Carbon Capture and Utilization Update
Abstract
In recent years, carbon capture and utilization (CCU) has been proposed as a potential technological solution to the problems of greenhouse-gas emissions and the ever-growing energy demand. To combat climate change and ocean acidification as a result of anthropogenic CO2 emissions, efforts have already been put forth to capture and sequester CO2 from large point sources, especially power plants; however, the utilization of CO2 as a feedstock to make valuable chemicals, materials, and transportation fuels is potentially more desirable and provides a better and long-term solution than sequestration. The products of CO2 utilization can supplement or replace chemical feedstocks in the fine chemicals, pharmaceutical, and polymer industries. In this review, we first provide an overview of the current status of CO2-capture technologies and their associated challenges and opportunities with respect to efficiency and economy followed by an overview of various carbon-utilization approaches. The current status of combined CO2 capture and utilization, as a novel efficient and cost-effective approach, is also briefly discussed. We summarize the main challenges associated with the design, development, and large-scale deployment of CO2 capture and utilization processes to provide a perspective and roadmap for the development of new technologies and opportunities to accelerate their scale-up in the near future.
1 Introduction and Motivation
It is widely accepted that fossil fuels will remain the main source of energy for at least the next 50 years, and the CO2 emissions derived from such energy sources contribute greatly to global climate change.1 This will require the deployment of advanced low-carbon fossil-energy technologies in the short term. The December 2015 U.N. Climate Change Conference in Paris agreed on a long-term goal of keeping average warming below 2 °C, and this can be accomplished by considering two long-term emission goals: first, a peaking of emissions as soon as possible, and then, a goal of net greenhouse gas neutrality (expressed as a balance between anthropogenic emissions by sources and removals by sinks) in the second half of this century.2 Therefore, it is imperative to reduce such anthropogenic emissions.
On the other hand, with the increase in the world population, the demand for energy supply is expected to increase significantly over the next decades, and thus, new and renewable energy sources are required to meet this demand. Captured CO2 can be treated as a valuable feedstock for the production of many value-added chemicals and fuels, which thus provides a solution to both emission-control and energy-supply challenges.3
The concepts of CO2 capture, utilization, and sequestration (CCUS) are commonly used in the context of carbon management and climate change. Carbon capture and storage (CCS) refers to technologies that focus on the selective removal of CO2 from gas streams, its compression into a supercritical condition, and finally its transportation and sequestration in geologic formations, including depleted oil and gas reservoirs or oceans.4 Despite promises for mitigating large volumes of CO2 and despite extensive government incentives and regulatory drivers, the high cost of CCS has largely impacted its large-scale deployment. The high cost of CCS comes primarily from capture and compression, which accounts for 75 % of the total cost of CCS. According to the International Energy Agency (IEA) report published in 2013,5 CCS will greatly contribute to emissions reduction from all applicable processes in power generation and industrial applications (e.g., cement, iron and steel, oil refining, pulp and paper, and biofuels sectors) through implementing 3000 CCS projects around the world, with over 7000 Mt CO2 annually stored in the process.
As a more attractive alternative, carbon capture and utilization (CCU) technologies have recently received a great deal of attention for turning captured CO2, as a renewable carbon feedstock, into valuable products instead of permanently sequestering it. In fact, CCU treats captured CO2 as a renewable resource to complement or alternate the conventional petrochemical feedstocks.6 Moreover, the long-term effects of sequestration are not a concern for this approach. Despite the significant advantages offered by CCU in comparison to CCS, converting CO2 and utilizing it in chemical reactions is very challenging mainly because of the thermodynamically stable nature of CO2 itself.
The purpose of this paper is to review the most recent developments in the field of carbon capture and utilization while providing an overview of current challenges and future opportunities in the context of carbon management. Detailed descriptions of various capture and utilization technologies fall outside the scope of this review, and the interested reader is referred to previously published reviews that, in addition to providing a detailed description, address life-cycle analysis, risk assessments, and industrial ecology considerations of CCU technologies.7-11
2 CO2-Capture Options: Challenges and Opportunities
CO2-capture technologies are either related to direct CO2 removal from flue gas streams (referred to as postcombustion) or the development of advanced low carbon-intensive combustion systems (referred to as precombustion), which include an integrated gasification combined cycle (IGCC), as well as oxyfuel combustion that employs pure oxygen to reduce the carbon intensity of power generation.12 Technoeconomic analyses have indicated that the current CO2-capture technologies are energy intensive and result in a significant decrease in combustion efficiency as well as an increase in the electricity price. The choice of capture technology differs widely across industries, depending on the source of CO2 and the industrial processes generating CO2. Capturing CO2 from different sources has different energy penalties; for example, some industries such as ethanol-production plants produce high-concentration CO2 streams, whereas thermal power plants produce CO2 at very low concentrations, and thus much more energy is required for its recovery.13 The latter, however, is the largest source of CO2, which is a conundrum in the industry. Moreover, advanced supercritical CO2 combustion processes that employ recycled CO2 and operate over the supercritical pressure of CO2 have been recognized as additional promising methods to address anthropogenic CO2 emissions. The use of supercritical CO2 as a working fluid in a power cycle has been investigated in a variety of scenarios and has been found to make the plant more energy efficient relative to the conventional steam cycle.14 Studies have also shown an increase in steam turbine efficiency if the supercritical CO2 is compared to oil as a working fluid. The drawback of this process is that oxygen must be completely purified before use. Further, there are two pathways that can be used: liquid CO2 and gaseous CO2 recirculation, the former of which involves cryogenically treated CO2 in liquid form.
Typically, power plants alone account for approximately 45 % of the worldwide CO2 emissions, which thus offers significant opportunities for CCU and CCS options as main sources of captured CO2.15 The industrial deployment of postcombustion CO2-capture technologies will have a larger economic impact on the reduction of capture costs than the other options. However, in the best scenario, for a new power plant equipped with the current postcombustion capture technologies, the cost of capture is estimated to be approximately 56 USD t−1, which incurs an energy penalty of 62 % to the power plant.11 Carbon capture has already been commercialized in chemical production and the natural gas industry. Recently in 2014, SaskPower demonstrated postcombustion CO2 capture from coal-fired flue gas on an industrial scale in the Boundary Dam 110 MW Power Station.16 Shown in Figure 1 are the various capture routes that have been investigated in industry and academia within the past few decades and that will be discussed in the next section.
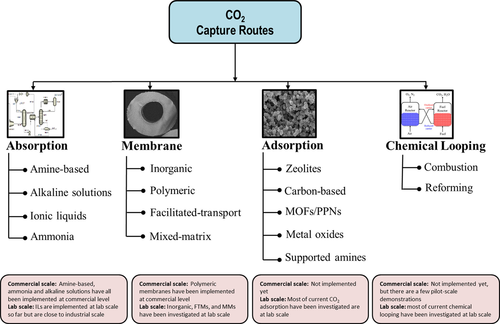
Various carbon-capture technologies and the corresponding materials currently under investigation.
