High-Entropy Materials for Water Electrolysis
Abstract
Green hydrogen production by renewables-powered water electrolysis holds the key to energy sustainability and a carbon-neutral future. The sluggish kinetics of water-splitting reactions, namely, hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), however, remains a bottleneck to the water electrolysis technology. High-entropy materials, due to their compositional flexibility, structural stability, and synergy between various elemental components, have recently aroused considerable interest in catalyzing the water-splitting reactions. Herein, a timely review of the recent achievements is provided in high-entropy materials for water electrolysis. An overview of different kinds of high-entropy materials for catalyzing the HER and OER half-reactions is introduced, followed by a discussion of theoretical and experimental efforts in understanding the fundamental origins of the enhanced catalytic performance observed on high-entropy catalysts. Various materials design strategies, including control of size and shape, construction of a porous structure, engineering of defect, and formation of hybrid/composite structure, to develop high-entropy catalysts with improved catalytic performance are highlighted. Finally, the remaining challenges are pointed out and the corresponding perspectives to address these challenges are put forward to promote the development of the research field of high-entropy water-splitting catalysts.
1 Introduction
Humanity demands clean energy to build a better life, support social progress, and achieve sustainable development. The continued use of traditional fossil fuels produces large quantities of carbon dioxide that has raised worldwide concerns about environmental issues, such as climate change. To address this, hydrogen (H2), as a cleaner alternative, has attracted renewed interest worldwide to be incorporated in the energy mix.[1] Green hydrogen, which is produced by water electrolysis powered by electricity generated from renewable energy sources (e.g., solar, wind, and hydro), has become the game changer.[2, 3] Currently, nearly 90% of H2 is produced by steam methane reforming and coal gasification, processes that utilize fossil fuels as the main energy supply and are therefore not sustainable.[4] In comparison, water electrolysis has the advantage of producing high-quality H2 with markedly reduced carbon footprints. However, to be economically competitive, the cost of green hydrogen production needs to be reduced. One major limiting factor for cost reduction of the water electrolysis technology is the sluggish reaction kinetics for the hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode.[5-7] These half-reactions require considerable overpotentials (η) to occur at appreciable reaction rates, accounting for large energy losses during the overall water electrolysis. This efficiency gap is currently mitigated by the use of electrocatalysts, with the best performance achieved on noble metal-based ones (e.g., those containing platinum, iridium, and ruthenium) which are both scarce and costly.[8-10] To bring down the cost, numerous research efforts have been devoted to the development of alternative catalyst candidates with no- or low-content noble metals, such as metals and alloys, intermetallic compounds, (oxy)hydroxides, oxides, halides, carbides, nitrides, sulfides, phosphides, and metal–organic frameworks (MOFs).
In this work, we provide a timely review of the recent developments in high-entropy materials for water electrolysis (Figure 1). We begin by an overview of different kinds of high-entropy materials for catalyzing the HER and OER half-reactions, such as HEAs, (oxy)hydroxides, oxides, and other types of materials candidates. We then summarize recent efforts in understanding the fundamental origins of the enhanced catalytic performance observed on high-entropy catalysts, including both theoretical and experimental results. We also highlight materials design strategies to develop high-entropy catalysts with improved water electrolysis performance, spanning endeavors from controlling size and shape, constructing porous structures, and engineering defects, to forming hybrid/composite structures. Finally, we present the challenges in the area of high-entropy water-splitting catalysts and put forward perspectives that may assist the development of this research field.
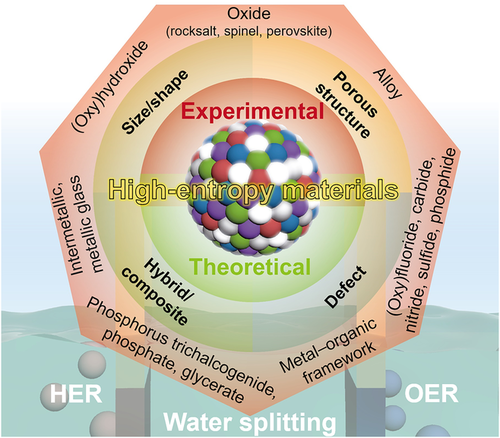
2 Overview of High-Entropy Materials for Water Electrolysis
2.1 High-Entropy Alloys
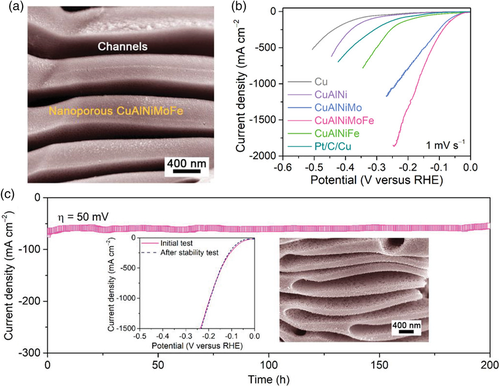
HEAs are the very first types of high-entropy materials and also the most widely investigated for the electrochemical water-splitting application.[19-25] Because of the high-entropy effect caused by the lattice distortion and slow diffusion as the number of components increases, HEAs possess distinctive characteristics such as extremely high mechanical strength and corrosion resistance under severe conditions, which are highly suited for electrocatalytic processes.[26] During the electrocatalysis, alloying can significantly modify the adsorption energy of reaction intermediates on the catalyst surface, thereby improving its catalytic performance. Using a facile alloying/dealloying method, Jin and co-workers uniformly blended Ir (≈20 at%) with another four different metals to form a quinary AlNiCoIrMo HEA with an ultrafine nanoligament size of ≈2 nm.[21] Thanks to the possible high-entropy effect, the AlNiCoIrMo HEA displayed outstanding performance toward the electrocatalytic OER and HER in acidic electrolytes as well as excellent structural and catalytic durability. Using a similar alloying/dealloying approach, Yao et al. fabricated a monolithic nanoporous CuAlNiMoFe multielemental alloy electrode consisting of CuNiMoFe HEA in situ formed at the surface of the body-centered cubic CuAlNiMoFe precipitates and seamlessly anchored on the hierarchical Cu skeleton (Figure 2a).[27] The HEA comprising dissimilar Cu, Ni, Mo, and Fe atoms served as bifunctional sites to facilitate water dissociation and promote adsorption/desorption of hydrogen intermediate (H*), whereas the hierarchical nanoporous Cu skeleton worked as a scaffold for fast ion/molecule transport and electron transfer. As a consequence, the monolithic nanoporous CuAlNiMoFe electrode exhibited excellent HER electrocatalysis, reaching current densities of 100 and 1840 mA cm−2 at overpotential of 183 mV in the neutral solution of 1 M phosphate buffer saline and 240 mV in the alkaline solution of 1 M KOH, respectively (Figure 2b). A robust durability was also observed during a 200 h continuous test (Figure 2c).
