing water, geothermal energy, and biofuels.[13-15] With the development of technology and industry, there are various categories of energy, such as chemical, electrical, mechanical, radiation, thermal, nuclear, and relativistic energy.[16]
Electricity is one of the vital energies needed in all aspects of human activities. The density of this energy is projected to increase from 250 Wh kg−1 in 2020 to 323 Wh kg−1 in 2030.[17] Even in 2060, total demand for electricity will reach 207.44 GWy (gigawatt years). This electricity can be obtained by converting various other types of energies.[18] However, as predicted by the World Energy Council, by 2050, the world will need to double its energy supply.[19] Therefore, most renewable energy except biofuels is supplied as electricity (electric power), which creates a high demand for reliable devices for electrochemical storage, including batteries (such as lithium-ion batteries[20]), fuel cells, and supercapacitors.[21]
Supercapacitors are storage devices that have very high capacity in a small size. Supercapacitors can be determined by several parameters,[22, 23] including technological improvement, model verification and validation, and the design of electrical circuits containing supercapacitors.[24] In particular, supercapacitors have advantages over batteries in their storage speed, including the ratio of low discharge time of supercapacitors to lithium-ion batteries is 1–10 s:10–60 min and the ratio of enhanced cyclic stability of supercapacitors to lithium-ion batteries is 30 000 h:500 h.[25-27] Recent advances in supercapacitors in terms of electrode materials and electrolytes have the potential to fill the gap between batteries and fuel cells. Thus, there is a need to develop sustainable and high-power energy conversion and energy storage systems such as supercapacitors for applications ranging from electronics to electric vehicles (EVs) with very low exhaust emissions.[21, 22] Figure 1a illustrates the number of publications (papers, books, patents, and scientific reports) on Google Scholar (accessed in August 2021) for the keyword supercapacitor.
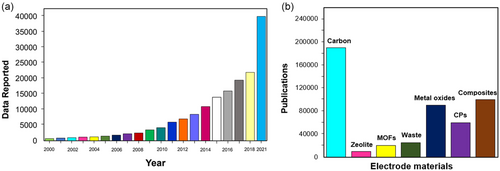
The development of supercapacitors has been carried out using various basic energy-producing materials such as carbon, zeolites, metal-organic frameworks (MOFs), environmental waste, metal oxides (e.g., MnO2[28]), Conducting Polimers (CPs), and composites. Supercapacitors can be used to meet high currents and specific power (10 000 W kg−1) in less than 1 min; hence, they can be used alone or in combination with other energy storage devices, namely batteries.[28-30] Various types of supercapacitors have been used to meet power and energy requirements.[31, 32] However, the ideal building materials for supercapacitor devices must be produced using simple, low-cost, and environmentally friendly synthesis methods. These things can be fulfilled from biomass due to the fact that biomass is widely available as a renewable feedstock, does not emit greenhouse gases, and involves simple synthesis.[33]
Biomass is one of the most abundant renewable resources on Earth and can be utilized for various applications such as the capture of CO2, hydrogen storage,[34-36] dye-sensitized solar cells,[37-39] water treatment,[40] and energy storage[40, 41] because of its wide availability,[42-45] sustainable capabilities, renewable nature, unique structure, and low cost. In addition, the abundant amount of carbon compounds and the well-oriented channel for the metabolic process make this biomass-based electrode material suitable for rapid ion transport in electrochemical charging applications. Currently, the use of biomass as a carbon source in electrode devices has become a hot topic in the field of energy conversion and storage (Figure 2).[46-51]
EVs are vehicles that use electric motors for propulsion, reducing or eliminating the need for traditional combustion engines and fossil fuels.[52] These vehicles are powered by either a battery or external sources of electricity, such as charging stations.[52] With the increasing demand for EVs as a sustainable alternative to traditional combustion engine vehicles, advanced and efficient energy storage technologies have become more crucial. The use of carbon nanotubes (CNTs) in EV applications holds great promise for improving the performance and efficiency of these vehicles. In addition to their mechanical benefits, the excellent electrical and thermal conductivity of CNTs makes them attractive for use in batteries and capacitors for EVs. The high surface area of CNTs also offers opportunities for enhanced energy storage and faster charging, addressing some of the key challenges in EV technology.[51-55] As research and development in this field continue to progress, the integration of CNTs in EVs holds great promise for revolutionizing the automotive industry and making EVs more efficient, reliable, and sustainable.[55] CNTs have great potential in EV lubrication systems that can reduce damage and wear, play an important role in improving supercapacitor electrode materials used in EVs, and contribute to various systems in EVs.[56] In general, the application of CNT supercapacitors is designed by functionalization using various materials so as to improve the specific capacitance and energy density of supercapacitors.[57] Figure 3 illustrates the research and development in the integration of CNTs for EVs.
Hence, research on biomass as a carbon source not only becomes an alternative in the utilization of biomass resources, but also gives us a better understanding of the relationship between structure and electrochemical properties, which can ultimately help in creating a more efficient design of biomass-based carbon materials for practical application. So far in the literature,[58] there have been several research reviews on the potential of biomass-derived materials and nanomaterials as biomass derivatives for use in electrochemical energy storage.[59] However, there have been very few reviews on the development of activated carbon composites from biomass and CNTs as supercapacitors.
This review is organized to initially examine the material components as composite using biomass-derived carbon and CNTs. The compulsory parts of this subject involve converting biomass to carbon material. Furthermore, it analyzes the characteristics and suitability of CNTs as electrodes for supercapacitors or other storage applications. In addition, it involves comprehending the development of supercapacitors and the application of CNTs in EVs. The second segment of this article highlights numerous kinds of synthesizing activated carbon and CNTs, as well as their merits and drawbacks. Thus, discussing some of the potentials of activated carbon composites and CNTs for supercapacitors and storage applications in EVs is very important. It becomes the authors’ insight that this review will be useful for investigating various approaches to the various synthesis methods of CNTs to achieve enhanced outcomes, optimized purity, and decreased expenses.