2.1 Absorption-based CO2 capture
The most mature separation method in the oil and chemical industries involves absorption by chemical or physical solvents.3 This technology has been used intensively for both postcombustion (with chemical solvents) and precombustion capture (with physical solvents). Chemical absorption by aqueous ammonia, amine-based solvents such as monoethanolamine (MEA), diethanolamine (DEA), and N-methyldiethanolamine (MDEA) and alkaline solvents such as Ca(OH)2 and NaOH is the most common method for postcombustion capture in various industries, including cement, iron and steel, power plants, and oil refineries.17-20 The well-established commercial physical absorption technologies for precombustion CO2 capture include Selexol, Rectisol, Purisol, and Fluor. Recently, ionic liquids (ILs) have been identified as suitable alternatives to the conventional physical solvents used in the above processes as a result of their inherent properties, which include low volatility, low vapor pressure, high thermal stability at elevated temperatures, and low regeneration energy requirements.21-23 However, their low working capacity is a main obstacle toward their widespread use in CO2 capture.
Although absorption is a mature and well-established separation method that results in high capture efficiency, the energy penalty incurred is very high mainly because of the high energy requirements for the solvent regeneration option.3 Although heat integration can reduce the need for energy in some industries such as power plants, other industries such as cement or iron and steel cannot provide such heat integration. Besides, other known operational limitations such as corrosion and a large volume of water makeup represent significant barriers. Typically, chemical absorption relies upon thermal swing regeneration, and thus, optimal selection of solvents with an optimum combination of thermal and physical properties is of paramount importance in developing energy-efficient absorption-based CO2 capture. For instance, piperazine (PZ) and PZ derivatives have been suggested as alternatives to conventional chemical solvents on the basis of their superior performance such as fast reaction kinetics, low chemical reactivity, and low regeneration energy.24 Another opportunity is to enhance the working capacity of ILs through incorporation of functional groups (such as perfluoroalkyl groups, amine and amino acid groups, or carboxylate anions), which could eventually pave the way toward their utilization as solvents for CO2 absorption.3 The trade-off between heat of reaction and kinetics is another important consideration. Typically, for chemical absorption the employment of solvents with a high heat of absorption (>60 kJ mol−1) could reduce energy consumption, whereas the use of thermally stable solvents with low regeneration energy requirements can greatly improve the thermodynamic efficiency of the separation process.4 Poisoning by impurities present in flue gas or other effluent streams is another challenge that reduces the stability of chemical solvents, and thus, tolerance to impurities in addition to resistance to solvent oxidation and vapor losses should be considered as key performance metrics in developing novel aqueous solvents for CO2 absorption. Increasing the concentration of amines in aqueous solutions could also help to increase the capture capacity of amine solutions.
In addition to advancements in materials development and optimal selection of solvents with better energy performance, process improvements are of equal importance for scaling up the next generation of absorption technologies. In that regard, next-generation absorption processes with optimized process configurations and intensifications that provide heat integration strategies such as interheated strippers (to recover effectively the heat from the stripper overhead) and intercooled absorbers (to provide more reversible absorber operation with greater rich and lean loading) could offer a competitive CO2-capture solution.
2.2 CO2 capture by membrane separation
Generally, the use of membranes for gas-separation applications can offer an approach that is more energy efficient and environmentally friendly than other separation methods. Furthermore, membrane-based CO2 separation is usually operated under continuous, steady-state conditions, and a pressure difference across the membrane drives the permeation process. The material of the membrane in addition to its configuration, morphology, composition, and operating conditions are all key factors that dictate its gas-separation performance to a great extent. Membrane separation for the removal of CO2 from power plant flue gas streams has been the subject of many studies.25-28 The application of membrane technology for postcombustion CO2 capture is very challenging mainly because of the low pressure of flue gas streams. In contract, membranes are more suitable for high-pressure precombustion processes such as IGCC. Notably, unlike other capture routes, membrane-based CO2 separation involves multistage operation and streams recycling, which are often perceived as challenges that make this operation difficult and complex.
Various porous inorganic membranes typically composed of zeolites, metal–organic frameworks (MOFs), carbon molecular sieves (CMS), ceramics, and a few oxides (e.g., alumina, titania, zirconia) have been extensively studied for CO2 capture from flue gas or other effluent streams. Although inorganic membranes can withstand high temperatures and often have mechanical stability, their high costs still hamper their commercialization. Inorganic membranes have not yet been demonstrated on a large scale, and in fact, they are still far from scale-up. The main challenges associated with their large-scale utilization stem from their fabrication routes, which are very expensive, and their long-term stability and reliability.
Conversely, polymeric membranes that can be easily formulated in hollow-fiber modules have shown tremendous potential for large-scale industrial applications. In addition, inorganic membranes cannot reach the high packing density offered by polymeric membranes, as in the case of polymeric composite hollow-fiber membranes assembled in the modules, which have a filtration area that reaches up to 10 000 m2 in a 1 m3 reactor at a low production cost, crack-free thin membranes, and large-scale production.29-32 However, polymeric composite membranes have low separation performance relative to inorganic membranes. For example, CO2 capture by current polymeric membranes still suffers from several drawbacks, such as low CO2/N2 selectivity and permeability for postcombustion processes, the trade-off limitation between permeability and selectivity, swelling, aging, sensitivity to impurities, and mechanical stability, especially for high-pressure operations. For polymeric membranes to be cost effective for postcombustion CO2 capture, a minimal CO2/N2 selectivity of 200 is required and a relatively high permeability must be maintained.33 High permeability eliminates the need for a high membrane area for an acceptable separation rate, which thus reduces the capital costs of the separation process. One way to address the challenges inherent with traditional polymeric membranes is to utilize polymers of intrinsic microporosity (PIMs) that contain large free pores within the polymer matrix that allow for higher CO2 flux. Polymeric membranes have been commercialized for CO2 capture in natural gas sweetening. Membrane Technology & Research, Inc. (MTR) has implemented a pilot-scale membrane-based process for postcombustion CO2 capture from flue gas coming from a 880 MW pulverized coal power plant with a capture rate of 90 %.34
Facilitated-transport membranes (FTMs) such as liquid membranes, ion-exchange membranes, and fixed-carrier membranes have proven to be highly selective for CO2; however, they are subject to poisoning by trace amounts of acid gases, such as NOx and SOx, present in the flue and suffer from long-term stability.35-37 Among various commercially available membrane types, hollow-fiber membranes provide the highest surface-to-volume ratio, optimum geometry for high production rates, and provide more compact modules than flat-sheet or spiral-wound units.35 Composite hollow-fiber membranes comprising a thin selective layer with a thickness lower than a micrometer supported on a highly porous polymeric substructure provide opportunities for advanced membrane development.38
Mixed-matrix membranes (MMMs) formed by dispersing highly selective molecular-sieve particles such as zeolites, carbon nanotubes, layered silicates, and MOFs in a polymer matrix are promising contactors that combine the scaling up and processing of polymeric membranes with the advantages of separation performance of molecular-sieving materials. In comparison to porous zeolites, MOFs as fillers exhibit better properties such as higher pore volume and lower density, better affinity to polymer chains, and easily tuning cavities in terms of size and shape by choosing appropriate ligands with different functionalities. MMMs provide a solution to go beyond the known upper-bound trade-off limit of polymeric membranes as well as the inherent obstacles associated with the costs and processing of inorganic membranes.39-45 However, they are still in their early stages of development and are far from industrial deployment. Besides, their current fabrication processes are costly and complex.