2.2 High-Entropy Metal (oxy)hydroxides
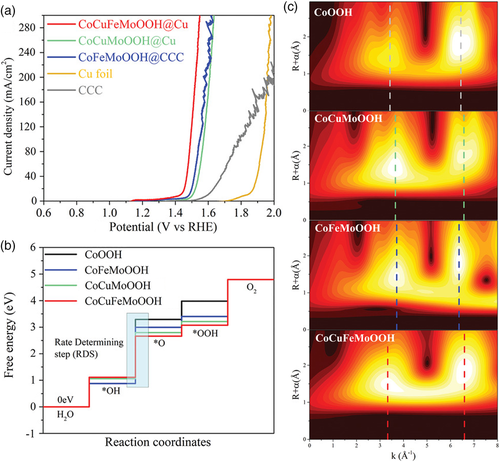
Hydroxides and oxyhydroxides are promising candidates for catalyzing the water electrolysis reactions, in particular, the anodic OER.[28, 29] Constructing high-entropy (oxy)hydroxides is expected to open up new opportunities for efficient water-splitting catalysis.[30-32] Gu et al. first introduced the concept of high entropy into layered double hydroxides (LDHs) and obtained a series of ultrathin high-entropy LDHs through a hydrothermal method followed by plasma post-treatment.[30] The high-entropy LDHs were composed of five near-equimolar metal cations having a similar ionic radius (e.g., Fe3+, Cr3+, Al3+, Co2+, Ni2+, Cu2+, and Zn2+) and thus minimized lattice mismatch. Benefiting from the synergistic effect of multicomponents, high-entropy LDH with components of Fe–Cr–Co–Ni–Cu showed good OER activity in 1 M KOH with an overpotential of only 330 mV at 10 mA cm−2 (η10). Zhang et al. prepared quaternary CoCuFeMoOOH oxyhydroxide supported on Cu foil through a combined hydrothermal and electrochemical treatment method.[31] Notably, CoCuFeMoOOH presented a superb OER activity with a low overpotential of 199 mV to reach 10 mA cm−2, much smaller than those of CoFeMoOOH (253 mV) and CoCuMoOOH (273 mV) (Figure 3a). Density functional theory (DFT) calculations revealed that the quaternary system exhibited a lower theoretical overpotential for the rate-determining step compared with the ternary systems (Figure 3b). Experimentation from wavelet transform analysis of X-ray absorption spectra implies the severe structural disorder with the increasing number of doping elements in oxyhydroxide (Figure 3c). These results suggest the usefulness of high-entropy design in enhancing the OER performance of (oxy)hydroxides.
2.3 High-Entropy Metal Oxides
Metal oxides, such as rocksalt oxide (AO),[33] spinel oxide (AB2O4),[34] and perovskite oxide (ABO3),[35, 36] are another large group of functional materials for water electrolysis. One major advantage of these oxides lies in their compositional versatility, which allows the substitution of a large number of elements in the periodic table of elements, thereby making the construction of high-entropy oxides highly viable. Very recently, there has been growing interest in designing high-entropy rocksalt,[37, 38] spinel,[39-42] or perovskite[43, 44] oxides as water-splitting electrocatalysts. For instance, Liu and co-workers synthesized a rocksalt-type high-entropy oxide Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O by an electrospinning method and achieved enhanced OER performance compared with unary metal oxides.[37] In another study, spinel-type high-entropy oxide (Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)3O4 was fabricated as a mesoporous thin film via a wet chemical soft-templating method.[42] Compared with a dense thin film, the mesostructured (Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)3O4 thin film displayed much improved OER activity, which is attributed to the larger number of catalytically active sites and the better gas exchange and electron transfer at the electrode/electrolyte interface facilitated by mesoporosity.
Nguyen et al. prepared a series of perovskite-type high-entropy oxides using a simple coprecipitation approach and studied the effect of elemental composition on the OER performance.[43] The B-site lattices consist of five consecutive first-row transition metals, including Cr, Mn, Fe, Co, and Ni, which have quite similar ionic radii. Both equimolar and nonequimolar (where the concentration of one of the five transition metals is doubled) high-entropy perovskite oxides outperformed the single-B-site counterparts, and the optimized La(CrMnFeCo2Ni)O3 high-entropy oxides displayed an excellent OER activity (η10 = 325 mV in 1 M KOH) and good electrochemical stability. More recently, Sun and colleagues studied in more detail the fundamental relationship between configurational entropy and reaction mechanism and activity of perovskite oxide OER catalysts (Figure 4).[44] They found that configurational entropy is closely correlated with the intercation charge transfer, creation of oxygen hole states around the Fermi level (EF), and formation of surface oxygen vacancies, which contributed to mechanism transformation to a more favorable lattice oxygen mechanism (LOM) compared with the conventional adsorbate evolution mechanism (AEM). The best OER activity (η10 = 320 mV in 1 M KOH) was observed on a high-entropy perovskite oxide (La0.6Sr0.4Co0.2Fe0.2Mn0.2Ni0.2Mg0.2O3) with optimum configurational entropy. These works open new dimensions for the development of high-entropy oxide catalysts for highly efficient water electrolysis.

