Nanotechnology Research and Development in Upstream Oil and Gas
Abstract
Research in nanomaterials has brought many performance enhancements to the materials used in oil and gas well drilling, cementing, production, and enhanced oil recovery (EOR). While the products of these investigations and product development processes vary widely in terms of technological readiness, improvements in performance with nanoparticles are being witnessed in the field at this current time. Step-change innovation in the industry through advances in nanomaterials is anticipated to find a strong footing in the development of smart self-sensing cements, production technologies, EOR technologies, nanoparticle sensors, sensor networks, and downhole power and automation. Herein, a brief overview of the applications of nanoparticles as they relate to upstream oil and gas is provided and where the current state of the art is in high-performance oil well construction, production enhancement, and reservoir management materials is evaluated.
1 Introduction
Nanoparticles have been shown to provide many improvements in material performances when included in existing material systems used in the construction and production of oil and gas wells.1 Research in petroleum well drilling, design, stimulation, and reservoir production management has demonstrated that nanoparticles can make industrial materials tougher,2 well completion and production processes more efficient,3 and provide the possibility for real-time sensing of a well's structural health4 and productivity. Nanoparticles have found potential applications in the industry due to a variety of different favorable physical properties they impart into materials through their high surface area-to-volume ratios,5 high surface activities,6 high aspect ratios (in the case of nanotubes),7 enhanced surface-dependent properties such as thermal conductivities,8 electrical conductivities,9 and the ability of some nanoparticles (such as sub-20 nm iron oxides) to have superparamagnetic properties.10 Nanoparticles, as those used in enhanced oil recovery (EOR), have been observed to display a structural disjoining pressure effect.11 This is recognized as a dominant mechanism in surface spreading by nanoparticles in chemical EOR applications.12 The structural disjoining pressure effect is believed to be responsible for higher efficiencies in oil displacement in oil wet reservoir rocks.13
The materials used to drill, build, and produce oil wells are required to be chemically resilient, temperature and pressure tolerant, and durable for years on end. Globally, the average age of an on-shore oil well ranges from 20 to 30 years.14 In some high-capacity on-shore wells, production can last for 50 years or more. With growing energy demands, more challenging environments are going to be encountered when petroleum wells are drilled to greater depths, leading to higher pressures, higher temperatures, and more complex protruding formations with the potential for more corrosive gases such as CO2 and hydrogen sulfide (H2S).15 Hence, materials in upstream oil and gas will be engineered to increasingly higher standards to meet these demanding well conditions. The high performance promised by nanomaterials has in many instances met these objectives. Step-change disruptions to the industry with nanotechnology are a distinct possibility and the appropriate advancements in nanomaterials for subsurface power and energy storage,16 sensors,17 and structural materials6 are being actively pursued by many research groups.
The drilling domain has as its primary drivers safety, efficiency, and speed. Wells drilled with fluids containing nanoparticles such as nanosilica offer the possibility of an improved rate of penetration (ROP) through difficult water-reactive rock formations, such as shales.18 Nano-zinc oxides which have been shown to outperform standard H2S scavengers can be used in drilling to eliminate potentially deadly H2S emissions from certain treacherous rock formations, helping to insure the safety of drilling personnel.19
Cements in well construction are also witnessing numerous advances in the implementation of nanoparticle designs. Nanoscale magnesium oxide expansive agents mitigate pervasive volumetric shrinkage issues in Portland cements (PCs) which threaten the long-term stability of the well.6, 20 Nanotubes21 and nanosilicas22 have been shown to reinforce cements with superior tolerance to excessive loads, corrosion resistance, and higher strength under simulated well conditions of high temperatures and pressures. With the foundations for improved strain tolerances and piezo-sensitivity in cements laid out, further engineering of these materials can lead to more durable wells, with lower risks of zonal isolation breaches and cements which can provide information to an operator when structural stresses within the well are excessive and problematic.
Production technology and reservoir management are anticipated to benefit greatly from advances in nanochemistry. Product performance achievements have been shown in hydraulic fracturing, where, for example, viscoelastic surfactant-based fracturing fluids display greatly improved leak-off and a higher thermal stability upon the use of ZnO nanoparticles.23 Free-flowing powders of emulsified acids stabilized with hydrophobic nanosilicas present the possibility of improved high-temperature acid fracturing performance. The control of materials at the nanoscale allows the engineer to design materials with molecular-scale stimulus–responsive behavior. For example, these encapsulated acid emulsions can be triggered with surfactant to release acid at the demand of the operator.5
EOR methods involving nanoparticles have shown promise for effects in providing a higher superior emulsion stability24 and for their propensity to displace hydrocarbons embedded within porous media.13 Multimetallic nanocatalysts have also shown the possibility of catalytic EOR for heavy oils and bitumens.25 If these technologies come to fruition, it is anticipated that the environment, production, and transportation costs of oil sands could be greatly reduced.26
The implementation of advanced nanotechnologies in oil and gas is expected to result in higher efficiencies and lower costs, leading to a reduction in the carbon footprint of oil extraction and higher safety standards. The objectives of this article are to 1) describe oil and gas applications of nanotechnology, 2) capture the most recent advances in this topic, and 3) highlight new and emerging areas of nanoparticle research that can contribute to the development of new oil and gas technologies. The application areas discussed herein are classified into four domains: drilling, cementing, production, and EOR. In what follows, we will give an overview of these applications and the nanomaterials (Figure 1) that are being investigated and developed to improve material performances.
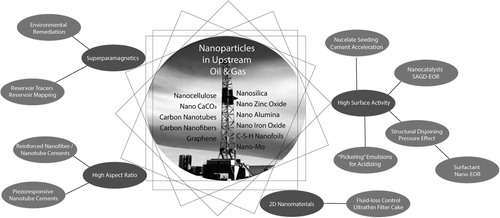
2 Nanomaterials in Drilling Fluids
Nanoparticles have been reported to bring significantly enhanced performance to drilling fluids and help address current challenges.27 The large surface area-to-volume ratio results in superior or even unexpected performances for surface-dependent nanomaterials. The utilization of various nanoparticles as a rheological modifiers and fluid-loss reducers in water-based muds (WBMs), has been studied for several years. Superior rheological properties have been obtained using very low concentrations of nanoparticles (<1.0 wt%). The nanometric dimensions of nanomaterials have also been shown to be of use in plugging pore throats in shale with greater efficacy than microparticles, leading to a significant reduction in fluid invasion as well as thinner filter cakes.28
During drilling operations, drilling fluids must have the rheological properties required to carry cuttings from beneath the drill bit up the annulus of the well for separation at the well surface. In addition, the fluids provide cooling for the drill bit and reduce friction between the drill string and the formation being drilled. At the same time, the fluids should maintain these properties while stabilizing the wellbore without causing irreversible damage to the rock formation.29 This is especially important when drilling reservoir sections, where formation damage can change the permeability which can reduce the ROP and directly impact oil and gas production efficiency.30
The beneficial use of nanoparticles in drilling fluids (typically referred to as muds or drilling muds) has been demonstrated in all types of drilling fluid systems. The basic mud systems (in the absence of nanoparticles) have been developed over the years to include WBMs, oil-based muds (OBMs), and synthetic-based muds (SBMs).29 OBMs are typically diesel based, whereas SBMs can be based on a variety of different but more environmentally friendly fluids (including glycol, glycerins, and glucosides).29 These different drilling fluid systems have different cost benefits and material advantages which are taken into consideration when the drilling engineer selects the fluid for a given section. For example, WBMs are often favored mud systems when drilling through aquifer sections of wells to minimize the environmental impact of the drilling process. In the following sections, we highlight some of the improvements realized with the use of nanoparticles in the design of novel drilling fluids (Figure 2).
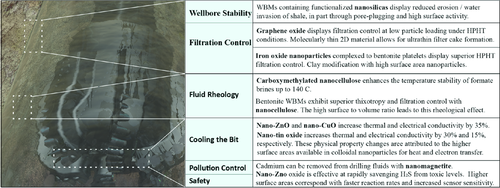
2.1 Wellbore Stability
When drilling through shale-producing formations, water invasion into the shale can weaken the wellbore and lead to difficulties in drilling, such as stuck pipe and hole collapse. WBM formulations that include nanosilicas have been shown to offer improved shale inhibition over some of the commercially available non-nanoparticle-based shale inhibitors. Because the extremely low permeability of shales (nano-Darcy) does not allow the fluid to permeate the formation as more permeable rock formation does, conventional filter-cake-forming technologies are not options in drilling through shales with WBMs.