In the context of membrane CO2 capture, the future opportunities should therefore focus on composite membranes that take advantage of both polymeric and inorganic constituents and that are capable of surpassing the current best-performing membranes. Strategies to improve composite systems by alternate chemistries, re-engineering the material systems, and processing techniques will provide critical insight into the barriers to engineering sophisticated composite systems for future membrane-based CO2 separation.
2.3 Adsorption-based CO2 capture
CO2 capture over porous solid materials offers a promising approach to remove CO2 selectively from gas streams in various industries. To date, a variety of adsorbents have been extensively evaluated for CO2 capture from precombustion and postcombustion gas effluents. Generally, the adsorbents used are classified as either high-temperature or low-temperature materials. The primary classes of high-temperature materials include hydrotalcites, alkali or alkaline-earth oxides such as calcium oxides, alkali silicates and zirconates, as well as double salts, whereas low-temperature adsorbents cover conventional porous materials such as zeolites, carbon-based materials (e.g., activated carbon, carbon nanotubes, carbon nanofibers, graphene), and molecular sieves, as well as recent classes such as MOFs, porous polymer networks (PPNs), and covalent organic frameworks (COFs).46-51 The high-temperature adsorbents are all chemisorbents, whereas the low-temperature adsorbents are chiefly physisorbents.52 Supported amines are among the low-temperature adsorbents, but they are chemisorbents that have strong interactions with CO2.
The efficiency and economics of adsorption processes such as pressure/temperature swing adsorption (PSA/TSA) are largely dictated by the characteristics of the adsorbents in addition to process design and operation factors.53, 54 Generally, for any gas-separation application, adsorbents are required to satisfy several criteria to be efficient for large-scale separation. These metrics include high working capacity and selectivity, low cost, low regeneration requirements, long-term stability, and fast kinetics.52 In addition to the physiochemical properties of the adsorbents, cycle configuration, number of steps, cycle time, operating pressures or temperatures, and number of beds are some of the other important process parameters that need to be optimized for optimum capture efficiency.
In the context of adsorptive CO2 capture, the majority of laboratory-scale studies often overlook the performance of adsorbents under practical conditions. For instance, competitive water adsorption; the structural, mechanical, and chemical stability to moisture that often exists in gas effluents; and the stability in the presence of other pollutants such as SOx, NOx (especially for postcombustion process), and fly ash are often not investigated. Retaining working capacity over many adsorption–desorption cycles and thermal management are other important criteria that are often overlooked in the design or screening of the adsorbents. In addition, most of the current studies fail to consider the ultimate process performance metrics in developing new materials. Although the conventional zeolite 13X material is still the best choice in terms of capture costs for postcombustion under dry flue gas conditions, relative to that of the best MOFs, such as HKUST-1 and Mg-MOF-74,55, 56 its water coadsorption is still problematic and requires a guard bed or a dehydration unit before the PSA or TSA unit. Further, although several MOF materials show outstanding capacity and selectivity towards CO2, their large-scale production and water stability remain a challenge for their wide-spread adoption in industry. The design of composite adsorbents (such as zeolite or MOF-functionalized amines) could address the known problems of conventional adsorbents and enable a cost-effective and highly efficient capture approach for postcombustion CO2 capture.
Adsorption-based CO2 capture by PSA has attracted a great deal of attention owing to the simple process, low energy required, and low cost.57 However, low recovery for CO2 is still challenging for this approach.54 Generally, for postcombustion CO2 capture, vacuum swing adsorption (VSA) and TSA are more appropriate than PSA, whereas (mainly because of large pressure drops in flue gas application), PSA is more promising for the precombustion process.58-61 Attrition of adsorbent particles is another common issue with the process operation. The use of structured adsorbents such as monoliths or hollow fibers can address both pressure-drop and attrition problems and still allow for rapid operation of cycles. Rapid swing cycles can enhance process throughput, which thus allows for smaller adsorbent inventory and column size. The TSA process for CO2 capture is still far from large-scale implementation as a result of the high energy requirement for adsorbent regeneration and the long cooling step time. Novel approaches that offer heat-management options such as hollow-fiber adsorbents with a cooling medium flown in the bore side or monolithic structures with optimum thermal management could address these scale-up challenges.43, 62-64 Moreover, in terms of process design, modifications to the original design that include novel indirect heating techniques such as heating jackets, heat exchangers, or coils could be implemented to reduce the energy consumption further and/or to shorten the required cooling time in the TSA process.58, 65-68
Although adsorption-based separation can address most of the limitations of the absorption processes, the current technologies developed/proposed so far are not cost effective at their current stages of development. Furthermore, no large-scale operation has been fully deployed yet.69-71 The design, development, and evaluation of high-performance adsorbents should be tightly coupled with evaluation of their practical performances and process considerations. Moreover, cycle design, configuration, and optimization of any cyclic process should take into account the characteristics of the specific adsorbent to be eventually used.
2.4 CO2 capture by chemical looping
Chemical-looping combustion (CLC) and chemical-looping reforming (CLR) are two processes that are considered to be potentially cost-effective CO2-capture options with minimum energy losses, in which both CO2 and H2O are inherently separated from flue gas.72 They can also largely minimize NOx formation during the reaction. For precombustion CO2 capture, chemical looping can be combined with IGCC to produce syngas as a valuable byproduct. These technologies use a metal oxide as an oxygen carrier to circulate oxygen between the air and fuel reactors; thus, their large-scale applications are highly dependent upon the availability of suitable oxygen carriers. High oxidation/reduction activity, mechanical stability (in fluidized beds), resistance to agglomeration, high melting point (to withstand the reaction temperature and avoid agglomeration), long-term stability under repeated oxidation/reduction, as well as cost and environmental impacts are key characteristics of metal oxides (mainly transition-metal oxides such as Fe, Cu, Co, Mo, Mn, Cr, Nb, V, Ce, and In oxides) for chemical-looping processes.72 Of these properties, reactivity in both oxidation and reduction cycles is the most important criterion that should be considered. Moreover, these oxides should be capable of completely combusting the fuel to achieve maximum combustion efficiency. None of the current oxygen carriers investigated so far are capable of fulfilling all of the above requirements at once.