2.4 Other Types of High-Entropy Materials
Following the flourishing development of HEAs and high-entropy oxides, the concept of high-entropy stabilization has been further extended to a wide variety of material systems in the context of water electrolysis, including intermetallics,[45]fluorides,[46, 47] oxyfluorides,[48, 49] carbides,[50] nitrides,[51] sulfides,[52-54] phosphides,[55, 56] phosphorus trichalcogenides,[57] phosphates,[58] glycerates,[59] and MOFs.[60, 61]
Intermetallic compounds represent a unique family of metals that feature long-range atomic ordering between sublattices but random mixing within each sublattice.[62] Jia et al. developed a novel alloy design strategy to manufacture a nonprecious metal-based high-entropy intermetallic compound (FeCoNiAlTi) (Figure 5a).[45] By leveraging both the chemical synergistic and structural site-isolation effects of the dissimilar metal atoms, the electronic structure of FeCoNiAlTi was controllably tuned to achieve highly efficient HER catalysis in the alkaline solution.
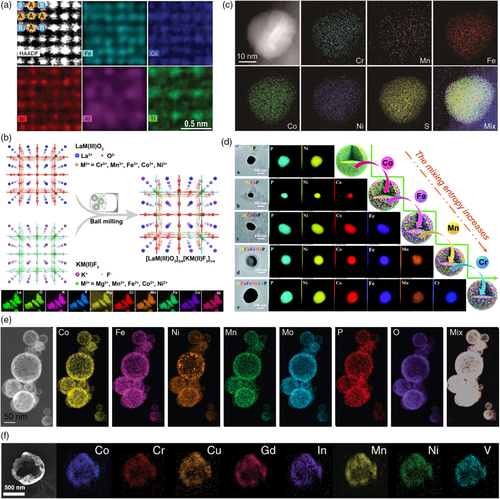
High-entropy fluorides of both rutile-type (AF2) and perovskite-type (ABF3) structures have been reported for the alkaline OER.[46, 47] Different divalent metal ions, such as Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, and Zn2+, can be incorporated and highly dispersed into the fluoride lattice to form a solid solution. A rutile-type high-entropy fluoride incorporating all these seven cations, (CuNiFeCoZnMnMg)F2, showed promising OER activity in 1 M KOH (η10 = 292 mV), outperforming a noble-metal IrO2 benchmark (η10 = 340 mV).[47] For perovskite fluorides, the OER performance can be further optimized by cation substitution at the A site, as exemplified by the K1–xNax(MgMnFeCoNi)F3 compounds.[46] Blending high-entropy perovskite fluoride [K(MgMnFeCoNi)F3 denoted as KM(II)F3] with high-entropy perovskite oxide [La(CrMnFeCoNi)O3 denoted as LaM(III)O3] at an appropriate molar ratio of 3:1 could further enhance the configurational entropy of the system to form a high-entropy perovskite-type oxyfluoride, [LaM(III)O3]3/4[KM(II)F3]1/4 (Figure 5b), which demonstrated better OER activity and stability than IrO2.[49]
Transition metal-based nonoxide inorganic compounds such as carbides, nitrides, sulfides, and phosphides are inherently good HER catalysts. Their corresponding high-entropy counterparts have recently demonstrated even improved HER performance.[50, 51, 54-56] For example, the HER activity of transition metal phosphides increases with the number of metal elements present, in the order of NiP < NiCoP < NiCoFeP < NiCoFeMnP < high-entropy NiCoFeMnCrP (Figure 5d),[55] which highlights the great efficacy of the synergistic high-entropy effect. Decent catalytic performance toward the OER was also observed on high-entropy sulfide catalysts. Using a pulse thermal decomposition approach, Cui et al. overcame the immiscibility of multiple metallic constituents in sulfide materials and obtained a quinary (CrMnFeCoNi)Sx high-entropy sulfide (Figure 5c).[52] Computational results revealed a migration in the charge state among multiple metallic atoms, which could modify the adsorption energy to optimize the OER catalysis, leading to an exceptional OER activity (low overpotential of 295 mV at 100 mA cm−2 in 1 M KOH) superior to the unary, binary, ternary, and quaternary sulfide counterparts. More complex transition metal compounds such as phosphates and glycerates can also be developed into the high-entropy form to deliver unprecedented OER catalysis.[58, 59] Qiao et al. proposed a high-temperature fly-through process to fabricate high-entropy phosphate CoFeNiMnMoPi (Figure 5e).[58] The homogenous mixing of multimetallic elements contributed to a high OER activity in 1 M KOH (η10 = 270 mV).
MOFs have received considerable interest in electrochemical water splitting due to their stable architecture, periodic porous structure, and abundant metal active sites.[64-66] Taking advantage of high-entropy materials and MOFs, constructing high-entropy MOFs (HE-MOFs) will likely afford new possibilities and intriguing properties in water electrolysis.[60, 61] By adopting a solution-phase method, Mu et al. successfully prepared anHE-MOF in which five different metal species (Co2+, Ni2+, Cu2+, Mn2+, and Fe3+) with near-equimolar distribution were randomly dispersed and coordinated with 1,4-benzendicarboxylic acid.[60] Due to the strong synergistic effect, the as-synthesized HE-MOF exhibited excellent electrocatalytic performance toward the OER (η10 = 245 mV in 1 M KOH). In another study, the same metal species were coordinated with organic ligands of 2,5-thiophenedicarboxylic acid and 4,4-bipyridine under solvothermal conditions to form an HE-MOF.[61] The introduction of multimetal ions caused a partial lattice distortion of the resultant HE-MOF, which gave rise to the formation of an unsaturated coordination environment for the metal active centers, leading to improved OER activity relative to the pristine MOF with a single-metal site.