Formation mineralogy is important when it comes to the design of drilling fluids for use with shales. Shale is a sedimentary rock composed of silicates and aluminosilicates such as quartz, clay minerals, carbonates, and other minerals such as pyrite. The clay content in shales makes them reactive to water-based drilling fluids. Different varieties of clays react differently to water. For example, a shale with a high smectite content will typically have a higher reactivity to water than a shale with a higher illite content.31
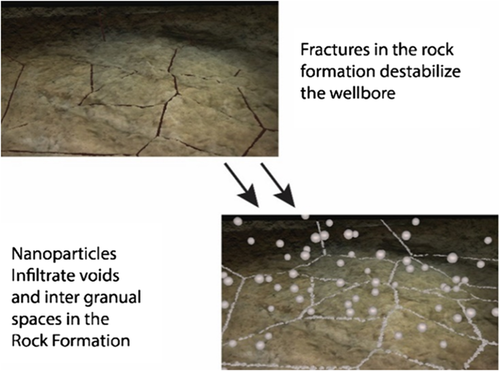
To address some of the issues in drilling through water-sensitive shale formations, nanosized particles can be designed to penetrate deeper into the formation and plug the shale pores to stop water invasion (Figure 3). High concentrations (from 5 to 50 wt%) of nanosilicas show plugging of shale core samples with a composition of between 17% and 20% smectite.32 These high concentrations of nanosilicas limit the economic viability of these products in the field, so a more active kind of nanosilica has been sought and applied for use in drilling operations. At concentrations below 3 wt%, nanosilicas functionalized with alkylamines show improved inhibition activity of shale (with 28% smectite/illite mixed layers) in WBMs. When allowed to interact synergistically with polymers, such as polyanionic cellulose, these nanosilicas show even greater efficacy, inhibiting clays in shales from reacting when in sea water-based drilling fluids.33 These nanoparticle systems also benefit from milder, less caustic fluid requirements over silicate muds, well known to inhibit shales. While these silicate muds require a pH in excess of 12, nanosilica drilling fluids can be adjusted below 10.18
Wellbore strengthening is a critical component sought in drilling through all formations. A strong wellbore is as important for oil or gas wells constructed through shales as it is for limestones and sandstones. Wellbore strengthening during drilling operations has also been achieved by the use of ferric hydroxide nanoparticles. A study by Nwaoji et al. utilized these nanoparticles as a loss circulation material (LCM) that resulted in a stronger wellbore and minimized fluid losses.34 The ferric hydroxide nanoparticle formulations were exposed to sandstone core samples. Cores display ultimate compressive strength values 70% higher than unexposed cores (comparing 16.5 and 27.5 MPa).
2.2 Filtration Control of Drilling Fluids with Nanoparticles
Rock formations which have higher permeability than typical shales, such as sandstones and limestones, require additives to prevent filtration of the drilling fluid into the formation. Since drilling fluids are typically weighted such that there is a positive overbalance between the drilling fluid and the formation's pore pressure, a filter cake at the rock–fluid interface is built to prevent fluid loss from the drilling mud. It is important that this filter cake (also known as the “mud cake”) is as impermeable to fluid loss from the drilling fluid as possible and that it builds this essential impermeability quickly. It is also critical for subsequent cementing operations that the mud cake be thin to ease in the removal of the mud cake after drilling operations. The well is drilled in intervals. After an interval is completed, a spacer fluid is pushed through the annulus of the well between the casing and formation. This fluid is flushed through the well to displace the drilling mud, clean the annulus of drilling mud, and remove the mud cake. A thick mud cake left on the formation after spacer treatment and after the placement of the cement can interfere with cement bonding to the rock formation. This is a potential leak pathway for formation fluids along the length of the well and a source of mechanical weakness and debonding of the cement from the formation.
Some nanomaterials have shown synergies with clays, such as bentonite, to not only reduce the filtration of fluid from the drilling mud into the formation but also enhance the rheological properties of the fluid. Ferric oxide nanoparticles in bentonite muds show promising results in this regard. Ferric oxide nanoparticles are believed to intercalate the bentonite clay platelets displacing the complexed ions (calcium and/or sodium) at high temperatures ultimately with the effect of increasing filtration control. A loading of 0.5 wt% of nano-Fe2O3 provides a 22% reduction in filter-cake thickness and a 17% decrease in filtrate volume where the test was performed at 120 °C.35 The performance of Fe2O3 nanoparticles from 3 to 30 nm has been tested to show the enhanced benefit of filtration control in drilling fluids at temperatures up to 200 °C, showing a 28% decrease in filtrate volume compared with the control without iron nanoparticles.36
WBMs including xanthan and graphene oxide (GO) have also shown value for filtration control in sandstone core samples. Comparing WBMs containing GO and commercially available drilling fluids, the filter cake formed from the standard American Petroleum Institute (API) test for fluid-loss control is approximately an order of magnitude thinner in the case of WBMs with GO. The amount of filtrate collected during the test is also slightly lower than with the commercially available additive.28
2.3 Rheological Optimization with Nanoparticles
Another drilling domain that shows promise from the use of nanoparticles is the design of drilling fluids for harsh environments where temperatures above 120 °C impact drilling fluid shear response due to thermal degradation of the viscosifiers in the fluid. Oxidation-resistant materials, polymers, and nanomaterials are of substantial value when drilling fluids are used with high brine concentrations. High brine concentrations are used in drilling fluids and completion fluids to densify the fluids in place of solid particles, such as barite. Since barite particulates can irreversibly plug pore throats and reduce the permeability of rock formations, these additives are often avoided in reservoir sections/intervals of the well. The implementation of soluble high-density salts (such as calcium chloride, sodium formate, sodium bromide, and zinc bromide) is often recommended when the effect of formation damage is important. Cuttings from the drilling process need to be carried, suspended, and transported by these fluids, and so yield points in excess of 1 Pa are often sought and achieved with conventional additives such as guar gum, xanthan, and crosslinked starch. Oxidative damage to these additives, however, becomes a significant problem in temperatures in excess of 120 °C in brines.
Cellulose nanofibers (CNFs) have shown potential as rheology modifiers in brines. Heggset et al. have demonstrated that functionalization of nanocellulose through controlled oxidation with 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) increases the temperature tolerance of WBMs based in cesium formate and sodium formate brines over both standard polymers, such as guar gum and xanthan, and nanocellulose without functionalization.37 CNFs as well as cellulose nanocrystals (CNCs) have demonstrated the ability to increase yield points in WBMs containing bentonite. It is believed that the CNCs form assembled complexes with bentonite platelets to increase yield point and fluid thixotropy, which were further enhanced through synergetic interactions with polyanionic cellulose.38 When CNCs with carboxylate (cCNC) functionalities and cationic CNCs (caCNC) are used in low-solid WBMs, further enhancements are observed in filtration control and rheology. It has been found that while CNFs and CNCs show potential for rheological modification of fluids, their use in filtration control is more modest and they are often outperformed by other nanoparticles such as iron oxide nanoparticles,36 GO,28 and nanosilica.33
2.4 Enhancement of Other Properties of Drilling Fluids with Nanoparticles
Other aspects which are important in drilling include thermal conductivity and electrical conductivity. Drilling requires a thermally conductive drilling fluid to transport heat away from the drill bit. Resistivity logging is a technique often preferred for imaging the wellbore which requires an electrically conductive drilling fluid. The effect of nanoparticles on the thermal and electrical properties of drilling fluids has also been investigated. The high surface area-to-volume ratios of nanomaterials are believed to contribute strongly to high heat transfer rates along with increased electron conduction. Enhancements have been shown for nanosized tin oxide38 and 50 nm copper/zinc oxide in polyanionic cellulose WBMs where up to 53% increases in thermal conductivity are observed with concentrations of nanoparticles between 1 and 5 × 10−3%. Electrical conductivity increases were also observed with the 50 nm ZnO nanoparticles.8
H2S released by some rock formations during drilling is a major safety concern for drilling operations. The primary concern with H2S is the hazard that it brings to field personnel. Exposure to 600 ppm levels of H2S is known to be fatal within 3–5 min. In addition to toxicity, H2S is extremely corrosive to equipment used in well construction. The dominant commercial materials available for scavenging H2S are zinc compounds such as zinc oxide (ZnO), zinc carbonate,39 and iron oxide (Fe3O4).40 Zinc oxide consumes H2S, converting it to zinc sulfide. The surface area-to-volume ratio of nanosized zinc oxide particles allows the reaction with H2S to occur with much more favorable kinetics than bulk zinc oxide. Nanoparticle samples can completely remove 800 ppm of H2S from mud after 15 min at room temperature. This compares well with bulk ZnO which removes 2.5 % of H2S in 90 min.19
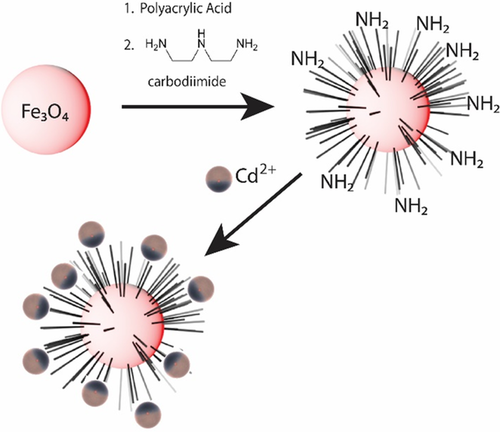
Drilling fluid wastes are an environmental concern in the drilling industry, and the effects of drilling activities using nonaqueous fluids on the metal contents of marine sediments are being addressed by some groups. It is known that the drilling fluid discharged in the mud pit at the drilling rig can contain a considerable amount of toxic heavy metal. Metals of this kind are a concern when they are not contained because of their potential to contaminate soils and permeate into ground water.41 Functionalized nanomagnetic particles of Fe3O4 have been shown to provide an effective means of fishing out harmful pollutants such as cadmium from WBMs and OBMs.10 This nanoparticle acts as a magnetic nanoadsorbent in the removal of cadmium from drilling muds. It has at its core nano-Fe3O4 which is decorated with polyacrylic acid chains, which are covalently bound to the iron oxide core and functionalized with amino groups at their ends (Figure 4).42 When these nanoparticles are added to a WBM with 5 ppm cadmium, 90% of the cadmium is removed from the mud within seconds.10
Field trials of nanoparticle additives in drilling fluids are under way, meeting with some success in improving the drilling fluid performance.43 Cost drivers are key in all sectors of the business and lowering the cost of nanoparticles will be key to their use in the field as they proceed into commercialization.