Another challenge associated with chemical-looping processes is the high-pressure operation required to achieve high overall efficiency, though high pressures may be favorable for CCS applications. Recent energy analyses have indicated that the calcium-looping postcombustion process in particular can incur an efficiency penalty of only 6–8 %.73-76 Such a low energy penalty stems from improvements in the original design of the two-bed (carbonator and calciner) Ca-looping process such as incorporating an extra heat-recovery bed to exchange heat between the CO2 stream and the solid particles entering the calciner. Currently, most of the chemical-looping technologies considered for the power-generation sector are at the laboratory or concept stage of development with a few pilot-scale studies currently under investigation,77-79 and it is projected that their full deployment will not take place before 2030.5 The technical hurdles from materials development and process design should be overcome to improve the current state-of-the-art chemical-looping technologies. In that regard, novel chemical-looping processes based on composite metal oxides such as Ca/Cu that provide the possibility of coupling endothermic and exothermic reactions in the same solid matrix could result in higher capture efficiency and lower equipment cost.
2.5 Direct capture of CO2 from air
Direct removal of CO2 from ambient air, referred to as direct air capture (DAC), has recently gained significant attention among researchers, because it could minimize the problems associated with transporting large volumes of CO2 from point-source emitters to sites suitable for geological sequestration.80-84 In addition, unlike conventional capture processes that target only large-point sources and can, at best, slow the rate of increase in the atmospheric CO2 concentration, DAC, if widely adopted, can reduce atmospheric CO2 levels. Although the concept is essentially similar to that of adsorption-based CO2 capture, owing to the ultradilute nature of CO2 in air (≈400 ppm), the DAC technology has daunting technological challenges. As a result of the ultralow concentration of CO2, materials with strong binding affinities (sharp uptake at low partial pressures) and high CO2/N2 selectivities are required for this technology. Various aqueous hydroxides such as calcium hydroxide solution, NaOH and KOH solutions, and solid materials including alkali and alkali-supported carbonates, anionic-exchange resins, amine-functionalized metal oxides, and MOFs have been evaluated for DAC.84 Notably, not every high-performance material that works well for large point source CO2 capture would necessarily perform at an acceptable level for the DAC process, mainly because of the differences pointed out above.
Recent thermodynamic analyses have indicated that the TSA process is thermodynamically more efficient than the PSA process for DAC applications, as the heat of adsorption or adsorbate affinity increases at dilute CO2 concentrations.85 At this point, the estimated DAC cost is significantly higher than that of capture from large point sources (30–1000 and 30–100 USD ton−1, respectively). Such a huge uncertainty in the design considerations and economic analysis of the DAC process must be addressed by clearly laying out the underlying assumptions. Moreover, to apply air capture on a large scale, materials that are low costing with high durability are required. Although still in the early stages of development, any DAC process must minimize the cost for its adoption and implementation in society. Using a minimal amount of energy, ideally from a distributed renewable source such as solar thermal energy, would be a potential pathway toward enhancing the feasibility of the DAC process.
2.6 CO2 capture by hybrid processes
One of the promising approaches that offers a cost-effective, scalable, and sustainable capture path involves hybrid separations that combine two or more capture subsystems. The concept of hybrid processes in gas separation and reaction has been previously applied to several applications. Hybrid technologies take advantages of having two or more separation units in parallel or in series with the aim to enhance separation efficiency while at the same time decreasing the overall cost of separation. The feasibility of several hybrid concepts for CO2 capture such as membrane–PSA and membrane–distillation have been investigated.86-90 A promising hybrid system with potential energy savings has recently been developed by American Air Liquide, by which subambient-temperature (−50 to −20 °C) CO2 capture is performed through a hybrid membrane–cryogenic distillation process.91 This hybrid process aims at retrofitting existing pulverized coal-fired power plants and is sought to enhance both the productivity and selectivity of the membrane module, which thus reduces both the energy and capital costs of the capture process. For this technology to be widely adopted at a large scale, a good heat-integration strategy is crucial, as all of the feed gas needs to be cooled to subambient temperatures.
For hybrid membrane–PSA systems, the idea is to use the high-pressure membrane permeation stream in the pressurization and high-pressure adsorption steps of a typical PSA process. In addition, membrane permeation could be incorporated into the blowdown step of the PSA cycle so that the operating pressure of the PSA can be used as the driving force for membrane permeation. Both these options can result in significant savings by eliminating the need for high-duty pumps.
Another example of such an integrated system is the hybrid pressure–temperature swing adsorption process (PTSA), which can be operated at moderate pressures and temperatures and thus leads to a lower energy cost for capture.92, 93 For this configuration, the need for the high vacuum level that is typically required in PSA to achieve high-purity CO2 or the high temperature that is often essential in TSA to achieve high recovery can be dramatically avoided, which thus leads to less expensive operation, faster cycles, and longer service life of the adsorbent.92 The general idea for designing PTSA is to obtain effective mass transfer during the adsorption step and effective heat transfer during the desorption step.94
Overall, on the basis of the current status of state-of-the-art technologies, hybrid processes that combine two or more capture routes should be considered as a novel approach to improve separation efficiency and cost. However, it is imperative that the future of related research focuses on the complete understanding of these hybrid processes from the perspectives of feasibility, process design, materials selection, environmental impact, and overall cost reductions by taking into account uncertainty factors.
2.7 Summary
In summary, despite significant progress within the past few years, most CO2-capture technologies still have a long way to go to become commercially available in various industries. It appears that a bridge between materials scientists and engineers is crucial to fill the gap between characteristics of the materials and process performance. Thus, investigation of the relationship between properties of the materials and the hybrid process parameters is crucial to build a unique and comprehensive strategy for the design of highly efficient and cost-effective next-generation capture technologies that greatly contribute to reducing CO2 emissions. Table 1 provides a summary of the current challenges and future opportunities related to various CO2-capture methods discussed in this section.
