Surface Amalgam on Magnesium Electrode: Protective Coating or Not?
Abstract
A better control of the metal–electrolyte interface is mandatory to help the development of rechargeable magnesium batteries. Protecting the magnesium surface by a coating layer to avoid its passivation with conventional liquid electrolytes is a promising strategy. Herein, in contact with a mercury droplet, a crystalline amalgam layer is created on the surface of magnesium. After the creation of this surface coating, a remarkable improvement of the plating/stripping process is observed with magnesium bis(trifluoromethanesulfonyl)imide/dimethoxyethane electrolyte, whereas strong surface passivation hindering electrochemical cycling occurs when using bare magnesium. The detailed investigation of the evolution of the amalgam layer during cycling, however, shows substantial chemical and morphological electrochemical changes, eventually showing that such a layer does not act as a protecting coating, but rather as an independent electrode material deposited on an inert Mg substrate.
1 Introduction
In the search for performing post-lithium rechargeable batteries, the use of metallic magnesium negative electrodes and of double-charged magnesium ions as electrochemical shuttle species is a promising strategy. Magnesium is abundant on the Earth, safe to use, and exhibits a quite low redox potential that could eventually lead to high-energy-density systems if coupled with a suitable positive electrode material. However, in contact with conventional nonaqueous liquid electrolytes, magnesium is prone to surface passivation, blocking the transfer of Mg2+ ions. As a result, unlike lithium, the passivation layer significantly impedes the reversible plating/stripping process of magnesium.[1-4] To counter this major drawback, two pathways have been proposed to develop better magnesium batteries: searching for innovative electrolytes compatible with magnesium electrode[5, 6] or protecting the surface of magnesium to enable the use of conventional electrolytes.
In 2017, the groups of Nazar and Archer proposed to protect lithium or sodium electrodes through the surface chemical reduction of metal salts in solution, followed by an alloying reaction between the alkali and the as-deposited metals.[7, 8] The obtained surface layer is actually a composite film, made of alloy(s) and alkali salts. The electrochemical behavior of the protected electrodes is significantly improved, with especially less dendritic growth.[9] Although dendrites could be also formed with magnesium electrodes, this protocol was afterward adapted to magnesium electrodes mainly to address the incompatibility with particular electrolytes. The reduction of the metal salt could be performed prior to battery assembly by immersing the magnesium electrode in a metal salt solution, but also directly in the battery if the salt is added to the liquid electrolyte.[10-13]
Recently, the use of liquid gallium was proposed to protect the surface of magnesium electrodes.[14, 15] Taking advantage of the low melting point of gallium, it is possible to spread droplets on the surface of the magnesium electrodes. The subsequent chemical reaction leads to the formation of a top layer mainly composed of crystalline Mg2Ga5, and enhanced cycling behavior was achieved with these as-protected electrodes in magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2)/dimethoxyethane (DME).
In a similar spirit, we report here an alloying surface reaction of magnesium electrode with mercury. Obviously the toxicity of mercury precludes from any possible practical application. However, it seems important to determine if other alloy-type coatings can be obtained on the surface of magnesium electrode, positively influencing the electrochemical behavior. Recently, the group of He and Ding applied an amalgam process on the surface of lithium, sodium, and potassium electrodes.[16-18] The direct reaction of mercury droplets on lithium foil induces the formation of a micrometric layer composed of LiHg3 and LiHg amalgams. Similarly on potassium foils, an amalgam layer of K7Hg31 and K2Hg7 is obtained. The protocol is adapted in the case of sodium electrode to avoid a too strong exothermic reaction, with sodium being dissolved in mercury to prepare the amalgam and the latter being afterward spread onto the sodium foil. In all cases, the as-protected electrodes exhibit an improved electrochemical behavior, especially with less-polarized and longlasting plating/stripping tests in symmetrical cells. However, the delithiation of the amalgam after long stripping questions the chemical stability of the layer and consequently its protective role. Indeed, it is believed that a coating should remain as much as possible upon electrochemical cycling. If the chemical nature evolves during cycling, such as change from Li3Hg to LiHg,[16] the layer acts as an electrode material itself. This is what we propose to investigate in the case of magnesium in this work.