2 Biomass-Derived Carbon for Supercapacitor
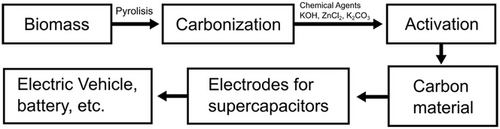
Biomass compounds, especially those consisting of carbohydrates, have the potential as a sustainable carbon source. In recent years, lignin, carbohydrates, cellulose, chitin, protein, etc. have been widely used as carbon sources. Carbon resources for this biomass can also come from raw materials containing carbon nanofibers (CNFs), biomass, and several composites such as palm shells, palm oil, bamboo, rubber wood, rice straw, or coconut shells.[59-64] There are several methods that can be used to convert biomass into carbon, such as carbonization methods (e.g., pyrolysis and hydrothermal carbonization) and activation methods (e.g., physical and chemical activation). Physical and chemical activation methods or both are illustrated in Figure 4, where each method has a conversion efficiency, namely, chemical activation 50–60%,[65] physical activation 30–50%,[65] HTC 50–70%,[65] pyrolysis 50–75%,[65] and hydrothermal carbonization 35–55%.[65] These methods have been widely used to convert biomass into carbon materials that have added value.[65]
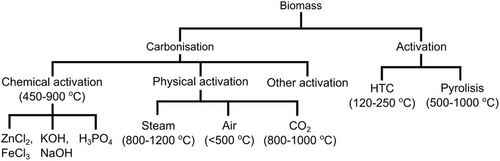
Pyrolysis and hydrothermal carbonization are commonly used methods for carbonizing biomass. Pyrolysis is carried out in an inert state or limited oxygen at high temperatures, while hydrothermal carbonization refers to a thermochemical process to convert biomass into carbonaceous materials.[66-68] Hydrothermal carbonization can be controlled by varying the operating conditions to obtain solid, liquid, and gaseous products in the desired ratio. Hydrothermal carbonization is carried out at a relatively lower temperature of around 180–250 °C and a pressure range of 2–10 MPa.[69-71] The use of hydrothermal carbonization for the conversion of biomass into carbonaceous materials has received great attention for use in various applications such as catalysis,[71, 72] CO2 capture,[73, 74] and energy storage.[70, 75-80] Therefore, biomass is very effective as a supercapacitor raw material. Recent research has shown that simple carbonization or activation methods can be used to activate carbon contained in biomass. The best method to obtain electrocapacitive performance is chemical activation methods, such as potassium hydroxide, where biomass that has a mesoporous pore type will produce a high capacitance value. This type of mesoporous pore will later become a gap in expanding the surface of activated carbon as a supercapacitor electrode, which is influenced by several factors such as carbonization temperature, activation method, activator concentration, activation temperature, and the type of material used.[81] Carbon derived from biomass can be further processed into CNTs to improve its application as a composite component in supercapacitors.
3 CNTs as Composite
CNTs have the potential to be used as activated carbon composites in the manufacture of supercapacitors. Modification of activated carbon produced by biomass and CNTs can increase the capacitance of supercapacitor cells.[81] CNTs are allotropic carbon molecules whose structure can be visualized as cylinders made of rolled graphene layers.[82] In recent years, CNTs have become an attraction for researchers because of their unique properties, namely, having high thermal conductivity,[83] the ability to carry large electric currents,[84] high mechanical,[85] and thermal stability. With its extraordinary mechanical and thermal properties, it affects the strength, stiffness, wear resistance, and conductivity of the composite when used as a matrix.[86] CNTs have potential in engineering applications due to their mechanical, physical, electrical, and geometric characteristics, which consist of small diameter and high aspect ratio.[87] This shows that the percentage of CNTs in the matrix directly improves the mechanical, thermal, and electrical properties of composite materials. In Atanu Roy's research, the asymmetric supercapacitor (ASC) exhibited a maximum specific capacitance of 197.7 F g−1 at a scan rate of 2 mV s−1 within a potential window of 1.8 V.[88, 89] The ASC demonstrated a specific capacitance of 190.5 F g−1 and an energy density of 85.7 Wh kg−1, while the power density reached 1.6 kW kg−1 at a current density of 1 A g−1.[90]
CNTs consist of two categories, namely single graphene walls (SWCNTs) and multiple walls (MWCNTs),[91] as shown in Table 1. The arrangement of these CNTs consists of two or more concentric cylindrical shells of graphene sheets arranged around a central hollow core with interlayer separation, such as on graphite. In contrast, SWCNTs consist of a single graphene cylinder. Both SWCNTs and MWCNTs have physical characteristics in the form of solids and are microcrystals with high aspect ratios of 1000 or more, even though their diameters are close to the molecular dimensions. The diameters of SWCNTs and MWCNTs range from 0.4 to 10 nm, while their lengths usually reach the micrometer range, although there has been a synthesis of centimeter-long CNTs.[92] Table 1 shows and describes the comparison between the theoretical and experimental properties of CNTs.[93] SWCNTs have higher specific stiffness and strength than MWCNTs.[94]
| Property | CNTs | Graphite | |
|---|---|---|---|
| SWCNT | MWCNT | ||
| Specific gravity | 0.8 g cm−3 for SWCNT; | 1.8 g cm−3 (theoretical) | 2.26 g cm−3 |
| Elastic modulus | 1 TPa | 10–60 GPa | 1 TPa (in-plane) |
| Strength | 50–5000 GPa | 10–60 GPa | |
| resistivity | 5–50 μΩ cm | 50 μΩ cm (in-plane) | |
| Thermal conductivity | 3000 W m−1 K−1 (theoretical) |
3000 W m−1 K−1 (in-plane) 6 W m−1 K−1 (c-axis) |
|
| Magnetic susceptibility | 22 × 106 EMU g−1 (perpendicular with plane), 0.5 × 106 EMU g−1 (parallel with plane) | ||
| Thermal expansion | Negligible (theoretical) |
−1 × 10−6 K−1 (in-plane), 29 × 10−6 K−1 (c-axis) |
|
| Thermal stability | >700 °C (in air); 2800 °C (in vacuum) | 450–650 °C (in air) | |
| Specific surface area | 10–20 m2 g | ||
| Layer structure | A single layer of graphene | Multiple layers of graphene | |
| Synthesis | A catalyst is required for its synthesis | Can be produced without a catalyst | |
| Bulk synthesis | Bulk synthesis is difficult as it requires proper control over growth and atmospheric condition | Bulk synthesis is easy | |
| Purity | Purity is high | Purity is poor | |
| Chance of defect | The chance of defect is less, but once it occurs, it is difficult to improve | The chance of defect is higher during functionalization | |
| Flexibility | It can be easily twisted and is more pliable | It cannot be easily twisted | |
| Accumulation | Less accumulation in the body | More accumulation in the body | |
| Characterization | Characterization and evaluation are easy | It has a very complex structure | |
| 3D structure |
|
|
|
Based on research by Han and Fina in 2011,[95] the thermal conductivity would increase significantly by adding a small amount of CNTs to the nanocomposite.[95] The right simulation for predicting the thermal transport properties is by molecular dynamics (MD).[96] According to Zhang et al.[97] thermal conductivity depends on chirality and zigzag. Chiral in CNTs has the highest and lowest points in thermal conductivity, respectively. Table 2 describes the thermal conductivity of CNTs analyzed using different methods of MD and continuum mechanics (CM).