3 Nanomaterial-Based Enhancements in Cement
PC is a dynamic, complex, nonequilibrium composite material with structural hierarchy existing from the nanoscale to the macroscale.44 It is arguably the most important material in petroleum well construction. It seals the casing to the rock formation to provide structure to the oil well and prevent the flow of fluids from one well section or interval of the well to another. Cementing operations start in a well after drilling a given section of a well and after a metal pipe, known as casing, is placed in the wellbore. To place the cement in the intended location, the cement slurry is typically poured down through the inside of the casing and then up through the annulus between the rock formations and the exterior surface of the casing, where it is left to transition from liquid to a set solid with appropriate mechanical properties.45
The existing oil well cements used widely in the industry are often not well suited for the extreme conditions encountered in many well environments without specialized additive technologies. Some of the material shortcomings of oil well grade PCs are shown in Figure 5 along with the nanomaterials which offer solutions to the problems encountered. The limitations with cements are principally shrinkage, strength retrogression, brittleness, corrosion susceptibility, and gas migration.45 These issues are discussed in more detail in the sections that follow.
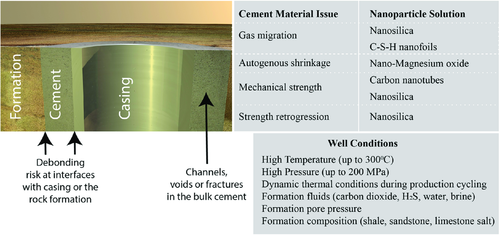
3.1 Autogenous Shrinkage and Nanomaterials
PCs shrink volumetrically (autogenous shrinkage) during setting, sometimes leading to the formation of channels in the cement, thereby allowing for fluid communication between zones in the oil and gas well.46 MgO is an additive which expands as it is converted into magesium hydroxide (Mg(OH)2) after exposure to water in the mixed cement slurry. The expansive properties of nanoscale MgO can be controlled through heat-treatment conditions (typically between 500 and 1400 °C) due to the influence of heat on the crystal structure and surface area of MgO. Typically, the higher the temperature the particles are heated to and the longer they are heated, the slower the reaction of MgO with water. This expansion can be further controlled through the synthesis of nanosized MgO. In this case, the expansion rate and extent are determined by the MgO calcining time and temperature, as well as the particle size of the MgO.
The reactivity of MgO must be adjusted to provide maximum expansion in the late plastic phase, at the beginnning of the hardening of the cement. This is why calcium oxide (CaO) systems, which tend to provide only early-stage expansion, are less favored than MgO systems. Given the variety of well conditions, MgO when used as an additive must be tailored to the temperature and pressure conditions of the well in which it is used. About 4% of MgO calcined at 700 °C, for example, has been found to be excellent at reducing shrinkage strains in a class G PC at 40 °C. At 100 °C, the same additive may not counter the effects of autogenous shrinkage as well.6, 20 An active area of research currently aims at providing a more generic additive solution to act against the shrinkage problem under a wide range of well conditions.
3.2 Nanoparticle Cement Set Accelerators and Gas Migration Additives
Cement set accelerators are important chemical additives in oil well cements. They offer the possibility of shortening the time duration it takes for the cement to transition from liquid (slurry) into the solid phase. This period of time, known as “waiting on cement” (WOC), incurs costs with each minute, much of which can be mitigated with good precision cement design. Cement accelerators are also used to reduce the risk of a phenomenon known as gas migration which is of critical importance to zonal isolation. Inorganic salts such as calcium chloride are often used as cement accelerators for these cases. These accelerators, however, are known to have the negative side effect of increasing the cement porosity. Due to its high surface activity, nanosilica has been proposed as a good replacement material as a cement additive.2, 47 Nanosilica displays a distinct advantage to commonly used accelerators48 because it decreases the porosity and increases the mechanical strength of set cement49 by up to 35%. The surface area-to-volume ratio of nanosilica along with the C—S—H nucleation, growth, and seeding observed in cements laden with nanosilica makes nanosilica a valuable accelerator and fortifying nanomaterial in PC. The C—S—H formation is accelerated, in part, due to the acceleration of the pozzolan reaction of hydrated lime (Ca(OH)2) and SiO2.50 For these properties, nanosilica finds potential applications in a number of different oil and gas well cementing scenarios including the cementing of lower temperature zones within a well. Cement close to the surface of wells in deep-water oil wells can be required to be set at low temperatures from 10 to 15 °C.
Nanosilica is also known to help with gas migration issues. Gas migration can occur in cements after the cements have been placed in the well and the hydration reactions in the cements render weak gel strength. The gel strength development results in the loss of the hydrostatic head provided by the cement slurry density on the surrounding rock formation. When this occurs, the cement becomes susceptible to the pore pressures from the formation which can cause channeling in the cement before the cement builds enough mechanical strength to resist the influx of fluid/gas from the formation. Nanosilica and C—S—H nuclei have been shown to reduce the risk of gas migration by shortening the time (transition time) between when the cement is a weak gel to when it is a strong set structural material.2, 47, 48, 50, 51
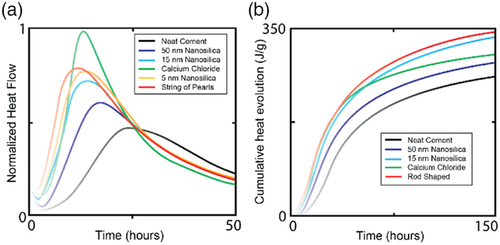
There are many different sizes and shapes of nanosilica that can be synthesized or purchased commercially. The size and shape of the materials can have a major effect on the influence of the material of cement hydration. Figure 6 shows how different shapes and sizes of nanosilica can influence acceleration of cement setting. The nanosilica tested imparts different setting kinetics on the cement where smaller the silica particle, the greater the effect on hydration and hence, the shorter WOC time. About 5 nm nanosilica displays the fastest setting with cigar-shaped and string-of-pearl-shaped nanosilica close behind. The larger the size of the spherical nanosilica, the slower the setting of the cement. The hydration of cement with nanosilica is believed to follow a C—S—H seeding mechanism. The reaction of water with the anhydrous tricalcium silicate and dicalcium silicate in cement is promoted and accelerated by the presence of nanosilica seeds in the cement to grow in larger quantities per unit time due to the high surface activity of the seeding nanoparticles.