Capture technology |
Challenges |
Opportunities |
|---|---|---|
absorption |
equipment corrosion |
improvement in commercially available absorption technologies |
|
amine degradation |
the use of ILs |
|
high regeneration energy requirement |
the use of advanced amines |
|
high overall energy penalty |
|
|
environmental impact |
|
membrane |
energy intensive for postcombustion application |
composite hollow-fiber membranes |
|
high fabrication cost of novel membranes |
MMMs |
|
not suitable for high-temperature applications |
hybrid membrane–cryogenic processes |
|
trade-off between purity and recovery |
|
|
low selectivity |
|
adsorption |
long-term stability to impurities and moisture |
composite adsorbents |
|
thermal management |
structured adsorbents |
|
pressure drop and adsorbent attrition |
rapid swing cycles |
|
|
hybrid membrane–PSA processes |
chemical looping |
high-pressure operation |
composite oxides as oxygen carriers |
|
efficient and stable oxygen-carrier materials |
process-design modifications |
direct air capture |
ultradilute CO2 content |
DAC coupled with renewable energy sources |
|
energy intensive |
structured adsorbents |
|
development of durable materials |
|
hybrid capture processes |
less studied |
membrane–distillation |
|
enhancement of synergy and process optimization |
membrane–PSA |
|
development of hybrid materials |
PTSA |
3 CO2 Utilization Options: Challenges and Opportunities
The utilization of CO2 can be considered as a viable option for providing a renewable energy source for the production of various valuable products. The process is required to be economically viable, safe, and ecofriendly.95 The primary utilization route can be classified as enhanced oil/gas recovery, chemical conversion, mineralization, and desalination. Figure 2 shows various ways in which CO2 can be utilized. Notably, the U.S. Department of Energy (DOE) categorizes CO2-utilization technologies into four main research areas that the CCS program supports: cement, polycarbonate plastics, mineralization, and enhanced hydrocarbon recovery.
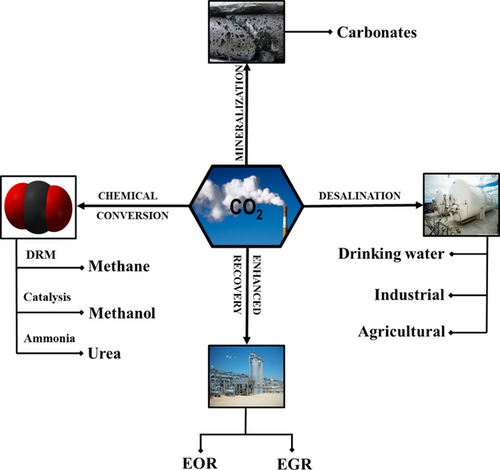
Various carbon-utilization pathways.
CO2 is typically formed as a byproduct during ammonia synthesis. Additionally, it is produced during the synthesis of ethylene oxide in oil refinery and during the fermentation process.3 As a raw material, CO2 is commonly used in the beverages industry, in food conservation, in urea production, in water treatment, in enhanced oil recovery, in chemical production, and in polymer synthesis with a current global usage of 232 Mt year−1.4, 96 However, currently, less than 1 % of CO2 emitted into the atmosphere is utilized as a raw material in the above industries.97, 98 Efforts should be put forth to utilize the already-captured CO2 into valuable commodities such as transportation fuels and fine chemicals.
3.1 Enhanced oil/gas recovery
Enhanced oil/gas recovery (EOR/EGR) refers to a procedure in which a substance is injected into a reservoir to repressurize rock formation and to release any oil/gas that may have been trapped in the formation. During the CO2 EOR process, the injected CO2 mixes with the oil and releases it from its otherwise hard-to-recover rock formation. This stream is then pumped to the surface, and the CO2 emerging with the oil is separated and resupplied into the cycle to repeat the process. This process often yields more barrels per reservoir than the traditional oil-recovery methods.99, 100 Basically, CO2 flooding is one of the most common and efficient methods used in EOR, as it mixes with the oil, which expands it and makes it lighter and easier to recover.101 Most CO2 EOR systems use naturally occurring CO2, but lately, research has focused on using CO2 captured from potentially hazardous gas streams, such as flue gas and other industrial gas effluents.102 Two commonly used CO2 EOR methods are continuous gas injection (CGI) and water alternating gas (WAG), and the latter method yields better oil recovery.103 In CO2 EOR, the addition of an intermediate hydrocarbon such as propane can improve displacement efficiency and the diffusion coefficient, which thereby further increases the recovery efficiency.104 In general, the efficiency of CO2 EOR depends largely upon the temperature and pressure of the reservoir involved.105
There are numerous challenges faced by CO2 EOR methods. For instance, owing to the heterogeneity of the rock formation between the wells, fluid properties and capillary pressure reduce the effectiveness of CO2 flooding.106 Furthermore, a large number of parameters such as fluid production rates, compensated neutron log (CNL), and production log are required for efficient execution.106 Despite these shortfalls, CO2 EOR/EGR has drawn significant attention and is predicted to rise rapidly in the near future. Overall, CO2 EOR/EGR is a promising approach in enhanced oil/gas recovery, with applications in most type of reservoirs. Despite this, EOR currently contributes to only 3 % of CO2 utilization. Although advances in this field have been retarded by the price of CO2, its use is steadily growing, and numerous facilities having implemented this method in their reservoirs.107-109
3.2 CO2 as feedstock for production of fuels and chemicals
CO2 utilization is expected to overcome the known challenges associated with CCS such as high cost, public acceptance, and long-term uncertainty. Additionally, it makes CO2 capture worthy and can be substituted partially for fossil fuels as the main source of energy.110 It can open up new avenues for developing sustainable technologies that supplement the conventional fossil-based resources.
3.2.1 Fuels production
CO2 conversion into fuels is considered the best route in CO2 utilization. Methane, methanol, syngas, and alkanes are some of the compounds that can be produced by utilizing captured CO2 as a feedstock. The fuel produced can be used in various sectors, including fuel cells, power plants, and transportation.4 There are tremendous pathways for producing fuels by CO2 utilization. Given that CO2 is a thermodynamically stable molecule, its utilization requires the application of a large amount of heat and catalyst inventory to obtain high fuel yields.97 In the context of fuels production from captured CO2, hydrogenation and the dry reforming of methane (DRM) are the two most-important pathways.111
CO2 hydrogenation is a very promising route for CO2 utilization, mainly because it offers the possibility of recycling CO2, storing H2, producing fuel, and solving the issue of electric energy storage.98 DRM is also considered one of the most-important pathways for the production of methanol and a variety of other liquid fuels by the Fischer–Tropsch (FT) process.112-114 In the hydrogenation of CO2 into methane,115 methanol,116 carbon monoxide,115 and formic acid,117 the source of hydrogen from fossil fuel appears to be problematic, as this can itself lead to an increase in CO2 emissions to the atmosphere. However, renewable energy (e.g., solar, wind, biomass) can be alternatives to fossil sources to mitigate additional CO2 emissions during hydrogenation.3 Recently, Audi motor company's “e-gas” in Germany has produced 1000 Mt year−1 of methane by CO2 hydrogenation.118
For transportation, methane is not valuable as a fuel, because it has a low volumetric gas density. Besides, its global warming potential (GWP) is 30.97 Producing more methane will not be profitable to CO2 capture because of its availability (methane is plentiful in natural gas, shale gas, coal gas, and landfill gas). Rather, CO2 hydrogenation to methanol appears to be a better pathway.119 However, C−H bond activation over current (mainly Cu-based) catalysts for methanol production is very challenging, and the catalysts tested so far are not economically attractive.120-122 Although methanol has many applications in paints, plastics, combustion engines, and organic solvents,97 its production contributes to reducing CO2 emissions by only 0.1 %.123 CO2 conversion into CO from the reverse water–gas-shift (RWGS) reaction is one of the most-important routes for CO2 utilization, because CO is a raw material for the synthesis of methanol and hydrocarbon fuels through the FT reaction.122 Despite this, the endothermic nature of the RWGS reaction and the low conversion at moderate temperatures are the two main bottlenecks to deploying large-scale methanol production from CO2 through the FT process. Additionally, developing active catalysts that can accelerate the reaction kinetics and maximize the yield represents another significant barrier.