| Classified | Researcher | Year | Method | Chirality | Temperature [K] | Thermal Conductivity [W m−1K−1] | |
|---|---|---|---|---|---|---|---|
| MD | CM | ||||||
| Low thermal conductivity | Moreland et al.[200] | 2004 | MD, Brenner potential | – | (10,10) | 300 | 215–831 |
| Maruyama[201] | 2003 | MD, Tersoff-Brenner bond order potential | – |
(5,5) (8,8) (10,10) |
300 | 260–400 for (10,10) | |
| Medium thermal conductivity | Osman and Srivastava[202] | 2001 | MD, Tersoff-Brenner potential | – |
(5,5) (10,10) (15,5) |
300 | 1700 for (10,10) |
| Grujicic et al.[203] | 2004 | MD, Adaptive Intermolecular Reactive Empirical Bond Order (AIREBO) potential | – |
(10,10) (18,0) (14,6) |
300 | 1780 for (10,10) | |
| Lindsay et al.[204] | 2010 | Boltzmann transport equation | – | – | 300 | 2500 | |
| Che et al.[96] | 2000 | MD, equilibrium Green-Kubo method based on the Brenner potential | – | (10,10) | 300 | 2980 | |
| Zhang et al.[97] | 2004 | MD, nonequilibrium Green–Kubo method used on the Brenner potential | – |
(11,11) (20,0) (10,13) |
300 | 3700 for (11,11) | |
| High thermal conductivity | Ren et al.[205] | 2010 | Nonequilibrium MD method | – | (10,10) | 300 | 6000 |
| Berber et al.[206] | 2000 | MD | – | (10,10) | 300 | 6600 | |
| Donadio and Galli[207] | 2007 | MD and Boltzmann transport equation | – | (10,0) | 300 | 7000 | |
| Mir et al.[208] | 2012 | – | Solid continuum elements | (10,10) | 300 | 9750 | |
| Yao et al.[209] | 2005 | MD | – | (10,10) | 300 | 1–4 × 105 | |
4 Synthesis Methods for CNTs
There are many methods for synthesizing CNTs, including arc discharge, laser vaporization, and chemical vapor deposition (CVD) techniques. These methods are described further in the sections below.
4.1 Arc Discharge
The arc discharge method was the first method used by Iijima[98] in synthesizing CNTs. Arc discharge is a method of electric gas disconnection to produce plasma. This method places two graphite electrodes close to each other, about 1 mm, in an atmosphere of an inert gas, such as helium, at a pressure of 500 Torr.[99] The process also produces carbon soot containing fullerene molecules.[100] The carbon arc provides a good tool for generating the high temperatures used to solidify carbon atoms into plasma (3000 °C).[101-103] The yield of CNTs depends on the stability of the plasma formed between the electrodes, the current density, the pressure of the inert gas, and the cooling of the electrode and chamber.[101-103] Among the various inert gases, helium (He) gives the best results due to its high ionization potential.[104] Meanwhile, SWCNTs are produced by conducting electrodes with metals such as Ni, Fe, Co, Gd, and Y.[105] The disadvantage of this method is that it produces not only CNTs but also carbon impurities and encapsulated nanoparticles.[98] However, the advantages of SWCNTs and MWCNTs are that they are easy to synthesize and require relatively lower costs.[106] Meanwhile, in the production of MWCNTs, the conditions are optimized so that during arc evaporation, the amount of soot production is minimized, and 75% of the carbon evaporated from pure graphite anode deposits to the graphite cathode faces the surface. The arc deposits consist of a hard gray outer shell made of pyrolytic graphite and an interior made of a soft black powder containing about two-thirds of CNTs and one-third of graphite.[107]
Figure 5 shows a schematic diagram of an arc discharge apparatus. It consists of two electrodes, one filled with carbon precursor and catalyst and the other a pure graphite rod. The chamber is filled with gas or liquid. An electric arc is generated between the electrodes, creating a high-temperature plasma that vaporizes the carbon precursor. Carbon vapor condenses on the cathode, forming a deposit containing CNTs. After purification, the deposit is examined under an electron microscope to study its structure.
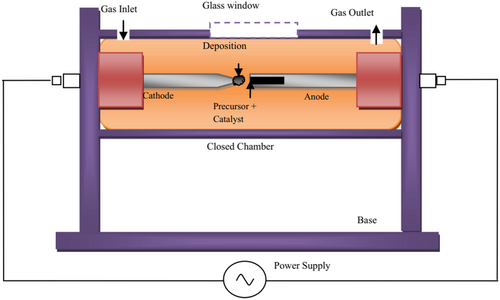
Arc discharge is a simple method that can be used to produce structurally excellent high-quality CNTs. In this method, CNTs are produced at the cathode surface, and the electrode distance is not constant, so the resulting current flow is not uniform, the electric field is not homogeneous, the carbon vapor density and temperature distribution are not uniform, and impurities always accompany the resulting CNTs. Various attempts have been made to overcome this problem, with many studies aimed at producing CNTs that are stable and have highly efficient discharge.[108-110] In the arc discharge method, the synthesis of MWCNTs does not require a catalyst, but certain types of catalysts are required for the synthesis of SWCNTs. The first report on the synthesis of SWCNTs was made by Ijima and Ichihashi.[111] In this method, holes are made in the graphite anode, which is filled with a composite mixture of metal powder and graphite, while the cathode is pure graphite. The catalysts used in the production of SWCNTs are transition metals such as Fe, Co, and Ni, and rare earth metals such as Y and Gd,[112-116] while composite catalysts such as Fe/Ni and Co/Ni have also been used for the synthesis of SWCNTs.[117]
Liu et al.[118] used a semicontinuous hydrogen arc discharge method to increase the production of SWCNTs. They obtained 2 g h−1 SWCNT bundles, and the production of SWCNTs in the ropes was about 30%, with a diameter of about 1.72 nm. The vapor pressure of the catalyst metal is an important factor in the enhancement of SWCNTs. The high helium pressure provides an advantage for the co-evaporation of metal and carbon to reduce the vapor pressure of the catalyst metal.[118] Arc discharge methods usually involve high-purity graphite electrodes, metal powders (only for producing SWCNTs), and high-purity He and Ar gases. Thus, the costs associated with the production of SWCNTs and MWCNTs are high.
4.2 Laser Vaporization
The laser vaporization method uses the same process as the arc discharge method. Both methods involve the condensation of the carbon formed from the evaporation of graphite. When metals such as Ni, Co, and Pt are added to the sample, SWCNTs/MWCNTs are formed.[119] In this method, graphite is placed in a quartz tube surrounded by a furnace, which heats it to 800–1500 °C. 500 Torr of argon gas is passed through the tube to carry soot to form a water-cooled Cu collector. The conversion of graphite into CNTs in this method can reach more than 70–90%. The disadvantage of this method is that it requires very high production costs due to the high power and expensive laser required. However, this method can yield conversion of graphite into SWCNTs as much as 50% in condensation vapor in a heat flow tube (occurring at 1200 °C).[119]
The arc discharge method involves a vacuum chamber filled with inert gas or hydrogen, with graphite electrodes acting as cathode and anode (Figure 6). A DC voltage creates a strong electric arc, vaporizing the anode and depositing CNTs on the cathode. MWNT purity and yield depend on chamber pressure, with higher yields observed at 150–400 Torr for organic gases. Pulsed arcs and liquid nitrogen environments can also be used for MWNT production.