2 Results and Discussion
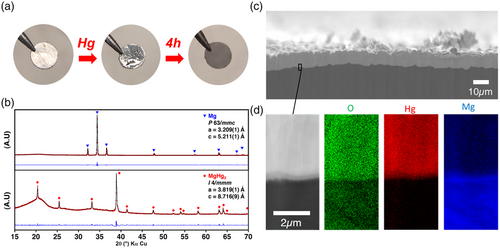
Controlling the amount of mercury poured onto the magnesium disc was not straightforward, especially due to its particularly important surface tension. However, by adding and/or subsequently wiping out some mercury, the mass increase could be tuned experimentally from 19% to 97%. About 4 to 10 h later, depending on the amount of mercury, the initial metal shine turns dull gray. Characterizations of the electrodes are gathered in Figure 1. XRD patterns are significantly modified after the amalgam process, with the absence of the diffraction peaks of Mg metal. Instead, the new set of peaks can be indexed with the MgHg2 amalgam (I4/mmm space group, a = 3.838 Å and c = 8.799 Å, after profile matching refinement). The cross-section SEM image shows a compact layer formed on the surface of the magnesium substrate. Starting from the surface, a first layer with a thickness of about 10 μm can be distinguished. A closer look at the interface reveals the presence of a sublayer measuring about 500 nm. No cracks or porosities are visible in both layers. SAM images show the distribution of mercury and magnesium on both sides of the interface, demonstrating that mercury reacts only with the top surface of the magnesium substrate, without diffusing deeper into the bulk. Overall, a first and thick layer can be attributed to MgHg2 while a thin sublayer at the interface between Mg and MgHg2 can potentially be assigned to Mg-richer amalgam[20] (Mg–Hg phase diagram provided in Figure S1, Supporting Information). Considering the initial dimensions of the magnesium electrode (250 μm, 7 mm diameter), and assuming a layer only made of dense MgHg2, the observed experimental mass increases correspond to layer thicknesses ranging from 8 to 40 μm, in fairly good agreement with the SEM image. The amount of consumed magnesium during the amalgam process is limited (Figure S2, Supporting Information).
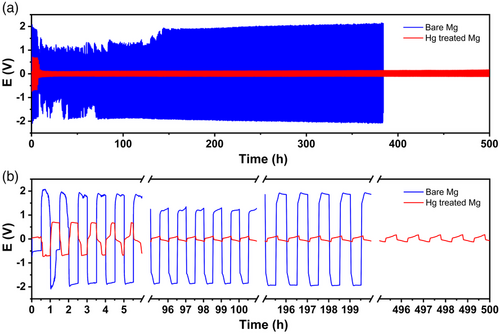
We first verified the possibility to plate and strip magnesium ions on and off the Hg-treated magnesium through cyclic voltammetry with magnesium metal as counter electrode. As magnesium is a relatively hard metal, it cannot be easily flattened on a current collector like lithium or sodium metals. Therefore, it appears important to apply the amalgam process on both sides of the magnesium electrode to avoid any contact between the electrolyte and bare magnesium. Unambiguously, the anodic and cathodic peaks could be assigned to magnesium ions’ plating and stripping (Figure S3, Supporting Information). However the strong surface passivation precludes the long-term use of magnesium metal. Consequently, polarization tests in symmetric cells were carried out. Figure 2 compares the plating/stripping process performed at a current density of 100 μA cm−2 for bare and treated magnesium electrodes. As expected, a large overpotential up to 2 V is immediately observed with bare magnesium electrodes. On the contrary, an impressive reduced (0.1 V) and stable profile is obtained when Hg-treated electrodes are considered. It is worth noting that higher values around 0.7 V are reached during the first cycles of plating/stripping. This activation sequences is difficult to analyze but could result from a change of morphology of the amalgam layer or a modification of the crystal structure.

This alloying reaction is reversible as the proportion of MgHg decreases after stripping.