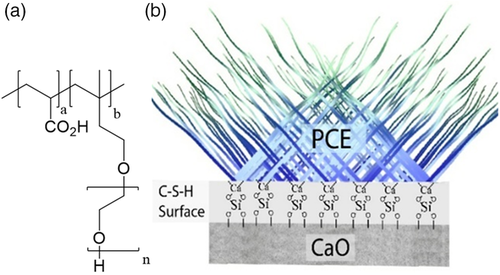
Other promising new materials which display the ability to accelerate the hydration of cement and porosity/permeability reduction52 of set cement are the so-called C—S—H nanofoils. These promising new materials are made as stabilized complexes with polycarboxylated ethers (PCEs) (Figure 7). PCEs are known as superplasticizers in the concrete industry and have remarkable properties on their own for their ability to dramatically reduce the viscosity (disperse) of cement slurries. Nanofoil-PCE nanocomposites are prepared by the coprecipitation of sodium silicate and calcium nitrate in the presence of a PCE. Larger PCE concentrations are associated with smaller sizes of amorphous/semicrystalline C—S—H nanofoils.53
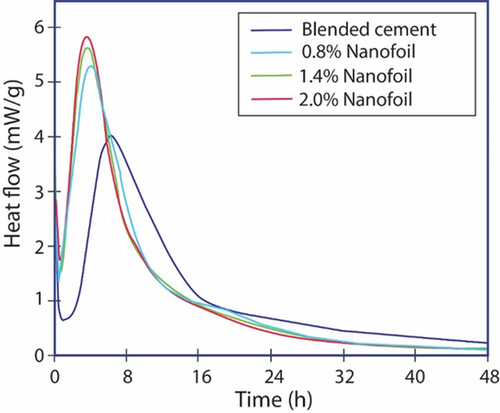
C—S—H nanofoils of 50–60 nm serve as effective nuclei for the hydration acceleration in cement.54 Examination of the time dependence on the compressive strength of PCs blended with fly ash indicates rapid early strength as measured through isothermal calorimetry of different concentrations of C—S—H nanofoils, showing excellent cement acceleration characteristics (Figure 8). It has been shown that the nanofoils not only accelerate the hydration of tricalcium and dicalcium silicates in cement but also accelerate the pozzolanic reaction between fly ash and Portlandite.53
3.3 Mechanical Strength and Nanomaterials
Mechanical shocks such as those taking place during perforations and hydraulic fracturing can impart undesired damage to cements in wells.55 The thermal environment of the cement will often be variable during production cycles, leading to the differential thermal expansion and compression of the casing onto the cement. This is a well-known problem in the industry and also leads to the development of fractures in the cement sheath. Breaches in the cement used in well construction can lead to the migration of hydrocarbons and water to annular spaces where they can mix and build up pressure. As hot reservoir fluids are produced, these trapped annular fluids will expand and often burst or crush the casing, leading to catastrophic damage. Consequently, when these kinds of pressure buildups occur and are detected, the well is often shut in if remedial treatments of the well fail. At best, failures of these kinds lead to losses in unrealized revenue. However, loss of life is also a consequence of operational failures of this kind, as encountered in the Deepwater Horizon incident.56 For these and other operational reasons, the mechanical properties of the set cement are targeted to have the highest possible tensile strength and the lowest possible Young's modulus. Nanoparticles have been shown to provide tensile strength enhancement ranging from 15%21 to 40%.7
In addition to the enhancements observed with nanosilicas, carbon nanotubes, due to their high aspect ratios, show some propensity for mechanical reinforcement of cement. However, almost all of the research reported to date with carbon nanotubes in cements has a requirement in the procedure to sonicate the nanomaterials for an extended period prior to mixing with the cement. If nanotubes are added to cement-mixed water without sonication, the resultant set cement material can actually suffer from reduced strength.47 This imposes a clear limitation of the procedure and use of the material due to scale-up issues encountered when handling the quantities of materials required for field deployments. The low concentrations of nanotubes required to show enhancement, however, are encouraging on economic grounds. Recently, carbon nanotubes have been grown from the surface of the cement clinker through a chemical vapor deposition (CVD) process. This method precludes the need for sonication as the nanotubes are already prepositioned on the cement grains prior to adding water. The resulting set cements utilizing this modified clinker have shown enhancements of 15% in tensile strength.21
Nanosilica is believed to contribute to increased strength and permeability reduction through the chemical reaction between SiO2 and Ca(OH)2 dissolved in water from the cement phases as well as the packing factor improvement due to the small size of the silicas.57 Nanosilica has been observed to produce a more dense and compact cement matrix than other silicas. The capillary porosity, which is defined as the total pore space occupied by water, has been observed to be reduced over time by over 200% in cements containing nanosilica compared with plain cements and cements containing fumed silica.58 Nanoindentation studies of PCs cured under simulated wellbore conditions (80 °C, 10 MPa) have revealed stiffer, higher-density C—S—H pastes, determined by 29Si NMR to have a greater degree of silicate polymerization compared with samples without nanosilica.59 Long-term (28 days) NMR studies of ordinary PC pastes inclusive of nanosilica cured under ambient conditions exhibit longer silicate polymer chains than samples in the absence of nanosilica.60 Nanosilica has the effect of increasing the bulk compressive strength of the set cements.2
At temperatures above 150 °C, the strength of cement can decrease dramatically (strength retrogression), due to the dynamic conversion of the amorphous C—S—H into the higher-density crystalline product which is α-dicalcium silicate hydrate.61 This problem with strength retrogression is, in part, overcome through the addition of crystalline silica. Usually, sand is added to cement at a concentration of 35 % by weight of cement (bwoc) to improve its strength.62 Research has shown that nanosilica can be added to further strengthen the cement at high temperatures. For example, upon the addition of 4% bwoc of nanosilica to a standard 35% bwoc sand-containing cement, the compressive strength of the set cement has been found to increase by 25% compared with cement without the nanosilica.22
Research teams have also looked at using nanosilica to counter corrosion, which is a major issue for cements. Cements can suffer from exposure to formations which produce carbon dioxide, H2S, or other corrosive gases. Carbon dioxide can corrode cement by converting portlandite into calcium carbonate. If the cement is exposed to water, the calcium carbonate can dissolve and diffuse out of the cement to render increased porosity into the cement and reduced mechanical strength. The calcium leaching rate by carbon dioxide invasion can be dramatically reduced with nanosilica. This is because the nanosilica reduces the porosity of the cement, transforms portlandite into the C—S—H gel through a pozzolanic reaction, and modifies the internal structure of the C—S—H gel, increasing the average chain length of the silicate chains.63
3.4 Sensors in Cement
In addition to the use of nanomaterials to overcome durability and sustainability issues pertaining to oil well cementing, nanomaterials are also being sought for use as sensors. Conductive nanofibers have been shown to not only enhance the durability and downhole suitability of aluminosilicate cements, but they also increase the piezoresistive response of cement.64 Additive materials in cements, which enhance the piezoresponsivities in cements, offer the possibility of enhanced downhole sensing capabilities. The structural health of the cement inside an oil well could potentially be monitored remotely and in real time through this technology. Figure 9 illustrates how permanent sensors may be located on the outside of casing within an oil or gas well.
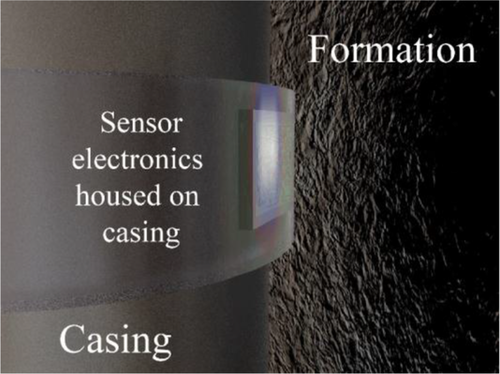
While there remain obstacles to the realization of this vision, there is much research and development effort in producing sensors that can report in real time the conditions throughout the well. Downhole energy-storage devices, such as supercapacitors, are also being investigated to be integrated to produce autonomous sensing capabilities.4 Cements can be made intrinsically sensing capable of external load through the addition of Fe2O3, carbon fibers, carbon black, carbon nanotubes, and carbon nanofibers to the cement prior to setting.65 Recent work66 suggests the feasibility of predicting the tensile stress on a cement containing 0.5% CNTs with separate algorithms for elastic response in cement as compared with strain-hardening and strain-softening responses observed after the material undergoes plastic deformation. Much of the research in this field has been devoted to construction cements and concrete but oil well cements have also gained some attention.67
4 Production Applications
4.1 Reservoir Sensing
Today, the industry relies on many well-monitoring methods to bring an understanding about how the reservoir is going to behave while producing petroleum fluids in both short and long terms. These methods include gathering of production data, near well-bore logging, and sampling of reservoir rock while drilling and reservoir simulation.68 To produce hydrocarbons from reservoirs most effectively, it is highly beneficial to increase the understanding of the reservoir architecture including reservoir flow paths, heterogeneities, and unswept areas of the reservoir. Surveys from these tests can lead to greater yields from extracting the amount of oil in place where a change in a few percentage points can correspond to enormous revenues. They are a powerful way of maximizing the yields from improved oil recovery (IOR) and EOR techniques.