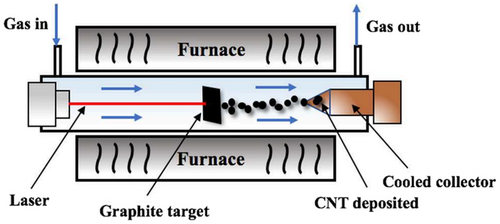
In 1996, the laser vaporization method was used to optimize the laser oven method to produce 70% SWCNTs.[120] Samples were prepared by laser vaporization method of graphite rods with a 50:50 mixture of Co and Ni powder at 1200 °C in an argon stream at a pressure of 500 torr followed by heating in a vacuum chamber at 1000 °C to sublimate C60 and other small fullerenes. A new method in the synthesis of CNTs by laser vaporization involves solid carbon in a liquid.[121] Various forms of carbon nanocage were also synthesized in this experiment. The growth mechanism is different from the precipitation mechanism because no metal catalyst is mixed with the target carbon.[122]
In another laser experiment, a porous target of Co–Ni graphite (nitrate) produced twice as much SWCNTs as a standard metal–carbon target.[123] It has been studied that ultrafast (subpicosecond) laser pulses can generate large numbers of SWCNTs. However, this laser vaporization method does not provide economic advantages because the process involves high-purity graphite rods and high laser power (in some cases, two laser beams are required), and the number of CNTs produced per day is not as high as the arc discharge method.[124]
4.3 CVD
CVD is popularly adopted and very different from two commonly used synthesis methods: arc discharge and laser vaporization.[123] In the CVD method, the synthesis of CNTs uses a metal catalyst to destroy the carbon source molecules.[124-127] Arc discharge and laser vaporization methods can be categorized as methods that require high temperatures (>3000 K) and the reaction occurs quickly, while CVD occurs at medium temperatures (700–1473 K) and the reaction lasts long (from minutes to hours). In arc discharge and laser vaporization methods, CNTs are produced separately.[128, 129]
In 1998, rapid advances in CVD development led to this method being recognized as a highly controlled technology in the production of CNTs. It is possible to manufacture high-quality MWCNTs and SWCNTs directly onto the substrate or as feedstock in bulk.[130-136] The main advantage of CVD is that CNTs can be used directly without purification unless the catalyst particles need to be removed. The CVD method produces CNTs by decomposing organic gas on a substrate coated with metal catalyst particles. Some of the CVD methods include thermal CVD, plasma-enhanced CVD (PECVD), and catalytic pyrolysis of hydrocarbons.
Figure 7 illustrates the CVD process for CNT synthesis. A CVD reactor typically includes a chamber filled with inert gas and a hydrocarbon (Figure 7a), such as methane for SWCNTs or ethylene/acetylene for MWCNTs. The substrate is heated to high temperatures (850–1000 °C for SWCNTs, 550–700 °C for MWCNTs). Hydrocarbons decompose, and carbon atoms dissolve in metal catalyst particles. Carbon accumulation leads to the formation of a semifullerene cap (Figures 7b,c), initiating nanotube growth. Removing catalysts and purifying the CNTs remain a challenge for producing high-quality materials.
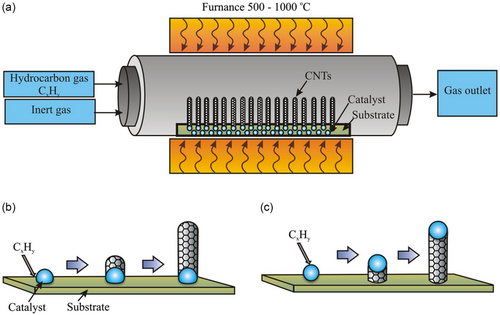
4.3.1 Thermal CVD
A thermal CVD reactor is an inexpensive and simple method of synthesizing CNTs using quartz tubes enclosed in a furnace. The substrate material can be Si, mica, silica quartz, or alumina. The nature and yield of the reaction obtained depend on a variety of different parameters, such as the nature of the catalytic metal and its supports, hydrocarbon source, gas flow, and reaction temperature. By selecting appropriate conditions such as physical (e.g., length, shape, diameter) and chemical properties (e.g., defects, graphitization), CNTs can be designed in advance. To produce MWNTs, most of the thermal CVD methods use acetylene (C2H2) or ethylene (C2H4 gas as the raw material with carbon and Fe, Ni, or Co nanoparticles as catalysts. The increase in temperature is usually in the range of 500–900 °C. At these temperatures, the carbon atoms dissolve in metal nanoparticles, eventually precipitating. The carbon then precipitates to form CNTs, whose diameter is determined by the size of the metal particles that act as catalysts. When other elements such as Cu, Cr, and Mn are used, only small amounts of CNTs are formed. Results regarding the synthesis of CNTs using the CVD method, which is divided into three classifications, are shown in Table 3.[137]
| Classified | Catalyst and substrate | Carbon source | Reaction condition | Product quality | References | |
|---|---|---|---|---|---|---|
| Temp. [°C] | Flow rate of gases [mL min−1] | |||||
| Low temperature | Fe, Co/Y, SiO2 | C2H2 | 700 | – | MWNTs (76%) | [210] |
|
Co, Fe, Cu/SiO2 Zeolite, clay |
C2H2 | 700 |
C2H2: ≈8 N2: ≈75 |
MWNTs (71–76%) And SWNTs (low yield) |
[211] | |
|
Co-Mo, Co-V Co-Fe/SiO2, Zeolite |
C2H2 | 700 |
C2H2: ≈30 N2: ≈300 |
MWNTs | [212] | |
| Co-Mo (1:2)/SiO2 | CO | 700 | CO: ≈100 | MWNTs (4%) and SWNTs (88%) | [213] | |
| Fe, Co, Zeolite powder | C2H5OH | 800 | C2H5OH vapor (5 Torr), Ar | SWNTs (>40 wt%) | [214] | |
| Mo, Co, Si, or quartz substrate | C2H5OH | 800 |
C2H5OH vapor (10 Torr), Ar-H2 (3%) |
Mat of SWNTs | ||
| Fe(CO)5 | CO | 800–1200 | CO (<10 atm) | SWNTs (in high yields) | [215] | |
| Fe(C5H5)2, Quartz | Coal gas | 850–950 | Coal: 50–200 | SWNTs (1–2 nm diameter) | [216] | |
|
(FePc)0.25(H2Pc)0.75 (FePc)0.06(H2Pc)0.92 (FePc)0.04(H2Pc)0.96 |
850–950 |
Ar: ≈~40 H2: ≈40 |
CNTs (high yield) SWNTs (2.4 nm diameter) |
[217] | ||
| Fe-Mo, Quartz | CH4 | 875 | CH4 : Ar (1:1 ratio) | DWNTs (in high yields) | [218] | |
| Medium temperature | Fe-Mo, Fe-Ru/Al2O3-SiO2 | CH4 | 900 | CH4: ≈6000 | SWNTs (42 wt%) | [219] |
| Fe/Al2O3 | CH4 | 900 | – | DWNTs (1–6 nm diameter) | [220] | |
| Fe, Si-substrate | C2H5OH | 900 | Ar + H2 (6%): ≈20 | SWNTs (4 cm length) | [221] | |
| Fe/Mo, SiO2/Si | CH4 | 900 |
CH4 (≈1500) H2 (125) |
SWNTs | [222] | |
|
Metallocene M(C5H5)2 (M = Fe, Co, Ni) |
C6H6 | 900 |
Ar (75%) H2 (25%) Ar + H2: ≈50 and 1000 |
MWNTs | [223] | |
| Co, Ni, Fe/MgO | H2/CH4 (4:1) | 1000 |
H2: ≈300 CH4: ≈75 |
SWNTs (70%–80%) | [224] | |
| Mg0.9Co0.1O | H2/CH4 | 1000 |
18 mol% CH4 CH4: ≈250 |
SWNTs/DWNTs: 64 wt%) (>80% 0.5–5 nm diameter) |
[225] | |
| Al/Fe/Mo, Si-substrate | C2H2 | 1000 | C2H2: ≈50–250 | SWNTs (1.3 nm diameter) | [226] | |
| Fe(CO)5 | CO | 1050 | CO (30 atm) | SWNTs (450 mg h−1) | [227] | |
|
Single or multi-Fe Co, Ni/Al2O3SiO2 |
C2H2 | 1080 |
C2H4: ≈30 N2: ≈80 |
Bundles of isolated SWNTs (0.7 or 0.2 nm diameter) |
[228] | |
| High temperature |
M(C5H5)2 (M = Fe, Co, Ni) Fe(CO)5 |
C2H2 | 1100 |
Ar: ≈975 H2: ≈25 C2H2: ≈50 |
SWNTs (diameter: 1 nm) | [138] |
| Fe(C5H5)2 | C6H6 and C4H4S | 1100–1200 | H2: ≈70–90 and 150–225 |
SWNTs (0.5–5 wt% Thiophene addition) MWNTs (higher than 5 wt%) |
[139] | |
| Mo, Quartz | CO | 1200 |
CO: ≈1200 (flow in the chamber) The CO pressure was held at ≈100 Torr for 1 h |
SWNTs (1–5 nm in diameter) |
[140] | |
4.3.2 Plasma-Enhanced CVD