In addition to the crystalline structure, the high-entropy concept has also seen applications in amorphous materials.[63, 67-73] Metallic glasses, one type of alloys with a disordered, amorphous microstructure, can provide tunable catalytic performance based on the individual properties of incorporated metals. Designing high-entropy metallic glasses may offer access to electrocatalytic properties arising from the dissimilar metal interactions.[63, 67-69] Glasscott et al. reported a generalized nanodroplet-mediated electrodeposition method to prepare high-entropy metal glasses containing up to 8 equimolar components with precisely controlled metal stoichiometry (Figure 5f).[63] A quinary CoFeLaNiPt high-entropy metal glass was demonstrated with good bifunctional activity for both the OER and HER, implying the potential use in water electrolysis. High-entropy amorphous alloys and oxides are also good candidates for the electrochemical water splitting.[70-73] High-entropy amorphous FeCoNiCrVB alloy was subject to surface reconstruction by means of cyclic voltammetry (CV) to cause selective leaching of metal elements, formation of oxyhydroxide active species, and introduction of core–shell nanoparticles, which facilitated mass/electron transfer at the catalyst interface and promoted the OER catalysis.[70] A similar electrochemical activation-induced surface reconstruction was also observed on a high-entropy amorphous FeCoNiMnBOx oxide, which formed a stable oxyhydroxide surface layer that contributed greatly to the OER performance.[72]
3 Fundamental Understanding of Active Sites in High-Entropy Materials
For the rational design of improved water-splitting electrocatalysts, the identification and tailoring of active sites are of utmost importance.[28] However, for a catalyst system as complex as the high-entropy materials, understanding the real active sites can manifest as a formidable challenge. Recently, there have been continuous efforts from theoretical, experimental, or both, to shed some light on this fundamental issue.
3.1 Theoretical Understanding
Using a low-temperature oil-phase synthesis approach, Li et al. prepared Pt18Ni26Fe15Co14Cu27 HEA with uniform and ultrasmall nanoparticles, which displayed excellent activity and stability toward the HER in alkaline media (Figure 6a–c).[74] A series of DFT calculations were then thoroughly performed to comprehend the alkaline HER process on the HEA catalyst. As illustrated by the site-dependent partial projected density of states (PDOSs) shown in Figure 6d, each compositional element in the HEA catalyst contributes quite differently to the electrocatalytic HER process. The surface Pt shows an obvious upshift toward the EF, which promotes electron transfer for the HEA surfaces. From the bulk to the surface, Fe sites demonstrate a site-independent electronic structure; Co sites show an alleviation of the eg–t2g splitting effect, indicating an increased electron transfer efficiency; Ni sites display a relatively stable d-band center to maintain the electroactive electron boosting center; and Cu sites closer to the surface exhibit a slight upshift trend of the 3d orbitals to support the electroactivity of the HEA. The synergy between these multiactive sites is therefore considered responsible for the high HER performance. DFT calculations for the energetic reaction pathways further suggest that the synergistic effect of various metal sites could promote the site-to-site electron transfer as well as the stabilization of the HER intermediates, thus leading to highly efficient HER kinetics (Figure 6e–g).
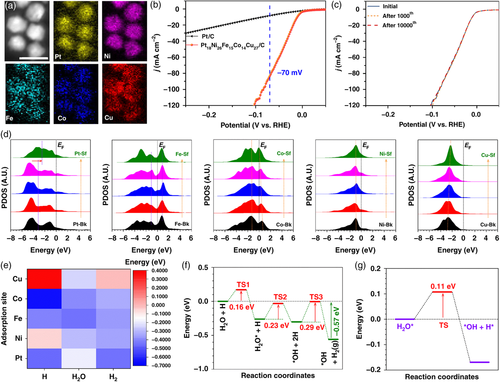
3.2 Experimental Understanding
Operando or in situ characterizations can reveal the catalyst information in real time during the water electrolysis and is therefore instrumental to understanding the actual active species of high-entropy catalysts. Raman spectroscopy is one of the most easily accessible tools for this purpose, which has enabled the observation of the structural evolution of high-entropy catalysts during the oxidative OER process, revealing the oxidized surface species as the real OER-active component.[53, 73, 75-77] For example, hydroxyl (OH) and superoxo (OO) intermediates were formed on the surface of a FeCoNiIrRu HEA during the acidic OER catalysis.[77] In an amorphous high-entropy NiFeCoMnAl oxide, the Ni sites were revealed to be the active sites, which evolved into β-NiOOH intermediates during the alkaline OER process.[73] In situ Raman spectra suggested the transformation of a FeNiCoCrMnS2 high-entropy sulfide into an oxyhydroxide, which was further corroborated by ex situ studies using transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS).[53] These results can have strong implications for the design of high-entropy materials as OER catalysts.
3.3 Combined theoretical and experimental understanding
Combining operando/in situ characterizations with theoretical calculations may provide a better understanding of the fundamental origins of the catalysis on high-entropy electrocatalysts. Feng et al. reported a carbon-supported ultrasmall NiCoFePtRh HEA (us-HEA) with superior HER activity and stability in an acidic solution. Operando X-ray absorption near-edge structure (XANES) measurements suggest that the Pt and Rh components are the main and direct active sites toward the acidic HER, whereas the Fe/Co/Ni sites play a role of modifying the electronic structures of Pt/Rh sites and enhancing the entropy of the us-HEA to improve the catalytic stability (Figure 7a–f).[78] At a potential of 0.05 V versus the reversible hydrogen electrode (RHE) where no HER takes place, the Rh site experienced a considerable shift in the absorption edge to a lower energy position, implying the gaining of electrons concomitant with the adsorption of hydrogen ions on the Rh site. In comparison, little change was found on the absorption edges of the other four metal sites. This could be explained by the fact that hydrogen has a stronger adsorption binding strength at the face-centered cubic sites with Rh nearby (Rh site) than those with only Fe/Co/Ni nearby (Fe/Co/Ni site) and with Pt nearby (Pt site) on the us-HEA (111) surface (Figure 7g). With the application of cathodic HER biases (−0.01 and −0.05 V vs. RHE), the XANES spectra of Fe, Co, and Ni remained unchanged, whereas the Rh XANES edge continued to shift slightly to lower energy, indicating that the number of hydrogen ions adsorbed on the Rh sites increases and that HER occurs directly on the Rh sites. Of note, the white line intensity of the Pt XANES edge declined dramatically with the initiation of the HER (at −0.01 V vs. RHE), which suggests that Pt sites are the main active sites for HER at this stage. DFT simulations were further performed to calculate the hydrogen adsorption free energy (ΔGH*) as a general descriptor for assessing the HER activity. The Pt site exhibited the smallest absolute ΔGH* (−0.004 eV) compared with the Rh and Fe/Co/Ni sites on the us-HEA (111) surface, which was also lower than that of pure Pt (−0.070 eV) (Figure 7h). The modified electronic structure of Pt in the us-HEA enables the Pt site to achieve an optimum Pt–H binding, which could favor the adsorption of hydrogen ions and release of H2 gas, eventually accelerating the HER kinetics.