The use of interwell reservoir tracers involves setting up a flow field in the subsurface between injection and extraction wells where the reservoir is to be mapped in the interwell zone. Tracers are introduced in the injection wells, with subsequent measurements of the tracer concentrations in the extraction wells. Existing tracer techniques include solvents (i.e., ethyl acetate),69 perfluorocarbon gases, and flourescent tracers (i.e., fluorobenzoic acids).70 Toxicity and environmental concerns limit the application of many of these chemicals. Furthermore, existing fluorescent chemical tracers display low sensitivities (often measuring to a sensitivity limit of hundreds of ppms), often due to fluorescence quenching and a fluorescence background from organic residues in crude oil.71
Figure 10 shows the use of tracers in an interwell scenario. In this illustration, two tracers are injected into two separate injector wells at two different locations within a reservoir. The tracers are collected at the production wells where the concentrations and time of travel through the reservoir can be determined. Tracer A and Tracer B can represent two different barcodes. As described in the following section, barcoded nanoparticle tracers can be differentiated as a difference in fluorescence signature,71 Raman signal,72 mass spectrum,73 or other chemical or physical characteristics of the tracer.
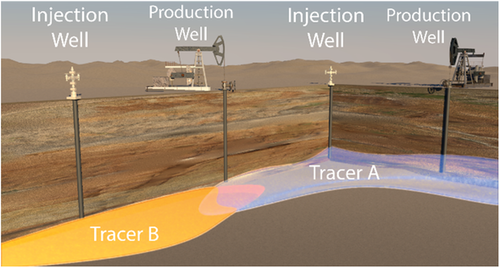
4.1.1 Nanoparticle Sensors for Reservoir Mapping
One of the ultimate goals of using these nanoparticles as reservoir tracers is to provide in-depth data about petroleum reservoirs. A long-term goal for nanoparticle reservoir tracers is that during their journey from the water injection well to an oil producer, the particles could provide reservoir sensing whereby they may record temperature, pressure, salinity, or any other important reservoir rock/fluid properties. One way to retrieve the measured data from the particles is the recovery of the particles at the producing wells. In addition, the nanoparticles can be used as vehicles for transporting specific chemicals to the reservoir such as wettability alteration chemicals, scale inhibitors, and water control agents. Despite the fact that this vision is ambitious, the literature has reported various applications toward making it possible.
The first oilfield-scale demonstration of the use of nanoparticles as sensors for oilfield reservoirs was reported in 2011.17 The particles were luminescent carbon dots with a size ranging from 5 to 10 nm.74 To measure the particles concentration, their response to light was utilized as they are fluorescent. Thus, their concentration was detected with an accuracy of 0.1 ppm using a fluorometer. Several experimental tests were used to assess the performance of the particles. These included visual tests to investigate the stability of the particles in oilfield brines, zeta potential measurements, and injection through porous reservoir cores (rocks) to investigate the mobility of the particles and their recovery potential. The authors reported that 96% of the particles were recovered after injection through a carbonate reservoir rock. After these promising lab tests, a field test was reported. The particles were injected into an oil-producing well at a concentration of 130 ppm. After the injection, the well was shut in for 24 h and afterward, the well was put on production where samples were collected and transported to the lab. The field results gave a recovery factor of 82%.17
Many fluorescent tracers that were tested thus far suffer from the drawback that the fluorescent signal can be obscured in the presence of polyaromatic hydrocarbon fluorescence in crude oil.75 For this reason, nanoparticle tracers have been in development which display a persistent luminescent signature that outlives the fluorescence of species from the reservoir. About 50–100 nm LGO:Cr nanoparticles show some promise as tracers of this type. They emit strong photoluminescence at 716 nm under ultraviolet (UV) excitation (254 nm). The fluorescence signal from the excitation lasts for an extensive time (of the order of minutes) and can be used to detect 1 ppb concentration of the nanoparticles.71 These materials are particularly interesting for the ability to detect the persistent luminescent signal with a portable device equipped with a charge-coupled device (CCD) camera.
Plasmonic nanostructures have been shown to display strong surface-enhanced Raman scattering (SERS) intensities,76 which make them excellent candidates in many different sensor applications.72 Silica-encapsulated silver nanoparticles have been shown to exhibit 10 ppb-level sensitivities to the adsorption of fluorescein and rhodamine dyes with Raman spectroscopy.72 This allows one to “bar tag” or label nanoparticles with dyes so that nanoparticles injected into the reservoir either at different times or at different locations can have a distinct spectroscopic signature, which assists reservoir engineers in detailing reservoir maps. Figure 11 shows the synthesis of Fe3O4/Ag@SiO2/Ag and Fe3O4/Ag@SiO2 nanoparticles. The nanocomposite samples are multifunctional and designed for low-concentration detectability. The silver nanoparticle component in these nanocomposites gives the SERS signal for detection of the particle, whereas the iron oxide nanoparticle component is superparamagnetic and allows the particles to be concentrated with magnetic fields for higher detectability.77
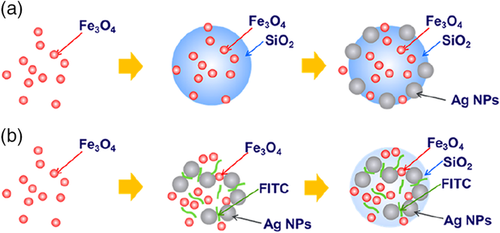
Reproduced with permission.77 Copyright 2019, American Chemical Society.
The use of nanoparticles as reservoir tracers is an exciting area of development toward the eventual possibility of bringing recordable stimulus–responsive nanoparticles into use for reservoir mapping. An appealing aspect of these technologies is that due to the potential for high revenue in oil recovery from reservoir mapping, there are fewer cost constraints in the design of nanoparticles as tracers than in many other applications of nanomaterials in upstream oil and gas. But there are many challenges that lie ahead in the development of these technologies. One such challenge is maintaining colloidal stability of the particles under the high saline environments that the particles are required to endure as well as the challenging temperature and pressure environments of the reservoir.78 Other challenges remain in transport through porous media at a high temperature and pressure.
4.2 Enhanced Oil Recovery
It has been estimated that only about one-third of the original oil in place (OOIP) in reservoirs is actually recovered. In heavy oil reservoirs, primary and secondary production methods at their economic limit leave behind 80–95% of OOIP.79 EOR methods offer the possibility of improving the production yield of wells through a variety of techniques including chemical injection, gas injection, thermal oil recovery (such as steam flooding),80 and microbial EOR.81 In all of these methods, the injected fluid travels from one or more injection wells through the reservoir to displace hydrocarbons toward an oil production well. Figure 12 shows a conceptual depiction of flooding as well as the nanotechnology applications for EOR purposes.
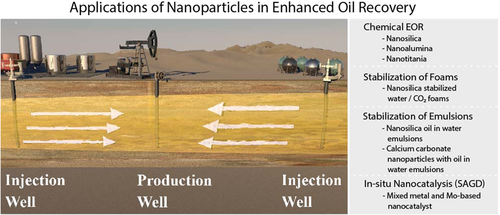
Various fluids, in chemical EOR, can be used for injection and include water alone, viscous polymer/water solutions,82 polymer/surfactant,83 alkali–surfactant–polymer (ASP) flooding solutions,84 and CO2 in water foams.85 In the following paragraphs, we shed light on the use of nanoparticles to help improve the existing chemical EOR technologies. After discussion of chemical EOR, emerging technologies utilizing nanocatalysts26 for use in thermal EOR, in steam-assisted gravity drainage (SAGD), are discussed. These nanocatalysts are used to enhance recovery by upgrading heavy oils and bitumens to lighter oils.