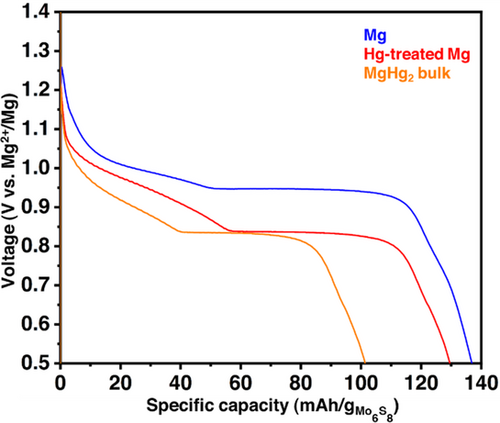
The last results indicate that the amalgam layer formed on the surface of the magnesium electrode does not really act as a protective coating as expected initially, but more as an independent mercury alloy electrode on top of a magnesium metal substrate. To finally state, full cells with Mo6S8 as positive electrode materials were assembled with either bare magnesium, Hg-treated magnesium, and MgHg2 self-standing electrode. As shown in Figure 4, besides the general shape of the profile in agreement with past reports using Mo6S8 electrode, the voltage of the cell containing the Hg-treated Mg electrode is virtually the same of that containing the MgHg2 bulk electrode and is sensibly lower, by more than 0.1 V, than the cell with the Mg metal anode. Consequently, it is reasonable to assume that the electrochemical reaction during the discharge at the MgHg2 bulk electrode, that is, a dealloying process releasing magnesium ions, also occurs at the surface of the Hg-treated magnesium electrode, without participation of the underlying Mg substrate to the electrochemical reaction.
The initial discharge with the Hg-treated magnesium electrode was then followed by a charge which clearly shows the characteristic plateaus of the Chevrel-phase Mo6S8, and then successive cycling was achieved (Figure S4, Supporting Information).
3 Conclusion
Taking inspiration from previous works on the protection of alkali metal electrodes after a surface amalgam process, a mercury-based coating containing mainly crystalline MgHg2 was successfully obtained after spreading a drop of mercury metal at the surface of magnesium electrode. Despite promising results in polarization test in symmetric cells, advanced characterizations after cycling revealed that MgHg2 amalgam turns into magnesium-richer amalgams during plating, without interacting with the underlying Mg substrate. Consequently, the layer cannot be considered as an efficient coating for magnesium electrode. However, the relatively easy process to create this layer should encourage the investigation of other alloy-based coatings. Recent attempts with gallium-based coating showed a tremendous enhancement of the electrochemical behavior, and other alloy elements, such as tin, lead, indium,[21] should be similarly investigated.
4 Experimental Section
Unless stated, all the operations were carried out in an argon-filled glovebox. Hg-treated electrodes were prepared by cutting discs (7 mm diameter) from a magnesium foil (Goodfellow, 99.9%, 250 μm) prior being scratched with a blade to remove the native oxide layer as much as possible. Then, drops of mercury were spread onto the surface of those magnesium electrode discs until it formed a homogeneous liquid layer.
X-ray diffraction (XRD) was performed with a Panalytical X’Pert Pro diffractometer operating with Cu Kα radiation. Samples were prepared in a glovebox under a protective airtight polymeric film to limit moisture reaction.
Cross-section scanning electron microscopy (SEM) and scanning Auger electron microscopy (SAM) images were obtained with Auger electron nano probe JAMP 9500 F (Jeol Ltd.). For this analysis, Hg-treated magnesium discs were mechanically cut and then polished using an Ar+-ion beam in a cross-section polisher IB-0901CP (Jeol Ltd.) operating at 4 keV.
MgHg2 powder was prepared by mixing magnesium powder and mercury drops in stoichiometric proportions in agate mortar. Chevrel-phase Mo6S8 powder was obtained through a well-established protocol.[19] For both MgHg2 and Mo6S8, XRD was performed to validate the preparation and then self-standing electrodes were obtained by mixing the active material with conductive carbon black powder (Timcal Super C65) and polytetrafluoroethylene binder (PTFE, Sigma-Aldrich, 40 μm particle size) in 70:20:10 weight proportions.
Mg(TFSI)2/DME electrolyte (0.5 mol L−1) was prepared by dissolving magnesium bis(trifluoromethanesulfonyl)imide salt (Solvionic, 99.5%, dried one night at 250 °C under dynamic vacuum prior use) in dimethoxyethane (Sigma-Aldrich, 99.5%, treated with molecular sieves prior to use). Coin cells (2032, 316L stainless steel) and one glass-fiber (Whatman, GF/A) surrounded by two polypropylene membranes (Celgard 2325) were used for the electrochemical tests.
Acknowledgements
The authors gratefully acknowledge financial support from the French National Research Agency (project MISTRALE, ANR-19-CE05-0013, Labex STORE-EX, ANR-10-LABX-76-01).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