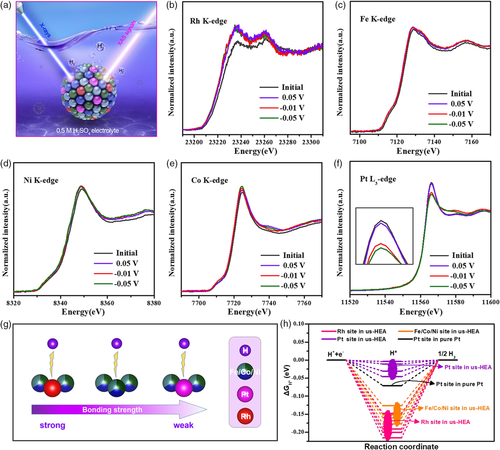
4 Rational Design of Improved High-Entropy Catalysts
In general, the electrocatalytic activity of a high-entropy material can be improved by taking into consideration the geometric and electronic effects, which aim to increase the number of the active sites available and accessible and to enhance the intrinsic activity of such active sites, respectively. In this section, strategies for designing high-entropy materials with improved water electrolysis performance are overviewed, including control over size and shape, construction of the porous structure, defect engineering, and formation of hybrid/composite structures.
4.1 Control Over Size and Shape
The conventional high-entropy materials, often formed through thermal approaches, usually have a bulk size with a small surface-area-to-volume ratio,[79] limiting their utility in electrochemical catalysis. Exploring novel synthesis strategies to translate these bulk materials into nanoparticles is of great importance to improve catalytic efficiency. Hu's group developed a high-temperature carbothermal shock approach to synthesize HEA nanoparticles with a wide range of compositional elements.[18] The particle size could be facilely controlled by adjusting the synthesis parameters including substrate, shock temperature and duration, and heating/cooling rate. Lu et al. proposed a rapid laser-pulsed irradiation method to assist the formation of tiny and uniform senary FeCoNiCuPtIr HEA nanocatalysts within just 1 ms.[80] By tuning the laser irradiation mode and laser impact duration, the particle size could be reduced to the sub-10 nm range. The FeCoNiCuPtIr sample processed in a rapid and pulsed manner was found to show the smallest size and best catalytic activities toward both the OER and HER. Despite the effectiveness and productiveness, these rapid methods often require specialized and sophisticated synthetic facilities that are not easily accessible to general researchers, implying the need for the development of more simplified synthesis protocols. Some conventional methodologies can be leveraged to achieve this purpose. For instance, a chemical coreduction route was reported to prepare ultrasmall NiCoFePtRh HEA nanoparticles with sizes down to the sub-2 nm range.[78] Using a solvothermal method followed by annealing, Wang's group obtained a high-entropy (CoCuFeMnNi)3O4 spinel oxide with one of the smallest nanometer sizes (≈5 nm) achieved thus far for high-entropy oxides, which exhibited promising activity in the alkaline OER catalysis.[40] The use of liquid-phase precursors was the key to attaining the high dispersion of five transition metal cations at the molecular level.
Apart from size reduction, designing special nanostructural shapes and morphologies is also critical to increase the number of active sites and thus improve the water electrolysis performance. Until now, high-entropy materials with nanosheet,[57, 60] nanowire,[81] nanoarray,[54] and nanosponge-like[69] morphologies have been reported. For instance, Song and colleagues successfully prepared a 2D nanosheet-structured high-entropy metal phosphorus trichalcogenide Co0.6(VMnNiZn)0.4PS3 with a thickness of about 3–4 atomic layers (≈2.8 nm) (Figure 8).[57] The 2D structure affords a large specific surface area and facilitates the exposure of the diversified metal edge sites, resulting in significantly enhanced HER activity in a 1 M KOH aqueous solution (η10 = 65.9 mV). In another example, a simple surface-dealloying process was applied to a PdPtCuNiP high-entropy metallic glass to create a nanosponge-like surface morphology, concomitant with the formation of nanopores, enlargement of surface area, and removal of nonfunctional Cu/Ni elements.[69] Such a surface nanostructure offered abundant active sites to deliver exceptional HER activity in both acidic and alkaline media (η10 = 62 and 32 mV in 0.5 M H2SO4 and 1 M KOH, respectively).