4.2.1 Chemical EOR and Nanoparticles
Water injection is the conventional solution for secondary oil/gas recovery but water does not wet the oils on these surfaces for many wells including unconventional heavy oils79 and hydrocarbons from porous, low-permeability oil-wet carbonate reservoirs.86 Chemical EOR, involving the injection of fluids containing polymers or surfactants, is an attractive EOR method for its potential to displace unrecovered oil. Chemical EOR aims to displace unrecovered oil by three different major methods. These are 1) polymer flooding methods, 2) surfactant flooding methods, and 3) foam EOR methods.87 Nanoparticles have found applications in all of these methods.
In polymer flooding, a water-soluble polymer is added to the flood water to increase the water viscosity and reduce its mobility through the permeable formation medium. This causes a reduction in the mobility ratio, leading to greater sweep efficiency. The volumetric sweep efficiency is defined as the ratio of the volume of oil contacted by the displacing agent to the volume of oil originally in place. Surfactant flooding uses a surface-active agent to reduce interfacial tension (IFT) and thus reduce the capillary forces which often trap oil in rock pores unless a sufficient viscous force is applied to displace the oil. Reducing the IFT reduces the viscous force required for oil displacement. In foam EOR, the primary purpose is to deliver gas to the reservoir to recover oil. Foam is the dispersion of surfactant-stabilized gas in water.87
A challenge in surfactant EOR, both in CO2 flooding and in micellar solutions, is that surfactants often adsorb strongly onto rock surfaces, destabilizing the EOR fluid dispersions and potentially leading to formation damage. This results in a loss in EOR efficiency.88 Colloidal nanoparticles have been shown to reduce the adsorption of surfactant molecules and in this way, increase oil recovery in comparison with cases where water without nanoparticles is used.89 Altering reservoir rocks through the use of nanoparticles has been reported to increase the amount of oil that can be recovered from a given core by changing the wettability from oil wet to water wet.90 Nanoparticles are believed to benefit from an additional mechanism of oil displacement, known as the structural disjoining pressure mechanism. In this mechanism, nanoparticles in the fluid form layered structures which contact the surface of oil-wet minerals with differential pressures applied through wedge-like films to spread the nanofluid over the surface, detaching the oily material coatings on the rock surface.11
Nanosilica shows promise for EOR due to their availability and lower cost in comparison with other nanoparticles. Core-flood studies have been reported using various sizes (7, 16, and 40 nm diameters) of nanosilica at different residual oil saturations.90 Laboratory results indicate that up to 8%, 17%, and 40%, additional oil can be recovered from Berea sandstone cores upon the use of these fluids of 7, 16, and 40 nm nanosilicas, respectively.90 Other studies have shown the salt tolerance of nanosilica in displacement of crude oil from Berea sandstone with an efficiency of 50% as compared with 17% for the brine in the absence of nanosilica.13 When nanosilica is introduced to Berea sandstone core samples containing crude oil, the water contact angle of the system is lowered, up to a reported 33% decrease (from 39° to 26°).91 The water-wetting efficiency has also been found to increase with increasing temperatures.91
Nanosilica is not the only nanoparticle system capable of improved efficiency in EOR. A recent study90 reports the comparisons utilizing 17–40 nm Al2O3, TiO2, and SiO2 for EOR purposes. The authors utilized a dispersant (vinyl pyrrolidone) with the Al2O3 and TiO2 nanoparticles and compared the performance of those nano-based fluids with nanosilica without dispersant. TiO2 nanoparticles were found to give the best wettability change as evident through contact angle measurements, and the core-flood experiments conducted with TiO2 gave the highest hydrocarbon recovery.90 Nanoparticles, however, have been found to require additional chemistry for increasing their suspension stability and disposability in brines and when migrating through porous media. There are two approaches which have been exploited to these ends. In one, surfactants are used with nanoparticles to increase their efficacy, and in the other, nanoparticles are specially functionalized for increased stability.24
4.2.2 Stabilization of Emulsions
Multiple synergies between surfactant additives, nanoparticles, and ions can be exploited for improved water wettability and improved nanoparticle stability.92 ASP EOR is a chemical EOR technique where high pH is believed to render a soap in situ in the presence of oil within the reservoir. ASP EOR has been explored in tandem with nanosilica to increase the water wettability of oil adsorbed onto mineral surfaces. It was found that the wettability of sandstone core samples depends on the coadsorption of nanoaggregate-surfactant complexes on the sandstone core where the presence of divalent cations assists in the wettability of the surfaces.93
The oil that is being recovered from the reservoir through these tertiary production methods can often emulsify due to mass and heat transfer, the low dynamic IFT between phases, and chemical reactions close to the oil–water interface. The presence of microemulsions is favored for oil recovery and the state of the art for surfactant EOR in achieving microemulsions typically uses alcohol-stabilized surfactants or branched surfactants with a finely tuned aqueous salinity. These microemulsion formulations are designed for stability. The chemistry must be tuned to prevent the formation of metastable macroemulsions which can be formed by the coalescing of microemulsion droplets which lead to surfactant-rich and polymer-rich phases that can plug the porous spaces within the rocks into which they are injected, leading to formation damage.84 Hydrophilic nanoparticles are used to stabilize oil in water (o/w) emulsions where the contact angle at the oil–water interface is less than 90°. In contrast, hydrophobic nanoparticles are used to stabilize water in oil emulsions (w/o) and their contact angle at the water–oil interface is more than 90°.
Stimulus–responsive nanosilica grafted with poly(2-(dimethylamino)ethylmethacrylate) (DMAEMA) have been shown to stabilize and break oil in water emulsions of crude oil. The switch between stabilizing and breaking the emulsions is pH responsive where a 0.1 wt% concentration of the nanoparticles gives a contact angle of 62° at pH = 7 and 81° at pH = 2. These functionalized nanosilica at concentrations of 0.1 wt% stabilizes emulsions at a neutral pH and breaks emulsions under acidic conditions (pH = 2). The stimulus–responsive nature of these materials offers the possibility of control over the emulsification of oil droplets such that formation damage could be mitigated. Testing of heavy oil recovery using DMAEMA nanosilica displayed superior oil recovery through a sandpack at 10 wt% OOIP as compared with less than 1% for DMAEMA in the absence of nanosilica.94
4.2.3 Stabilization of Foams
Gas injection is a widely used method for enhancing oil recovery. Gases used for injection can vary from nitrogen, air, methane, natural gas, or CO2.95 CO2 injection accounts for about 38 % of EOR in the United States.81 CO2 flooding is often the preferred method for oil recovery for light oil with viscosities less than 10 cP and at reservoir depths greater than 900 m. The depth must be high so that the reservoir pressure exceeds the minimum CO2/oil miscibility pressure. When heavier oils are being pursued, the use of CO2 alone as an EOR fluid, the low viscosity, and the resulting high mobility of the gas compared with oil (heavy oil can present viscosities as high as 10 000 cP) result in a phenomenon known as viscous fingering, leading to poor sweep efficiencies.96 The mobility of water is directly related to the viscosity of the fluids, and this must be equal to or lower than the oil to afford recovery/displacement of oil. To improve the viscosity of CO2, it can be foamed with water. The longer the viscosity stays high, the better the sweep efficiency. Thus, it is important to maintain foam viscosity for the longest period of time possible. As is determined to be the case in surfactant EOR, nanosilicas aid in reducing surfactant adsorption in foam EOR applications. Results of a study of nanosilica in sodium dodecyl sulfate (SDS) revealed a 75% reduction in surfactant adsorption onto kaolinite.97
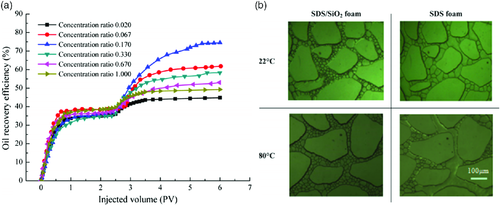
The stability of foams is dramatically impacted by the presence of oil, salinity, temperature, and pressure. About 15% additional OOIP was recovered with the use of methyl-functionalized 12 nm nanosilica to stabilize the water–CO2 foam.98 CO2–surfactant foams that are surface modified with nanoparticles (such as nanosilica) display enhanced stability over surfactant-stabilized foams. Similar to the nonfoamed surfactant EOR, nanoparticles in CO2-foamed EOR display a lower propensity for adsorption onto the formation and have been shown to increase CO2 foam stability when compared with cases where just surfactants are used. Figure 13 shows the foam half life of SDS as compared with SDS–nanosilica. The nanosilica used in these tests was of 20 nm diameter with a partially hydrophobic surface (contact angle with water was 122.2°). From the oil recovery efficiencies from sandpack recovery testing (Figure 13a), the optimal synergetic effect between nanosilica occurs when the SDS/SiO2 ratio is at 0.17 at 1.5 wt% nanosilica. The nanosilica surfactant outperforms SDS foam under high temperatures and pressures. Visual flooding experiments (Figure 13b) show that the nanosilica-stabilized foams have a superior stability at elevated temperatures, whereas the SDS surfactant-stabilized foam shows coarsening/coalescing of the foam.99
Reproduced with permission.99 Copyright 2016, American Chemical Society.