PECVD is a promising technique for synthesizing CNTs in the future. In this method, the synthesis of CNTs is carried out by positioning and aligning the CNTs vertically. Vertical alignment is an important application. This method is particularly useful in field emitters, which are being considered for use in flat panel displays. The conventional wisdom in selecting plasma processing is that the precursor is separated from the highly energetic electrons, and as a result, the substrate temperature can be much lower than that of the thermal CVD. Synthesis of CNTs has been carried out by various plasma techniques such as hot filament PECVD,[138-140] microwave PECVD,[141-145] DC (light discharge) PECVD,[146-148] inductively coupled PECVD,[149, 150] and radio frequency-PECVD,[151-153] it is clear from this method that PECVD is a method of synthesizing CNTs that can be controlled vertically and parallelly so as to achieve high production yields.
4.3.3 Catalytic Pyrolysis of Hydrocarbon
This method is commonly used for mass production of CNTs via CVD. The main advantage of this method is that no purification is required to separate the CNTs from the substrate. The simplest method is to inject the nanoparticle catalyst directly into the CVD chamber. Organometallic compounds are often used as precursors for catalysts.[154-159] In the heating process, these precursors become sublimated, and when the compound is decomposed/reduced by heat or hydrogen, nanoparticle catalysts are formed in situ. In the organometallic sublimation process and the production of nanotubes at different temperatures, the sublimation process uses a double-stage furnace. In general, the sublimation of metallocenes involves little control over the structural parameters of the nanotubes, such as length and diameter. However, the diameter of the structure can be changed by varying the concentration of reactive metallocenes to carbon in the gas phase.[160] A recent advancement of the dual-stage furnace is the use of a syringe pump and atomizer to continuously feed the molten carbon metallocenes (e.g., benzene, xylene, toluene, and n-hexane) feedstock solution into the single-stage furnace where the production of nanotubes occurs.[161] In some cases, nanotubes are developed on quartz (SiO2), either in the form of a substrate or a reactor wall. The advantage of this method is that the production of CNTs is only done in one step, so that it only requires a relatively lower cost.[160-163]
5 Development of Supercapacitor Application in EVs
Supercapacitors are devices that can store electrode/electrolyte charges and can complement the functions of conventional batteries and capacitors in storing energy and conducting electricity.[164-170] In line with various studies on electricity storage, such as life cycle, charging time, and power, supercapacitors are becoming one of the promising devices in various fields that require high electricity, such as hybrid EVs, and stable energy, such as computer chips and portable electronic devices.[171] Supercapacitors store energy in two ways: capacitive behaviors: the electric double-layer capacitance obtained from the accumulation of different electrostatic charges on the electrode/electrolyte surface and the apparent capacitance due to a rapid and reversible redox process.[172] The working principle of supercapacitors is based on energy storage and distribution of ions from the electrolyte to the electrode surface area.[173] Based on their energy storage mechanism, supercapacitors are classified into three classes: EDL capacitors, pseudocapacitors, and hybrid supercapacitors, as shown in Figure 8.
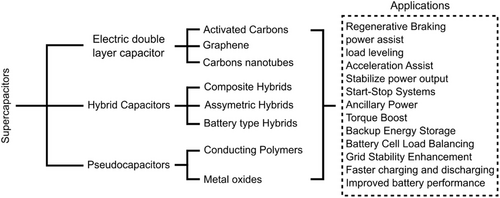
Based on the charge accumulation process, supercapacitors are divided into double-layer capacitors, hybrid capacitors, and pseudocapacitors.[174] A double-layer capacitor is a type of capacitive supercapacitor (non-Faradic) because when it is charged, there will be a combination of positive and negative charges on the electrode charge.[174] Hybrid capacitors are a type of capacitor that combines the properties of double-layer capacitors and pseudocapacitors.[174] While in a pseudocapacitor, chemical reactions occur in the active material of the electrode according to Faraday's law.[174]
In the manufacture of supercapacitors, the best combination of materials is between activated carbon and CNTs. The electrostatic charge on the activated carbon electrode/double-layer electrolyte interface results in high capacitance because activated carbon has a high specific surface area. However, the relatively low electronic conductivity of activated carbon causes the supercapacitor to have a high equivalent series resistance value and, therefore, can reduce the specific energy (E) and power (P) during charging and discharge. This shortcoming can be overcome by mixing activated carbon with CNTs, which have higher electronic conductivity, and CNTs have lower specific capacitance (Csp) values than activated carbon. A mixture of activated carbon and CNTs that undergo physical/chemical activation can increase the surface area and specific capacitance (Csp) of the electrodes.[175, 176]
EVs are gaining significant traction as a sustainable transportation solution, with their performance supported by various parameters such as the primary motor, battery, vehicle mass, wheel rolling radius, maximum speed, gradability, acceleration time, and mileage.[177] Among these, the battery is a crucial component, and its development faces several challenges, including low capacitance.[178]
To address this issue, CNT-based composites have emerged as a promising solution. These composites can provide higher energy storage capacity, enabling EVs to store more energy and extend their range.[176, 177] The unique properties of CNTs, such as their high surface area, electrical conductivity, and structural stability, make them attractive for battery applications. The integration of CNTs with other materials, such as conjugated polymers and metal nanoparticles, can further enhance the performance of these composites. The interaction between these components can lead to synergistic effects, improving charge transfer and transportation, which are crucial for efficient energy storage.[179] Moreover, composite materials in EVs offer additional benefits, such as serving as a lightweight and heat-resistant frame.[180] This not only reduces the vehicle's weight but also enhances its durability in various operating conditions.[179-181]
6 Prospective Potentials of CNTs in the Development of EVs
EVs are gaining traction as a potential solution to reduce greenhouse gas emissions and reliance on fossil fuels in the transportation sector.[174-176] The advantages of EVs include reduced emissions, less maintenance, improved energy security, higher efficiency, and minimized tailpipe emissions.[177, 178] However, there are also disadvantages that EVs have to overcome before they can completely replace internal combustion engine vehicles. Some disadvantages of EVs include limited range compared to conventional vehicles, higher initial cost, reduced availability of charging infrastructure, potential strain on the power grid, and lack of public awareness about the practicality and benefits of EVs.[177, 178] Additionally, EVs still face challenges related to the performance and cost of batteries, as well as potential environmental impacts associated with their production and disposal. As the demand for EVs continues to rise, the need for efficient and reliable energy storage systems becomes increasingly critical.