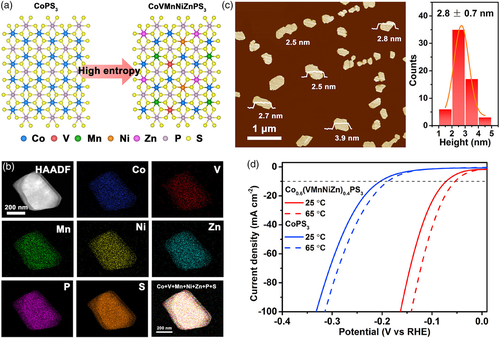
4.2 Construction of Porous Structure
The construction of porous structure, in particular, a hierarchical one, can not only increase the exposure and accessibility of active sites but also facilitate the mass transfer and ion diffusion, and is therefore highly desired for electrochemical water splitting. To date, although porous architectures have been reported for many high-entropy materials,[21, 82, 83] those featuring multiple length scales are still rarely seen. Li and co-authors recently developed a dealloying process to obtain a porous quaternary FeCoNiCu HEA with hierarchical porosity, which was made possible through selectively dissolving the FeCoNiCu HEA in a mildly acidic HNO3 solution (Figure 9).[84] During the dealloying process, the dendritic phase with a smaller Cu content was preferentially dissolved and the interdendritic phase with a larger Cu content was relatively stable and retained as the skeleton of the microscale porous structure. Meanwhile, a 3D secondary nanoporous structure with mesopore size was formed inside the dendritic-like skeleton, due likely to the leaching of the FeCoNi-enriched nanophase in the spinodal-modulated structure. Importantly, the formation of the unique hierarchically porous architecture could be manipulated by controlling the dealloying time. The dealloyed HEA with a hierarchical porosity displayed ultrahigh HER activity in 1 M KOH with a small η10 of 42.2 mV and a low Tafel slope of 31.7 mV dec−1, outperforming the commercial Pt/C benchmark. This work represents a paradigm change for developing efficient high-entropy electrocatalysts via properly designing a porous structure.

4.3 Defect Engineering
Defects play an essential role in improving the water electrolysis performance of high-entropy materials. Lattice defects such as lattice deformation (e.g., dislocations and stacking faults) could lead to an increase in the free-surface energy to bring down the activation energy, thus accelerating the electrochemical reaction kinetics.[85] For instance, a cathodic plasma electrolysis deposition method was reported to synthesize a 3D porous FeCoNiMnCu HEA with a dendrite structure self-assembled by nanoparticles that have highly deformed lattices, which contributed to high OER performance in the alkaline solution.[86] In high-entropy materials, lattice defects may also originate from the lattice strain due to the mismatch of different metal cations.[37] These defect sites could regulate the electronic structure of the active sites and/or maximize the exposure of the active sites, simultaneously facilitating the electrocatalytic water splitting.
Vacancies, including cation vacancies and anion vacancies, represent other types of defects beneficial to water-splitting catalysis. For example, plasma treatment has been reported as an effective way to introduce oxygen vacancies in high-entropy LDHs[30] and high-entropy rocksalt oxides[87] for improved OER and HER catalysis, respectively. Gu et al. applied a lithium manipulation strategy to incorporate the reductive Li into the lattice of Fe–Co–Ni–Cu–Zn high-entropy rocksalt oxide (Figure 10a,b).[38] The oxygen vacancy content was found to increase with the Li amount, with a maximum at a Li amount of 16.7% (i.e., equimolar for the six elements) (Figure 10c,d). Interestingly, the HER and OER catalytic activities trended well with the oxygen vacancy amount (Figure 10e,f), highlighting the importance of regulating anion deficiencies in designing highly active water-splitting electrocatalysts.
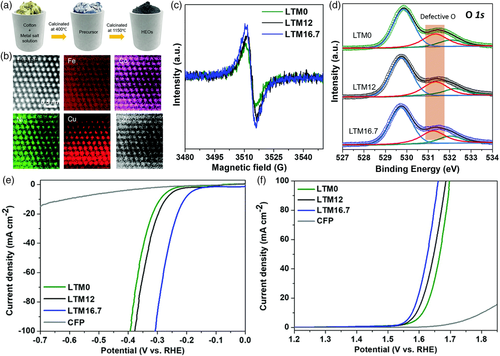
4.4 Formation of Hybrid/Composite Structure
Building multiphase hybrid or composite structures based on high-entropy materials can take advantage of the strong interaction between different material phases to bring improved electrocatalytic performance compared with the single-phase counterparts. For example, the dispersion of Pt clusters on a (AlCoFeMoCr)3O4 high-entropy spinel oxide substrate was found to promote the OER catalysis due to a strong interaction between Pt and (AlCoFeMoCr)3O4.[88] In addition, introducing Ag as an electron donor to a Co–Cu–Fe–Ag–Mo high-entropy (oxy)hydroxide could enhance the metal–oxygen hybridization and consequently lead to considerably improved OER performance.[32] Post-treatment of high-entropy materials represents one most straightforward way to fabricate composite structures. For example, phosphorization was performed under a hydrothermal condition to grow CoNi phosphates on a CoCrFeNiMo HEA, which significantly boosted the OER activity.[89] Acid etching was used to convert the superficial species of an amorphous FeCoNiPB HEA into a crystalline (FeCoNi)3O4−x high-entropy oxide.[90] The combination and strong interaction of the two high-entropy phases gave rise to notable electrochemical performance for both the OER and HER (η10 = 229 and 163 mV, respectively). In some rare cases, the presence of microdomains in the high-entropy material itself could bring new electrochemical phenomenon and possibly improved electrocatalysis. For instance, the formation of a Cr2O3 oxide microdomain in a CrFeCoNi HEA could modify the local coordination environment of the HEA matrix as well as promote the charge transfer and lead to the interfacial reconstruction and amorphization upon the leaching of Cr ion during the OER catalysis, eventually improving the catalytic activity.[91]
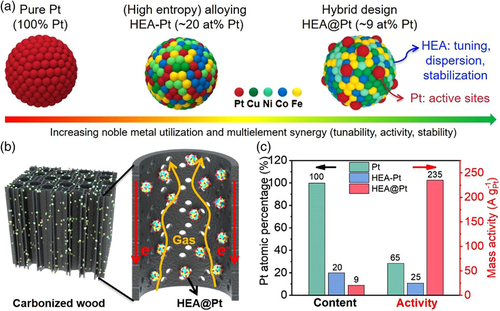
Constructing high-entropy-based hybrid structures is extremely useful for the better utilization of noble metal-containing catalysts. Yao and colleagues reported a hybrid catalyst design in which a small amount of noble metal Pt clusters (0.35 wt%) was uniformly dispersed and stabilized on the non-noble HEA (FeCoNiCu) core nanoparticles (HEA@Pt) (Figure 11a).[92] A rapid high-temperature shock approach was adopted to prepare the HEA nanoparticles loaded onto a hierarchically porous carbonized wood substrate (which allows for fast electron conduction, electrolyte permeation, and gas product release) (Figure 11b), followed by galvanic displacement to realize the anchoring and exposure of Pt on the HEA core. Remarkably, the HEA@Pt hybrid exhibited an excellent HER mass activity of 235 A g−1Pt at −0.05 V versus RHE, 3.6-fold and 9.4-fold greater than that of the pure Pt (65 A g−1Pt) and the homogeneous FeCoNiCuPt HEA (HEA-Pt) (25 A g−1Pt) (Figure 11c). The good catalytic performance is attributed to the high-entropy stabilization effect and the core–shell interaction of the hybrid catalyst. Such a design strategy was also successfully extended to the development of a low-Ir, HEA-based hybrid OER catalyst, FeCoNiCrMn@Ir, which underscores the universality of the HEA-based hybrid design strategy.