Recently, DRM has attracted significant research interest in terms of using CO2 for syngas production.124-126 Typically, the purity of syngas that is produced by DRM is higher than that produced by partial oxidation and steam reforming.127 In addition, the amount of unreacted methane in the DRM process is only 2 %, which is less than that in steam reforming, and this makes it possible to apply DRM at remote natural gas sites for the production of liquid fuels, which are easier to transport than gaseous fuels.113 Ni, Ni–Co, Ru, Ir, and Rh supported on silica, alumina, and lanthanum oxide have been extensively evaluated in the DRM reaction.128 Despite significant advances in the development of catalysts with high activity and optimum stability for DRM, finding a suitable catalyst for this reaction still remains a big hurdle, especially at high operation temperatures, as deactivation by coke formation is inevitable at high temperatures (>700 °C).129-133
Oxidative dehydrogenation of light alkanes to alkenes (ODA) with CO2 as a soft oxidant (instead of O2, which is typically used in dehydrogenation processes) is another attractive approach that can reduce the amount of coke formation and maintain the stability of catalysts at elevated temperature.134-138 Moreover, CO2 enhances the equilibrium conversion of the oxidative dehydrogenation of light alkanes by removing hydrogen through the RWGS reaction.139 However, care must be taken in monitoring the temperature, as excess heat can cause the olefins to undergo overoxidation, which yields carbon oxides and results in low selectivity.140 CO2 also forms the redox cycle and produces active oxygen species. The role of CO2 in the ODA and the mechanism of this reaction are unclear and are dependent on the nature of the active sites and the reducibility of the metal and its supporting material.141 Despite initial high activity, the catalysts investigated so far suffer from low stability.
It is apparent from the above discussion that the main hurdle in utilizing captured CO2 as a feedstock for the production of synthetic fuels lies in the design and development of novel catalysts that not only exhibit high catalytic activity under different reaction conditions but that are also resistant to coke formation and show long-term chemical and structural stability.
3.2.2 Chemicals production
In addition to synthetic fuels, CO2 can be used as a feedstock to produce a large array of fine chemicals. The most important applications are urea (≈160 Mt year−1), inorganic carbonates (≈60 Mt year−1), polyurethane (≈18 Mt year−1), acrylic acid and acrylates (10 Mt year−1), polycarbonates (4 Mt year−1), and alkylene carbonates (a few kt year−1).4 Urea, as a major fertilizer, has the largest market for CO2 utilization.4, 142 It is also widely used as a feedstock in polymer synthesis, pharmaceuticals, fine chemicals, and inorganic chemicals such as melamine and urea resins.143, 144
Organic carbonates such as acyclic (linear) carbonates [e.g., dimethyl carbonate (DMC), diallyl carbonate (DAC), diethyl carbonate (DEC), and diphenyl carbonate (DPC)], cyclic carbonates [e.g., ethylene carbonate (EC), propylene carbonate (PC), cyclohexene carbonate (CC), and styrene carbonate (SC)], and polycarbonates [e.g., poly(propylene carbonate) and bisphenol polycarbonate (BPA-PC)] that have many applications in pharmaceuticals, agrochemicals, polymers, lubricants, coating, and catalytic reactions are another class of chemicals that can be produced from captured CO2.95, 124 The challenges of this process arise from operation at high temperatures and pressures and the need for high catalyst inventory. Moreover, the separation of the catalyst from the products is also another challenge in this process.95, 124 In the production of polycarbonates from the reaction of CO2 with epoxides, commercially available Al-based catalysts are widely used, but they are not environmentally friendly. In this regard, the oxidative carboxylation route is an alternative with great potential to synthesize polycarbonates from CO2 and olefins.113 Polyurethane is another chemical produced by the reaction of CO2 with cyclic amines such as aziridines and azetidines or the N-analogues of epoxides.113, 145
Another important chemical that can be obtained through CO2 utilization is formic acid. Hydrogenation of CO2 into formic acid has recently attracted some interest mainly due to the mild reaction conditions, the lack of formation of byproducts, the ability to store hydrogen in liquid form, and the easy decomposition of formic acid into hydrogen and CO2.98, 143
Biological utilization of CO2 offers another pathway for the production of biodiesel and various biomass-derived commodity chemicals (used as food, silage, biogas, and fertilizer).146 The advantages offered by this approach include higher growth rate, shorter growth cycle, no competition on land with other plants, and the production of different valuable byproducts. However, captured CO2 should be purified prior to feeding into a photobioreactor to remove pollutants such as SOx, NOx, and heavy metals that are toxic to the growth of microalgae.9
In addition to EOR, utilizing CO2 as a technological fluid without conversion into chemicals has found applications in many industries, including the air-conditioning (as coolant), solvent, dry-washing, food-preservation, and beverage industries.4, 96, 98 Generally, EOR consumes 50 Mt year−1 CO2, whereas 8 Mt year−1 CO2 is consumed in the food and beverage industries.147
Overall, although there is a great market for turning captured CO2 into chemicals and fuels, the proposed laboratory-scale technologies are still far from industrial commercialization. The reason for this is partly because the materials investigated so far are expensive to make yet are not chemically stable and partly because, in most cases, CO2 conversion rates and overall yields of the main products are low and thus do not meet the requirements for large-scale deployment. Moreover, there still exists limited understanding of the reaction mechanisms involved in the chemical transformations of CO2. Evaluation of the process requirements and considerations is also overlooked in this field.