One potential solution to some of the disadvantages of EVs lies in the use of CNTs. CNTs have emerged as a promising material for enhancing energy storage in EVs. Their unique properties, such as high conductivity, large surface area, and mechanical strength, make them ideal candidates for improving the performance of battery systems in EVs.[179-181] With the presence of a metal catalyst, the synthesis method begins with the decomposition of hydrocarbon vapor so that when an interaction occurs, it will decompose into carbon and hydrogen. The carbon will dissolve in the metal catalyst, while the hydrogen will evaporate so that two types of CNT growth occur, namely tip growth and base growth.
Electric energy storage devices may be broadly characterized by two parameters. First is the energy density (how long the device can last) and power density (how much of that energy can be delivered from the device over a certain period of time).[180] By incorporating CNTs into the structure of supercapacitors, researchers have been able to significantly increase their specific capacitance and cyclic stability, enhance their energy storage capacity, and reduce manufacturing costs, thus extending the range of EVs and making them more affordable.[182-195] One of the key advantages of CNTs in EV storage is their ability to address the limited ranges of EVs. By enhancing the energy storage capacity, CNTs can enable EVs to achieve longer driving ranges, ultimately making them more practical for everyday use. In addition to improving energy density, incorporating CNTs contributes to faster charging times, addressing another significant concern in the industry. These advancements not only enhance the performance of EVs but also align with the growing emphasis on sustainability in the automotive sector. Referring to data, this review concludes the development of research trends in the field of CNT in storage applications over the past 5 years, which is shown in Table 4.
| No | Types of Trends | Description |
|---|---|---|
| 1 | Development of EVs | It can be seen that several researchers, such as Cano et al.[229] Feng et al.[230] Hannan et al.[231] Tian et al.[232] Sanguesa et al.[233] and Kouchachvili et al.[234] have undertaken research to develop EVs as a replacement for fossil fuel vehicles. |
| 2 | Application of lithium batteries as energy storage in EVs | Some researchers, including Schmuch et al.[235] Feng et al.[230] Zubi et al.[236] Manthiram,[237] and Kim et al.[238] reviewed the lithium batteries as energy stores in EVs in terms of performance, price, and working mechanisms. |
| 3 | Recycling lithium battery | Harper et al.[239] Zou et al.[240] and Chen et al.[241] highlighted the research to recycle lithium batteries. |
| 4 | Supercapacitor review and recent advancement | Muzaffar et al.[242] Poonam et al.[243] Yang et al.[244] and Simon and Gogotsi[245] reviewed supercapacitors from a material point of view. On the other side, Raza et al.[171] and Kouchachvili et al.[234] conducted advancements in supercapacitors |
| 5 | Development of larger power storage and faster recharging system | Koohi-Fayegh and Rosen,[246] Liu et al.[247] and Yamada et al.[248] have emphasized developing technologies to improve power storage and charging time. |
| 6 | Development of nanomaterials as part of supercapacitors | Yang et al.[244] showed that nanomaterials can be used to make high-voltage supercapacitors |
| 7 | Development of CNTs as part of supercapacitors | Results from Yang et al.[244] Fang et al.[249] and Zou et al.[240] proved that CNTs have a crucial role in the development of supercapacitors and batteries. |
| 8 | Fuel cells for EVs | Cano et al.[229] Dicks and Rand,[250] and Jiao et al.[251] showed the need for better fuel cells for EVs. |
| 9 | Carbon-based electrode for supercapacitor | Najib and Erdem,[252] Wang et al.[253] Kuang et al.[254] and Li et al.[255] showed research trends on the production of electrodes for supercapacitors from carbon-based materials. |
| 10 | The use of a variety of biomass in EVs | Wang et al.[256] Li et al.[257] Jin et al.[258] and Azwar et al.[34] suggested that biomass can be used as a raw material for the manufacture of electric cars. |
The potential of CNTs to revolutionize various aspects of EVs has captured the attention of researchers and industry experts worldwide. CNTs have their own properties, such as high conductivity, large surface area, and mechanical strength.[179-181] One of the most promising applications of CNTs lies in battery technology. CNTs can be incorporated into electrodes to improve their capacity, charging/discharging rates, and cycle life. This translates into EVs with extended range, faster charging times, and longer battery lifespans. Additionally, CNTs can enhance the thermal management capabilities of batteries, mitigating overheating issues and ensuring battery safety.
Beyond batteries, CNTs also hold immense potential in EV lubrication systems. CNTs can be dispersed into lubricants to reduce friction and wear, leading to improved energy efficiency, reduced maintenance costs, and extended component lifetimes. Furthermore, CNTs can enhance the heat transfer properties of lubricants, ensuring optimal lubrication performance under extreme operating conditions. Nanoadditives enhance numerous lubricant properties, including COF, antiwear (AW), thermal conductivity, viscosity, specific heat capacity, and density.[196]
In addition to batteries and lubricants, CNTs can also contribute to various ancillary systems in EVs. For instance, CNT-based composites can be employed in lightweight yet robust body structures, reducing vehicle weight and improving energy efficiency. CNTs can also be integrated into wiring harnesses and charging systems to enhance their conductivity and durability. Moreover, CNTs can be used in battery management systems to optimize performance and safety. The integration of CNTs into EVs holds tremendous promise for the future of sustainable transportation. As research and development efforts continue to advance, CNTs are poised to play an increasingly crucial role in realizing the full potential of EVs, paving the way for a cleaner, more efficient, and sustainable transportation future. These opportunities have encouraged the potential of CNTs in the development of EVs, as shown in Table 5.