5 Outlook and Perspectives
In this review, we have summarized the recent progress achieved for high-entropy materials in water electrolysis, focusing on an overview of materials candidates, fundamental insights into the catalytically active sites, and strategies for developing improved catalysts. Table 1 and 2 provide a summary of some of the best-performing high-entropy materials for the HER and OER catalysis, which highlights the great promise of high-entropy materials for use as highly active and stable water-splitting electrocatalysts. Despite these achievements, it should be noted that the research for high-entropy water-splitting electrocatalysts is still at its early stage and that grand challenges and enormous opportunities coexist. To promote the next stage of research and applications, we further put forward some perspectives as follows. 1) Caution should be exercised in designing high-entropy materials. High-entropy materials can often bring better electrocatalytic performance due to the synergistic interplay of different elements. This is extremely useful in developing low-noble-metal-based water-splitting catalysts because the use of noble metals can be significantly reduced in high-entropy materials, as exemplified by the Pt18Ni26Fe15Co14Cu27 HEA with an ultralow overpotential for the alkaline HER (11 mV at 10 mA cm−2 in 1 M KOH).[74] However, it should be noted that high-entropy materials do not necessarily offer improved water-splitting performance compared with lower-entropy counterparts. For example, a high-entropy metallic glass containing equimolar metal elements of Co, Fe, La, Ni, and Pt was found with an alkaline HER activity much lower than that of a Pt disk electrode (555 mV vs. 201 mV at 10 mA cm−2 in 0.1 M KOH).[63] Such a discrepancy in the alkaline HER activities of the two Pt-based high-entropy materials suggests that simply blending multiple elements in one material system to make a so-called high-entropy material will not necessarily guarantee high electrochemical performance and that the nature of the high-entropy materials as well as the specific interaction between the different elements should be considered during the catalyst design. Realizing this fact is important for decision-making at the initial stage of research because the research into high-entropy materials can be expensive given the many precursors and facilities required for synthesis, characterization, and testing. 2) Determining whether the material of interest is indeed a high-entropy one is critical. Although there are plenty of reports about high-entropy materials and the number of such reports continues to grow, it remains controversial if the many “so-called” high-entropy materials are really entropy stabilized or “just” simply showing high configurational entropy.[47] To facilitate the development of this new and emerging research area, we highly encourage better practices to be performed in the reporting of high-entropy materials for water electrolysis. In particular, from the perspective of materials science, the determination of the high-entropy nature of the materials developed should be considered an essence of the research and supported by solid and thorough experimental or theoretical proof. 3) Identifying the real active sites of a high-entropy water-splitting catalyst remains a formidable challenge and needs to be addressed. The vast compositional space inherent in the high-entropy materials means a bright future for tunable, designer water electrolysis catalysts. Currently, HEAs with more than ten constituent elements are being developed.[93, 94] One undeniable fact is that complexity also comes with the increase in the number of elements involved, not alone the intertwined interaction between these elements, whether catalytically active or inert. Currently, the effect of each component of the high-entropy materials on the overall catalytic activity remains poorly understood.[95] Such an understanding becomes even more difficult to obtain under realistic water-splitting conditions, which, however, is significantly important to improve the catalyst design. Operando and in situ studies are highly suggested to facilitate the understanding of the catalytic sites of high-entropy materials. 4) Continued efforts should be devoted to the research of high-entropy water-splitting catalysts from many different aspects. To fully leverage the high-entropy concept, new materials with expanded compositional space should be explored for water electrolysis purposes. New synthesis methods should be developed in a controlled manner to obtain high-entropy materials with targeted atomic arrangements and surface compositions that may serve as model catalysts for fundamental understanding.[96] Also importantly, high-throughput computational techniques should be exploited to aid the screening and design of potential high-entropy catalysts and the understanding of their catalytic behaviors.[97-100] All these endeavors should be combined toward an improved understanding of the structure–property relationships of high-entropy materials for water electrolysis. We expect to see more fascinating achievements in the development of high-entropy materials for water electrolysis and also other energy conversion and storage applications.