Similar surfactant-nanoparticle synergies in foam stabilization for EOR have been observed with hydrophilic nanosilica (40 nm diameter, contact angle with water = 39°) with the cationic surfactant cetyltrimethylammonium bromide (CTAB). It was found that increasing the ratio of CTAB/nanosilica (with an optimum ratio of 0.033) would increase foam stability, whereas CTAB produced a monolayer on the nanosilica rendering it more hydrophobic. Optimum contact angles for foam stability are between 60° and 90°. As the CTAB concentration was increased further relative to nanosilica, the foam stabilization effect diminished.100
4.2.4 Thermal EOR and Nanocatalysts
While nanoparticles are useful in enhancing oil recovery through surfactant and emulsion chemical EOR (which applies to heavy oils and light oils alike), thermal EOR methods for oil recovery are also being developed with a focus on the extraction of heavy oils and bitumens. Steam flooding is a process which involves the injection of steam into hydrocarbon-bearing formations to reduce the viscosity of heavy oils and bitumens as a means to enhance recovery. Typical recovery factors for this method are between 50% and 60%. The major limitation of steam flooding is that it is very energy intensive and costly. SAGD is a specific kind of steam injection which involves two horizontal wells, typically spaced with the injection well 5 m above the production well. Steam is injected into the upper well, reducing the viscosity of the hydrocarbons which drain into the lower production well and are then pumped to the surface.80
Because of the energy-intensive nature and environmental footprint of steam flooding and SAGD, the injection of catalysts is emerging as a promising technology for recovery and upgrading heavy oils with thermal EOR. Molybdenum-based nanoparticles show the possibility of achieving EOR of bitumen through in situ catalytic conversion of light oil from bitumen feedstock within oil sands. The EOR method used for these materials was through SAGD EOR at 3.5 MPa and 300–320 ºC.101 The studies revealed that enhanced recovery could be achieved through the decomposition of heavy oil into lighter products. The absence of a porous matrix (used in fixed-bed catalyst technologies) for these materials and their high surface area is believed to be the reason for their higher performances over other catalysts such as fixed-bed catalysts investigated in this kind of EOR.101 It has also been suggested that because nanocatalysts may permeate through the sands or formations into which they are injected and thus be recovered in the production well, it may be possible to recycle nanocatalysts.26 Furthermore, these concepts in upgrading heavy oils and bitumens are not limited to SAGD. External microwave heating is being actively investigated with nickle nanocatalysts to reduce the viscosity of heavy oils in EOR. A recently published report provides a proof of concept that nickel nanocatalysts with 2.45 GHz microwaves at 2 kW can upgrade (break C—S and C—O bonds in heavy oil sands) and could potentially be used as an in situ method for heavy oil upgrading.102
In summary, nanoparticles show promise in EOR. There are technical challenges that remain to realize the profitability of these technologies. Aggregation is an issue with many nanoparticles due to their high surface areas and the influence of such destabilizing conditions as temperature, pressure, salinity, and the influence of oil or other chemical species that may be present in the reservoir.91 In chemical EOR, the tunability of the nanoparticle surface chemistry, when achieved at low cost, offers the possibility of superior performance over existing surfactant technologies. Enhancing nanoparticle efficacy through stimuli–responsive technologies, when harnessed with the appropriate level of control, could potentially lead to superior solutions in terms of foam stability, microemulsion stability, and overall performance. In thermal EOR, nanocatalysts are interesting for their potential in upgrading heavy oils and bitumens but there is still considerable investigation required in how nanoparticles may be transported through porous media under well conditions while maintaining their stability. The catalytic activities of nanocatalysts are currently being developed to obtain the maximum possible recovery enhancement while limiting formation damage.
4.3 Application of Nanotechnology in the Production Domain
4.3.1 Nanoparticles and Hydraulic Fracturing
Several domains in the production sector have benefited from the use of nanotechnology. These include the design of hydraulic fracturing fluids, water control chemicals, fine migration prevention, and acidizing. The use of nanoparticles has been highlighted in the development of fracturing fluids where a great deal of literature exists on this topic.3, 103 Figure 14 shows nanoparticles which have been used and show a promise in these upstream applications. In a typical fracturing operation, a fracturing fluid is used to transmit hydraulic power in the form of dynamic stress on the rock downhole to break it down and create fractures. Once the fracture is created, proppants/sand particles are used to keep it open. To transport the particles into the fracture, the viscosity of the fracturing fluid is increased by adding crosslinkers. For hydraulic fracturing, ZnO nanoparticles have been proven for use with viscoelastic surfactant (VES)-based fluids to improve their leak-off properties. Without the addition of ZnO, these fluids were limited in applications of a 200 mD maximum permeability.23 Performance enhancements through the use of polymer-based fracturing fluids can be achieved with nanosilica. Guar-based polymers,104 polyacrylamide-based polymers,105 and guar polymers under high salinity conditions show better performance when nanosilica was incorporated in these fluids. Recent works23, 106 report the use of nanoparticles in packs during hydraulic fracturing treatments. Through van der Waals forces, the particles attach themselves to the proppant surfaces. When the reservoir-generated fines come into contact with the proppant-treated surface, they will be attracted and therefore, their propagation into the wellbore and perforations will be prevented.
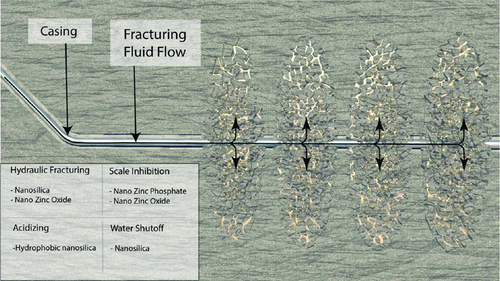
4.3.2 Nanoparticles and Water Shutoff
The production domain utilizes nanoparticles for the design of water shutoff gels. These gels are composed of a polymer and a crosslinker that react together to form a 3D gel structure which, in turn, should block water-producing rocks and allow more oil to be extracted. The literature usually refers to these as conformance improvement treatments (CITs). The use of nanoparticles in formulating these chemical technologies was reported to improve gel stability, enhance control over the gelation time, or improve gel propagation. We give examples of these technologies hereafter. One report107 suggests the use of silica and aluminum oxide nanoparticles to improve the stability of silicate gels which inherently lack stability without an additive. The addition of SiO2 nanoparticles with a size of 12 nm was found to decrease the gelation time of silicate-based CIT gels. Increasing concentrations of nanosilica allows the operator to tune the kinetics of the phase change of the liquid colloidal solution (sol) to the gel. This reaction is often referred to as a sol–gel reaction.
Another study108 reports the use of nanosilica particles as a water shutoff material due to their low average size (3–100 nm) which enables propagation through the reservoir rocks. The use of nanosilica to improve mechanical properties of gels generated by the crosslinking of partially hydrolyzed polyacrylamide (PHPA) with polyethyleneimine (PEI) is reported.109 Scanning electron microscopy (SEM) images show that the gels prepared without the addition of nanoparticles were thin and more rigid.110 Another study111 reported the use of dextran and PEI to form nanoparticles that can sequester chromium (Cr+3) and prolong the gelation time.