| Classifications | Types of research | Year | Materials | Main result |
|---|---|---|---|---|
| Development of battery in EV | Cobalt nanoparticles shielded in N-doped CNTs for high-areal-capacity Li–S batteries[259] | 2020 | Co- nitrogen-doped carbon nanotubes (NCNTs) | A maximum areal capacity of 2.45 mAh cm−2 was achieved and retained at 1.48 mAh cm−2 after 700 cycles (corresponding to a specific capacity of 872.3 and 525.0 mAh g−1, respectively) with an average drop of 0.072%. |
| Applications of long-length carbon nanotube (L-CNT) as conductive materials in high-energy-density pouch-type lithium-ion batteries[260] | 2020 | L-CNT containing LiNi5Co2Mn3O2 (LNCM) | With only 0.16% of L-CNT inside the LNCM positive electrode, it could reach 224 Wh kg−1 and 549 Wh L−1 in weight and volume energy density, respectively. Further, this high-energy-density LIB with L-CNT offers stable cyclability, which may constitute valuable progress in portable devices and EV applications. | |
| Nano-TiNb2O7/CNTs composite anode for enhanced lithium-ion battery[197] | 2018 | TiNb2O7/CNT | For example, it delivers a large reversible capacity (346 mAh g−1 at 0.1 °C) and a prominent rate capability (still 163 mAh g−1 at the ultra-large current rate of 30 °C). Exceptional cyclic stability is also demonstrated with over 100 cycles at 10 °C with a large capacity retention of 97.6%. These results reveal that the nanoTiNb2O7/CNT composites can be a promising anode material for lithium-ion batteries of EVs. | |
|
Performance evaluation of hybrid oscillating heat pipe with carbon nanotube nanofluids for EV battery cooling[261] |
2021 | Hybrid OHP charged with ethanol–water mixture-based CNT nanofluids | CNTs vary in the range of 0.05–0.5 wt%. Experimental results show that the vertical OHP charged with CNT nanofluids exhibited better start-up and heat transfer performance than the ethanol–water mixture. At the CNT concentration of 0.2 wt%, the average evaporator temperature and thermal resistance of the OHP could be reduced to 43.1 °C and 0.066 °C W−1. | |
| CNTs-based aerogels as cathodes for lithium–oxygen cells[262] | 2024 | CNT-based aerogels | The study resulted in a huge discharge capacity of 3967 mAh g−1. | |
| CNT-wrapped and amorphous nanocarbon shell-coated LiFePO4 microclew for high-power lithium-ion batteries[263] | 2023 | LiFePO4@C@CNT) | LiFePO4@C@CNT, with a reasonable 5 wt% carbon content and superior carbon structure, exhibits the lowest Rct value of 38 Ω and the highest specific capacity of 158.2 mAh g−1 at 0.1 C among the as-prepared products due to the highest efficient charge transfer and lithium-ion diffusion. After 1000 cycles, the discharge capacity of LiFePO4@C@CNT-5 can reach 129.6 mAh g−1 at 1 °C, showing excellent high-rate capability and long cycle life for LIBs. | |
| Development of complementary part in EV | High-ampacity and high-strength of electrical power transmission wires[264] | 2024 | CNT-NiDPL/Cu | CNT/Cu composite wire with NiDPL achieved both high strength (2.54 GPa) and ampacity (1.68 × 106 A cm−2). |
| Wireless EV charging application[265] | 2018 | MWCNT-Cu | At a frequency of 15 MHz, the resistance of the individual MWCNT-Cu coil was reduced to less than 40% of its original value for primitive Cu, leading to more than a fourfold increase in their quality factor. When MWCNT-Cu coils were used as the transmitting component, the transmission efficiency of the WPT system increased from 10.57% to 95.81% at a transmission distance of 4 cm and a frequency of 3.45 MHz | |
| Battery housings for batteries in EVs[266] | 2023 | UPPH-CF50-CNT0.5 | UPPH shows electromagnetic shielding, which can be improved by incorporating CNT. | |
| Battery pack casing for EVs[267] | 2020 | CNT | CNT has proved to be the best material considering all the performance standards. Optimization results conclude that the maximum equivalent stress can be reduced from 3.9243 to 3.2363 MPa, and the six-order resonance frequency can be increased from 722.65 to 788.71 Hz. | |
| Development of lubricant for a tribological system in EV | Rheological analysis of Joannesia princeps (J. princeps) oil for sustainable biolubricant in EV application[268] | 2021 | Plain oil and with MWCNTs | Shear viscosity of J. princeps oil was experimentally analyzed. The samples are prepared with MWCNT. A 14% improvement in viscosity at a lower shear rate was observed with MWCNT. |
| CNT for increasing the thermal conductivity of polyalphaolefin (PAO) as a lubricant[269] | 2001 | Polyalphaolefin with carbon nnotube | Particularly measured the effect of adding suspended CNTs and discovered a 160% increase in the thermal conductivity of polyalphaolefin (PAO). | |
| CNT grease with high electrical conductivity[270] | 2020 | MWCNT-OH | The addition of CNTs in greases also bolsters the electrical conductivity of grease. | |
| MWCNT for improving thermal conductivity[271] | 2020 | TiO2-MWCNT/ethylene glycol (EG)–water hybrid nanofluids | The largest increase in the thermal conductivity was 34.3% at a temperature of 60 °C and a concentration of 1 vol%. | |
| TiO2/CNT as nanocomposite grease to reduce friction and wear[272] | 2020 | TiO2/CNTs | TiO2/CNTs was 3 wt%, reducing the wear and friction coefficient by about 72.3% and 60%, respectively. Furthermore, the apparent viscosity and shear stress are increased by 48.2 and 74.2%, respectively. | |
| Novel tribological behavior of hybrid MWCNTs/MLNGPs as an additive on lithium grease[273] | 2017 | Hybrid MLNGPs/MWCNTs | The optimum percentage was found to be 1%, where wear scar diameter (WSD) was reduced by 66%, the friction coefficient was reduced by 91%. | |
| Tribological behavior of CNTs as an additive on lithium grease[274] | 2015 | CNT | The results show that the grease with CNTs exhibits good performance in AW and decreases the WSD by about 63%, decreases friction reduction by about 81.5%, and increases the extreme pressure properties and load-carrying capacity about 52% with only 1% wt. of CNTs added to lithium grease. |
The application of CNTs in supercapacitors is accompanied by functionalization using a variety of materials, thereby increasing the specific capacitance and energy density of the supercapacitors. Specifically, CNT applications can be found for energy storage and thermal battery management, and the most common applications of CNTs for supercapacitors are detailed in Table 6.
| Classified | Researcher | Year | Materials | Energy density [Wh kg−1] | Specific capacitance [F g−1] |
|---|---|---|---|---|---|
| Low specific capacitance | Ryu et al.[275] | 2012 | Enzyme catalyzed in situ PPy/MWCNT | – | 46.2 |
| Yang et al.[276] | 2018 | CNTs and Ti3C2 MXene | – | 134 | |
| Mofokeng et al.[277] | 2020 | N-CNT@CF | 5.5 | 155 | |
| Lu et al.[278] | 2012 | PPy/CNT | – | 164 | |
| Park et al.[279] | 2021 | Fe3O4/CNT | – | 187.1 | |
| Wang et al.[280] | 2015 | PPy/rGO/CNT | 14.3 | 199 | |
| Medium specific capacitance | Xie et al.[281] | 2020 | N-C/CNT | – | 322.1 |
| Mothkuri et al.[282] | 2021 | FCNT-MnO2 | 49.93 | 359.54 | |
| Heo et al.[283] | 2018 | SWNTs-coated Korean traditional paper | 114 | 366 | |
| Arshad et al.[284] | 2023 | CNT–NiO fiber fabrics | 28 | 402 | |
| Sharma et al.[285] | 2021 | VS2-MX-CNT-50//MWCNT | 7303 | 505 | |
| Zhou et al.[286] | 2015 | CNT@PPy@MnO2 | 38.42 | 530 | |
| Shen et al.[287] | 2018 | Functionalizing CNTs on a Ti thin film with graphene | 26.1 | 535.7 | |
| Yousefipour et al.[288] | 2022 | MMO/CNT | 29.6 | 571 | |
| Pujari et al.[289] | 2021 | MnS2/CNT | – | 855 | |
| High specific Capacitance | Hu et al.[290] | 2021 | NiCoO2/CNT@NF | – | 929 |
| Cheng et al.[291] | 2015 | CNTs on Ni thin films | – | 1200 | |
| Zhou et al.[292] | 2016 | Self-assembled CNTs/NiCoO2 system | – | 1360 | |
| Wang et al.[293] | 2022 | CNTs/NiCoO2 system (3D) | – | 1587 | |
| Mu et al.[294] | 2017 | CNT/rGO/MnMoO4 | 59.4 | 2374.9 |
Furthermore, CNTs can also play a crucial role in improving the electrode materials of supercapacitors used in EVs. The use of CNTs in electrode materials of supercapacitors enhances their capacitance and power delivery, providing an additional avenue for improving the efficiency of EV energy storage systems. In addition to their high electrical conductivity, CNTs also offer a large surface area for the deposition of active materials, facilitating efficient charge transfer and storage within the electrode materials.[183-185]
7 Discussion
CNTs still have various shortcomings, one of which is a slow production rate and low yield, making CNTs difficult to apply as a composite in EV loading. However, various methods for the CNT production process have emerged, as described in Table 7, along with several parameters and differences between each method.