| Catalyst | Electrolyte | Substrate | Loading [mg cm−2] | η 10 [mV] | Tafel slope [mV dec−1] | Stability | References |
|---|---|---|---|---|---|---|---|
| AlNiCoIrMo | 0.5 M H2SO4 | GCEa) | 0.0243 (Ir) | 250 | 33.2 | / | [21] |
| CoCrFeNiAl | 0.5 M H2SO4 | / | 0.3 | 73 | 39.7 | 12 h @ 10 mA cm−2 | [23] |
| PdFeCoNiCu | 1 M KOH | GCE | 0.142 | 18 | 39 | 15 days @ 10 mA cm−2 | [24] |
| FeCoNiCu | 1 M KOH | / | / | 42.2 | 31.7 | 120 h @ 500 mA cm−2 | [84] |
| CuAlNiMoFe | 1 M KOH | Nanoporous Cu | / | 56 @ 100 mA cm−2 | 60 | 200 h @ 60 mA cm−2 | [27] |
| FeCoNiAlTi intermetallic | 1 M KOH | / | / | 88.2 | 40.1 | 40 h @ 20 mA cm−2 | [45] |
| FeCoNiCuPtIr | 1 M KOH | CNPb) | / | 21 | 54.5 | / | [80] |
| PdPtCuNiP metallic glass | 1 M KOH | / | / | 32 | 37.4 | 100 h @ 20 mA cm−2 | [69] |
| V2Sn0.6(FeCoNi)0.6C | 6 M KOH | Self-supported | / | 184 @ 20 mA cm−2 | 37.9 | / | [50] |
| FeCoNiCuMnN | 1 M KOH | CCc) | / | 184 | 113 | 50 h @ 23 mA cm−2 | [51] |
| CoZnCdCuMnS | 1 M KOH | CC | 6.7 | 173 | 93.4 | 70 h @ 10 mA cm−2 | [54] |
| NiCoFeMnCrP | 1 M KOH | GCE | / | 220 | 94.5 | 24 h @ 10 mA cm−2 | [55] |
| Co0.6(VMnNiZn)0.4PS3 | 1 M KOH | CC | 0.175 | 65.9 | 65.5 | 12 h @ 10 mA cm−2 | [57] |
| (LiFeCoNiCuZn)O | 1 M KOH | CPd) | 2 | 207 | 79.4 | 1000 CV cycles | [38] |
| FeNiCoMnVOx | 1 M KOH | NFe) | 0.24 | 81 | 88 | 100 h @ 10 mA cm−2 | [87] |
- a) GCE: Glassy carbon electrode;
- b) CNP: Carbon nanotube paper;
- c) CC: Carbon cloth;
- d) CP: Carbon paper;
- e) NF: Nickel foam.
| Catalyst | Electrolyte | Substrate | Loading [mg cm−2] | η 10 [mV] | Tafel slope [mV dec−1] | Stability | References |
|---|---|---|---|---|---|---|---|
| AlNiCoIrMo | 0.5 M H2SO4 | GCE | 0.0243 (Ir) | 233 | 55.2 | 7000 CV cycles | [21] |
| AlCrCuFeNi | 1 M KOH | / | / | 270 | 77.5 | 35 h | [82] |
| FeCoNiCrVB | 1 M KOH | / | / | 237 | 24.2 | N.A. | [70] |
| Co–Fe–Ga–Ni–Zn | 1 M KOH | GCE | 0.981 | 370 | 71 | 10 h @ 1.5 V vs. RHE | [25] |
| CoCuFeMoOOH | 1 M KOH | Cu foil | 1 | 199 | 48.8 | 72 h @ 10, 20, and 50 mA cm−2 | [31] |
| Ag@CoCuFeAgMoOOH | 1 M KOH | Cu foil | 1 | 218 | 35.3 | 50 h @ 10 mA cm−2 | [32] |
| (FeCoNiCrMn)3O4 | 1 M KOH | CP | 1.1 | 288 | 60 | 95 h @ 10 mA cm−2 | [39] |
| (CoCuFeMnNi)3O4 | 1 M KOH | GCE | 0.204 | 350 | 59.5 | 12 h @ ≈15 mA cm−2 | [40] |
| La(CrMnFeCo2Ni)O3 | 1 M KOH | NF | ≈1.18 | 325 | 51.2 | 50 h @ 10 mA cm−2 | [43] |
| K0.8Na0.2(MgMnFeCoNi)F3 | 1 M KOH | GCE | 0.7 | 314 | 55 | 10 h @ 10 mA cm−2 | [46] |
| FeNiCoCrMnS2 | 1 M KOH | NF | 2 | 199 | 39.1 | 55 h @ 500 mA cm−2 | [53] |
| CoFeNiMnMoPi | 1 M KOH | GCE | 0.07 | 270 | 74 | 500 CV cycles | [58] |
| FeNiCoCrMn glycerate | 1 M KOH | NF | 2.5 | 229 | 40 | 36 h @ 10 mA cm−2 | [59] |
| MnFeCoNiCu MOF | 1 M KOH | GCE | 0.21 | 245 | 54 | 48 h @ 10 mA cm−2 | [60] |
| FeCoNiPB amorphous oxide | 1 M KOH | GCE | 0.2 | 235 | 53 | 40 h @ 10 mA cm−2 | [71] |
Acknowledgements
This work was supported by the Australian Research Council (ARC) Discovery Projects (grant nos. DP180100731 and DP180100568).
Open access publishing facilitated by Curtin University, as part of the Wiley - Curtin University agreement via the Council of Australian University Librarians.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Xiaomin Xu obtained his Ph.D. in chemical engineering from Curtin University, Australia. He is currently a research associate at Curtin University. His research interests are mainly focused on the development of functional materials for applications in electrochemical energy storage and conversion.

Zongping Shao is a John Curtin Distinguished Professor at Curtin University, Australia. He obtained his Ph.D. from the Dalian Institute of Chemical Physics, China, in 2000. He worked as a visiting scholar at Institut de Researches Sur La Catalyse, CNRS, France, and then a postdoctoral fellow at California Institute of Technology, USA, from 2000 to 2005. His current research interests include fuel cells, lithium-ion batteries, electrocatalysis, metal–air batteries, solar cells, and oxygen-permeable membranes.

San Ping Jiang is a professor at Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory, China. He obtained his Ph.D. from The City University, London, in 1988. Before 2010, he worked at Nanyang Technological University in Singapore. Since 2010, he is John Curtin Distinguished Professor at the WA School of Mines: Minerals, Energy and Chemical Engineering and Deputy Director of Fuels and Energy Technology Institute, Curtin University, Australia. His research interests encompass fuel cells, water electrolysis, supercapacitors, carbon dioxide reduction, single-atom catalysts, and nanostructured functional materials.