4.3.3 Nanoparticles and Acidizing
After hydraulic fracturing or in place of hydraulic fracturing, acids are sometimes used as stimulation fluids for well production enhancement. In these acidizing processes, the permeability of the production zone is increased through the etching of rock formation and boring of wormholes through the rocks using acids. On the acidizing application for well-stimulation purposes, hydrochloric acid (HCl) is used as a stimulation fluid to dissolve carbonate (CaCO3) rocks. One of the challenges of acidizing is that the acids may be too reactive with rock (especially carbonate rock formations) such that they dissolve the face of the rock without fracturing or unevenly etch wormholes into the rock. To overcome these issues, the industry has developed different retarder technologies to slow the acid's reactivity. One way to retard the dissolution reaction, between HCl and the reservoir rock, is to emulsify the acid in diesel. With time and temperature, the emulsion breaks down releasing the acid, allowing it to come in contact with the rock. The emulsion is formed by the use of a surfactant that acts as a stabilizer at the interface between the aqueous phase and the hydrocarbon phase. Standard surfactant-stabilized emulsions have limited temperature stability and therefore, are of value to enhance the thermal tolerance of stable emulsions. In this way, the acids can take more time to break and stimulate the reservoir to greater depths. The Pickering emulsion concept has been recently introduced for oil and gas stimulation applications. In this method, the nanoparticles can be used as a physical barrier between the acid and the diesel. Extensive lab evaluation has revealed that use of nanoparticle additives greatly enhances the stability of emulsified acids. Bulk thermal stability tests indicate that emulsion stability can be improved upon with the use of nanoparticles. Samples of acids emulsified with hydrophobic silica nanoparticles show enhanced retardation for emulsion breakage at temperatures up to 150 °C.5, 112
Microencapsulation of HCl with Pickering emulsions of 14 nm size hydrophobic nanosilica (from surface modification with polydimethylsiloxane) has been shown to render stable dry (acid-in-air) powders of HCl with excellent thermal stabilities (Figure 15). These acid-in-air emulsions are preferred for water-sensitive shales where water-reactive clays can limit the efficacy of water-based fluids. The release of acid from these powders is triggered by surfactants so the timing of the acid release can be modified to a desired rate. Testing of this technology has demonstrated that enhanced fracturing and uneven etching are achieved with this chemistry.

Reproduced with permission.5 Copyright 2017, American Chemical Society.
4.3.4 Scale Inhibition with Nanoparticles
The formation of scale deposits in well production tubing is a long-standing issue that the oil and gas industry faces. Scale inhibitors are used to prevent scale formation. Adsorption of the inhibitor on the reservoir rock is used as a strategy of scale inhibition. This is achieved by squeezing the scale inhibitor inside the reservoir rocks in the near wellbore area where adsorption of the inhibitor takes place. When the well is switched back to production, the inhibitor (phosphonate) is slowly released with the produced fluid. There are concerns with this approach such as lifespan of the treatment. To address these concerns and enable a slower release, inhibitor-precipitation methods have been put into application, a method where an inhibitor may be precipitated inside the rock as a metal salt. The precipitating phosphonate/calcium complex is a result of adjusting the salt concentration and pH of the injected solution. Limitations with the precipitation approach include formation damage and blockage of pores in the rock by the precipitation of solid materials.
To further increase the lifespan of scale inhibitor treatments and address concerns related to the blockage of pores with precipitates, a new nanotechnology-based concept was lab tested recently.113 Zinc and calcium metals were used to formulate metal-phosphonate nanoparticles that are synthesized with a size from 50 to 200 nm. Carbonate and sandstone porous media were treated with these metal-phosphonate nanoparticles to investigate their propagation in porous media and test their retention/release behavior. Several experimental techniques were used to investigate the performance difference between the nanoparticles of calcium/methylene phosphate (diethylenetriamine-pentamethylene phosphonic acid [DTPMP]) under different conditions. The authors compared the pore volume (PV) to breakthrough which is a measure of how long it took the inhibitor to be released from the porous media. In such an experiment, the concentration of the metal is monitored as a function of PV produced. Once the concentration drops below a minimum threshold value (around 1 mg L−1), the corresponding PV is recorded as the breakthrough PV (Figure 16). Clearly, better control over the inhibitor release is achieved by use of nanoparticles in carbonate rocks when compared with sandstone cores.113
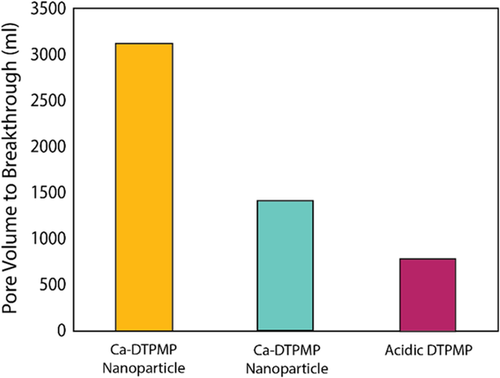
In summary, the production technology domain has made use of nanotechnology in several applications that showed promising results. Nanoparticle-based fracturing fluids were found to outperform other non-nanoparticle-based fluids, and this was verified in field applications of VES-based fluids combined with nanoparticles.23 Pickering emulsions represent another example where nanotechnology provided an opportunity to design better acidizing fluids. Some of the limitations in the field deployment and commercialization of these nanomaterials in production are based in cost. As the nanoparticles demonstrating performance advantages over conventional additive technologies are brought into larger-scale production, driving down costs, it is anticipated that commercialization of many of these technologies will follow.
5 Nanotechnology in Other Oil and Gas Applications
The earlier applications relate to the bulk use of nanoparticles in upstream oil and gas. However, there are many indirect uses of nanotechnology-related advances in the field. The rapid evolution of miniaturized and high-performance electronic devices has had a significant impact in the industry. Sensors will be ubiquitous in oilfields and the emergence of low-dimensional and low-power sensor networks based on nanomaterials in the future will enable several new detection technologies at various levels of applications in the field. Power delivery is another area where nanotechnology will have a significant impact. Form-fitted, fully powered sensors are important for EOR applications. Power delivery in the form of batteries and supercapacitors will become viable in downhole applications if new chemistries can be developed for operations across higher-temperature windows (≈150–200 ºC), higher pressures, and harsher environments. Recent developments have shown that these devices can be made to operate above 150 ºC using nanostructured electrodes and ionic liquid electrolytes.16 As high temperature and robust energy-storage devices with high energy, high power densities, and miniaturized features become available, the industry will greatly benefit in building high-precision detection devices in several upstream technologies. Fully autonomous and deployable sensors in EOR and other downhole operations would be the first technologies to benefit.
6 Conclusions
Nanotechnology provides huge potential in addressing technology challenges related to the upstream oil and gas industry with nanomaterials and devices. Some of the clear benefits of nanotechnology include the use of nanoparticles to provide incremental improvements to existing methods and procedures in extracting oil and gas, whereas some of the development in nanoparticles promises to be transformative. Step-change advances in the industry through developments in nanomaterials can occur in any of the domains of well construction, production, or reservoir management. With nanoparticles utilized in solving difficult industrial problems in upstream oil and gas operations, the benefit of nanoscale precision in product development is clear. A recent book by Schwab114 has vividly captured the impact of “The Fourth Industrial Revolution” where major technological developments across industries are driven by innovations in manufacturing,115 robotics,116 sensors,117 electronics, power, and materials. In this ensuing technological revolution, one can have little doubt that nanotechnology will be an essential component through all of the technological drivers that exist in the oil and gas and related industry.
Acknowledgements
The authors would like to thank Aramco Services Company and Saudi Aramco for permission to publish this work. The authors acknowledge significant contributions from Dr. Ghaithan Muntasheri in the preparation of this manuscript and thank him for his deep insights, inspiration, and direct contributions in several sections of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Peter J. Boul received his Ph.D. degree in chemistry from Rice University in 2002. From 2002 to 2004, he was a chateaubriand postdoctoral fellow at the Institut de Science et d'Ingénierie Supramoléculaires in Strasbourg, France. Following a fellowship at the University of Texas at Austin, he took a position at NASA (Johnson Space Center) in research and development with a focus on nanocomposites. He is currently a senior researcher at the Aramco Research Center (Aramco Services Company) in Houston where his work involves the development of advanced materials for the construction of oil and gas wells.

Pulickel M. Ajayan received his Ph.D. degree in materials science and engineering from Northwestern University in 1989. Shortly after, he spent time at NEC Corporation in Japan and at research laboratories at the Laboratoire de Physique des Solides in Orsay and the Max-Planck-Institute fur Metallforschung in Stuttgart. Later he joined the materials science and engineering faculty at Rensselaer Polytechnic Institute. Currently, he is the founding chair of the Materials Science and Nanoengineering Department and a professor of engineering at Rice University. His research interests are focused on functional nanostructured materials for a variety of applications.