| Process | Arc discharge | Laser vaporization | CVD |
|---|---|---|---|
| Temperature [°C] | 1700 | 1200 | 700–900 |
| Yield | Moderate (70%) | High (80–85%) | High (95–99%) |
| Diameter [nm] | 4–30 | 10–20 | 20–25 |
| Purity | High | High | High |
| Production rate | Low | Low | Low |
| Cost | High | High | Low |
| Process control | Difficult | Difficult | Easy |
| Energy requirement | High | High | Moderate |
| Advantages | Simple, inexpensive, high-quality nanotubes | High purity, synthesis in room temperature production | Simple, high purity, large-scale production |
| Disadvantages | High-temperature, less purified, tangled nanotubes | Limited method to the lab scale | Defect in MWNTs |
Based on the results, CVD was found to be the best method among others. Rapid advances in CVD development have led to this method being recognized as a highly controllable technology. The main advantage of CVD is that CNTs can be used directly without purification, unless the catalyst particles need to be removed. Several CVD methods include thermal CVD, PECVD, and catalytic pyrolysis of hydrocarbons.
8 Conclusion, Challenges, and Future Aspects
In conclusion, the development of supercapacitors has been carried out using various energy-producing basic materials such as carbon, zeolites, MOFs, environmental waste, metal oxides, CPs, and composites. The ideal building materials for supercapacitor devices should be produced from simple, low-cost, and environmentally friendly synthesis methods such as biomass. In general, biomass that has a mesoporous type of pore will produce a high capacitance value. There are several biomass materials that can be used, such as CNFs, biomass, and some composites. Based on this literature review, the best composites to be used as the base material for supercapacitors are mesoporous activated carbon and CNTs because they can increase cell capacitance.
CNTs are attractive because they have high thermal conductivity, the ability to carry large electric currents, and high mechanical and thermal stability. Various CNT synthesis methods, including arc discharge, laser vaporization, and CVD, have been widely used and reviewed in this study. However, based on explanations from various literature, the authors conclude that CVD is the best method for synthesizing CNTs. Rapid advances in CVD development have made this method recognizable as a highly controlled technology. The main advantage of CVD is that CNTs can be used directly without purification unless the catalyst particles need to be removed. Several CVD methods include thermal CVD, PECVD, and catalytic pyrolysis of hydrocarbons. However, as a new type of composite material, there are some challenges to its development. First, there is a disadvantage of the thermal CVD method that only small amounts of CNTs are formed in the process.[137] Second, there is the absence of robust quality control and assessment of CNTs produced by several research groups employing diverse methodologies, which challenges the acquisition of accurate product specifications.[197]
The application of CNTs in EV storage systems can help address some of the key challenges faced by the industry. These challenges include the limited range of EVs, long charging times, and concerns about the environmental impact of current battery technologies. This advancement in energy storage technology allows EVs to achieve higher energy densities, longer driving ranges, and faster charging times.
9 Future Outlook
This work focuses on the various synthesis methods of CNTs; however, further research is required to understand the characteristics of CNTs for some applications. More experimental studies are required for the best method suitable for each composite from a type of mesoporous activated carbon and CNTs, in terms of preparation and performance outcome that will be different on each synthesis method. Conducting analytical sampling of CNTs from various sources at an accredited laboratory would provide a precise assessment of the benefits and drawbacks of different methods. These findings would then assist us in investigating the use of various combined approaches to achieve enhanced outcomes, optimized purity, and decreased expenses. The future application of CNTs can be inspired by both modeling and experimental approaches.
As future aspects advance in the field of nanomaterials, the role of CNTs in energy storage systems for EVs is expected to become increasingly significant. The unique properties of CNTs offer a pathway for the development of next-generation energy storage solutions that can address the increasing demands for high-performance and sustainable energy technologies in the automotive industry.
Acknowledgements
The authors wish to acknowledge the World Class University Program by Universitas Pendidikan Indonesia and DAPT EQUITY Program from LPDP (grant nos. 3790/E3/DT.03.08/2023 and 238/UN40/HK.07.00/2023). Open Access funding was supported by The University of Tokyo.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Apri Wiyono obtained his bachelor's degree in education (B.Ed.) from Universitas Negeri Jakarta in 2014 and his master's degree in engineering (M.Eng.) from Universitas Indonesia in 2017. He is a lecturer and researcher in the Department of Mechanical Engineering Education and Automotive at Universitas Pendidikan Indonesia. His research focuses on energy conversion, renewable energy, and waste-to-energy technologies. He holds the position of chief editor at Journal of Advanced Research in Fluid Mechanics and Thermal Sciences and also Journal of Mechanical Engineering Education. Additionally, he is an expert staff at PT. Akar Tajuk and Trustees of the Bumi Scholars Tower Foundation.

Nurin Wahidah Mohd Zulkifli is an associate professor and head of mechanical engineering at the University of Malaya. She holds a mechanical engineering degree and Ph.D. from the University of Malaya and a master's from Monash University. Her expertise in combustion and fuel engineering is demonstrated by 112 research articles, 3868 citations, and an H-index of 38. In 2020, she was recognized among the top 2% of engineering researchers. She collaborates with international researchers and organizations like PROTON and PETRONAS while managing various grant projects.

Muhammad Aziz is currently an associate professor and principal investigator at the Institute of Industrial Science, The University of Tokyo, Tokyo, Japan. He received B. Eng., M. Eng., and D. Eng. from Kyushu University, Japan, in 2004, 2006, and 2008, respectively, in the field of mechanical engineering. His general research areas are advanced energy conversion systems, mainly focusing on mutual energy conversion, power generation, renewable energy utilization, process modeling, smart grid, electric vehicles, battery, and hydrogen production, storage, and utilization.