3.3 Nongeologic storage of CO2 (mineralization)
Nongeologic storage or mineral carbonation of CO2 results in the production of stable mineral carbonates by treating CO2 with metal oxides such as calcium and magnesium oxides that are naturally abundant in the form of mineral silicates.7 The carbonation of magnesium and calcium silicates through spontaneous reaction with atmospheric CO2 under ambient conditions is a naturally occurring process (known as natural weathering) that is thermodynamically favored yet very slow.4 Artificial improvement in the carbonation kinetics can be achieved by injecting fluids with a higher concentration of CO2 and by increasing the temperature. Despite significant efforts devoted to accelerating this reaction, the slow kinetics are still the main drawback in scaling up the mineralization process.4 Additionally, this process is energy intensive, as it requires the extraction, processing, and transportation of the rocks, as well as high pressures (10.0–15.0 MPa) and temperatures (150–600 °C) to achieve a carbonation efficiency higher than 80 %.148 Also, the duration of the carbonation reaction is very long (6–24 h), and the rocks should be mined (<37 μm). Large plant sizes and the need for additives to extract reactive species and separate (or dispose of) reaction products are other components with high cost penalties.148 In a sense, the mineralization process may be viewed as a sequestration method, because it aims at permanently fixing CO2, but unlike CCS, which suffers from leakage (geological storage of CO2), the carbonates are stable and safe.149 Also, the exothermic nature of the mineralization reaction along with the geothermal gradient (up to 20 °C km−1) contribute to a reduction in energy consumption. Moreover, as pure CO2 is not required for this process, flue gas can be used directly without removing impurities such as SOx and NOx.147
To address the operational and technological drawbacks associated with the direct carbonation process, indirect carbonation (indirect storage) can be implemented in multiple reactors. In this method, high carbonation efficiency and purity can be obtained in the presence of additives under mild conditions and short time periods.149, 150 Another advantage of this process is the production of diverse commodities such as magnesium/calcium carbonate, iron oxide, and silica that can compensate the process cost. However, as this process is complex, optimizing the operation conditions should be performed separately for each step.4 In addition, the energy cost is still a main obstacle that prohibits its commercialization.7, 149 To reduce energy costs, materials such as acetic acid, ammonium salts, and sodium hydroxide can be used instead of hydrochloric acid, which is typically used in this process.149, 151 Accelerating the reaction kinetics through advanced materials is one way to improve the efficiency of the process.150 Indirect mineral carbonation is considered to be the most useful process, and it has the potential to be scaled up in the near future.152 A near-complete in situ CO2 mineralization process in Basaltic rocks has recently been achieved within a two-year timeframe.153
The possibility to upgrade alkali-metal wastes into high commercial value-added products such as high-purity precipitated CaCO3 through carbonization is a promising approach that should be the focus of future research in this field.149-151, 154 As an example, carbonation of Ca-carrying cementitious materials through reaction with CO2 results in the development of high early-stage strength for building materials applications, and a CO2 uptake of 7–12 % is achieved in the process.155 To date, only a few projects based on the utilization of inorganic wastes have moved to the commercial or small-scale demonstration phase. For example, retrofitting a cement plant in Texas by SkyMine (Capitol Aggregates) to reduce its carbon emissions by 15 % (83 000 t year−1) through the direct transformation of flue gas into marketable products, such as sodium bicarbonate, hydrochloric acid, and bleach, is approaching the commercialization step. In another example, a pilot-scale demonstration of the mineral carbonation process on the basis of the utilization of coal fly ash to reduce CO2 emissions has been installed at a 2120 MW coal-fired power plant in Point of Rocks, WY, USA.156
3.4 Desalination and water production
As another promising utilization approach, captured CO2 can be used to remove total dissolved solids (TDS) and to transform brine into water.157-159 The resulting potable water can be utilized in places for which there is a deficiency.6 Whereas most desalination plants do not employ CO2 to perform desalination owing to economic constraints, new technologies are being developed for the cheap and efficient utilization of CO2 in this process. If sea water, mixed with ammonia (to weaken the salt molecules), is exposed to CO2, already-weak bonds start to form, which leads to the removal of the ions from the water phase.159 The products formed, Na2CO2 and NH4Cl, are heavy and, thus, can easily settle to the bottom of the tank. The latter NH4Cl can be recycled by thermal operations with calcium oxide or be used as a feedstock for the synthesis of ammonia and chlorine. The hydrate-forming method is another technique that is used for desalination, and it involves the formation of hydrates by using CO2 to separate the salts from water.160, 161 In this approach, CO2 can be in either the gas or liquid form. The CO2 hydrates are either dumped into the ocean or transported elsewhere.162 The ammonia–carbon dioxide forward osmosis process is yet another desalination technique that employs CO2.158 In this process, the driving force is osmotic pressure instead of hydraulic pressure in reverse osmosis, and by using a “draw” solution, the brine and fresh water are separated.
One problem commonly faced by the desalination processes is the brine waste that is generated in very large quantities during the process.163 Additionally, high salt concentrations, solvent chemical residues, and metal corrosion have the capacity to destabilize the local ecosystem.164 To overcome these problems, the use of three main units, namely, carbonation, filtration, and recovery, has been proposed for chloride and amine compounds.165
Desalination is unlikely to penetrate the market without any significant cost advantages. According to a recent DOE report published in 2013, the cost of potable water production with approximately 21 ppm TDS from 233 000 ppm brine by using three stages of CO2-based clathrate desalination is estimated to be 3.17 USD Kgal−1.kilogallon (1 gal≈3.8 L).166 Depending on the source of brine, the cost could vary significantly. The estimated desalination costs are currently higher than agricultural or municipal water costs, which thus makes CO2-based desalination technology less attractive to address the water market. In particular, given the high cost of providing agricultural-quality water by CO2-based desalination, it is highly unlikely that this technology could be adopted to supply agricultural water. Overall, although CO2 remains an attractive option for desalination, the current cost of potable water produced by the CO2-related technologies is far from being competitive.
3.5 Summary
In summary, the future prospects of carbon-utilization technologies are bright, and there is huge potential in various industries to market the utilization of captured CO2 as a renewable resource instead of permanently sequestering it underground or in the oceans. It is expected that with future research and development on the key components of CO2 utilization discussed above, the majority of proposed or emerging technologies related to CO2 utilization will continue to have lower costs, which will make more of the fine chemicals, fuels, and water markets addressable with sustainable CO2-based processes. Adopting these emerging technologies depends to a great extent upon their cost effectiveness. To date, only a small fraction of CO2 captured from gas effluents has actually been used to provide energy or to produce other value-added products. The estimated high costs along with the low efficiency of these technologies are the two key factors responsible for such a slow progress. Table 2 provides a summary of the current challenges and future opportunities associated with various CO2-utilization routes discussed in this section.
Utilization technology |
Challenges |
Opportunities |
|---|---|---|
chemical conversion |
high operating conditions |
dry reforming of methane |
|
complexity of reaction pathways |
catalytic reduction to formic acid and its derivatives |
|
stability of catalysts to coke formation |
noble-metal-doped transition-metal catalysts |
|
low conversion and product yield rates |
biological pathways to synthetic fuels |
|
catalyst regeneration |
oxidative dehydrogenation |
|
development of highly selective catalysts |
|
enhanced oil/gas recovery |
transportation of CO2 |
water alternating gas (WAG) system |
|
large number of parameters involved |
compensated neutron log (CNL) |
|
fluctuations in oil price |
|
mineralization |
slow kinetics |
indirect carbonation |
|
high-pressure and high-temperature operation |
utilization of inorganic wastes |
|
expensive to implement |
|
desalination |
equipment corrosion |
providing potable water to residential and municipal customers |
|
expensive operation |
possible implementation is various regions |
|
large amount of brine waste |
modified Solvay process |
4 Combined CO2 Capture and Utilization
Combining capture and utilization offers an efficient strategy to minimize the high energy requirements for direct chemicals/fuels production from waste-gas streams, especially if the capture and utilization are performed at the same temperature. Such process intensification will lead to smaller, cleaner, and more energy-efficient technologies. The concept of hybrid processes in gas separation and reaction has been previously applied to several applications. For instance, membrane reactors (MRs) combine membrane separation with a chemical reaction in one unit. MRs are capable of promoting a reaction process by selectively removing at least one of the products through the membrane from the reaction zone, which makes the equilibrium reaction shift to the product side.167-169 The sorption-enhanced reaction (SER) is a similar concept that combines adsorption with a reaction in a single unit. SER has been widely applied to the water–gas-shift (WGS) reaction for the production of high-purity hydrogen.170-176 In this process, the in situ capture of CO2 allows the thermodynamically constrained WGS process to operate at higher temperatures (i.e., 350 °C), at which the reaction kinetics are more favorable.175 Such novel concepts could be adopted for simultaneous CO2 capture and utilization in various industries. The schematic of the proposed capture–utilization process is illustrated in Figure 3.
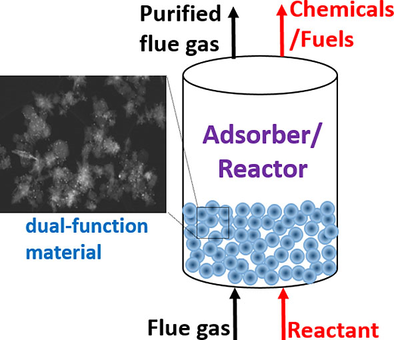
Schematic of the proposed combined carbon capture–utilization process.
The concept of producing chemicals and fuels directly from industrial flue gases over a dual-function material has been previously applied to the production of syngas (CO and H2), a useful reactant for methanol synthesis.114, 177, 178 In this process, called tri-reforming of methane, a synergetic combination of CO2 reforming, steam reforming, and partial oxidation of methane occurs in a single reactor at 850 °C with supported nickel catalysts.114, 180, 181 In another example, the in situ capture and methanation of CO2 has been studied over a dual-function material in the form of a coated monolith to produce synthetic CH4 by using H2 at 320 °C.179-182 Hydrogenation of CO2 is another example of combined capture and reduction that has been recently reported.183 Simultaneous capture and mineralization of coal combustion flue gas CO2 is another example of a hybrid process that has been demonstrated at a pilot scale in the USA.156
Such integrated systems could provide a solution to both the energy and environmental problems currently encountered worldwide. However, creation of novel in situ capture–conversion technologies requires advancements in both materials science and process engineering. Given the different natures of adsorption and catalysis, the fundamental aspects of hybrid adsorbent/catalyst characteristics in conjunction with process considerations and operation conditions should be carefully studied to obtain a highly efficient and cost-effective technology. Also, for the direct utilization of waste-gas streams, the resistivity of materials (especially catalysts) to impurities may pose a challenge, and efforts should be undertaken to ensure the reliability of the materials for a long service life.
5 Outlook
In this short review, the current challenges and future opportunities of carbon capture and utilization technologies were presented and discussed from perspectives of efficiency and cost. Indeed, recent years have witnessed significant advancements in the design and development of various CCU technologies with a few cases being deployed on an industrial scale. However, the majority of technology options being considered so far are still at the laboratory-scale stage of development. In both scenarios, commercial implementation of novel materials that outperform the current state-of-the-art materials in each respective technique will certainly decrease the energy requirements of both capture and utilization processes. However, the research and development of materials concepts should be coupled with process performance considerations to evaluate better their potential under real conditions. Having such a holistic view of both materials and processes and a mutual communication between materials scientists and engineers will help to accelerate dramatically the scale-up of CCU technologies. In addition, small-scale evaluation of materials or processes should take into account the large-scale implementation requirements to provide a realistic evaluation of the performance and to reduce the uncertainties in estimating the associated costs. Cost effectiveness is the ultimate factor determining the feasibility of the adoption of many emerging CCU technologies.
The long-term stability of the materials used in most CCU methods is an important consideration that not only impacts the system performance but also affects the economics of the process. In the context of utilization, the production of fuels and chemicals and the use of renewable energy sources in particular can bring down the total cost while providing a sustainable approach for the production of value-added products. In most technologies, system integration and process intensification will enable a cost-effective approach to improve the separation and thermodynamic efficiency; however, the complexity of the operation and other related issues should also be considered.
Hybrid processes that either couple CO2-capture subsystems or offer simultaneous capture and utilization approaches should be the focus of future research, as thermodynamic analyses of such systems have highlighted their energy efficacy and cost effectiveness (by reducing both capital and operating costs). Although the emerging hybrid systems appear to have many hallmarks of next-generation CCU technologies, more research (e.g., materials development, processes operation requirements) and development (e.g., synergistic assessment studies and process scale-up) are required for these emerging technologies to become commercially available in the near future. Any feasibility study should also include other considerations related to environmental impacts, risk assessment, and life-cycle analysis.
Acknowledgements
Funding was provided in part by the University of Missouri Research Board (UMRB).
Biographical Information
Ahmed Al-mamoori received his B.Sc. in chemical engineering from Al-Nahrain University, Baghdad in 2005 and his M.Sc. from Al-Nahrain University, Baghdad in 2009. He is currently pursuing his Ph.D. in chemical engineering at Missouri University of Science and Technology. His research interests include CO2 capture and utilization, adsorption, catalysis, and reaction engineering.
Biographical Information
Anirudh Krishnamurthy received his Btech. in chemical engineering from Alagappa College of Technology, Anna University, India in 2014 and is currently doing his M.S. in chemical engineering at Missouri University of Science and Technology. His research interests include CO2 capture, adsorbent materials, and volatile organic carbon emissions control.
Biographical Information
Dr. Rownaghi is an assistant research professor of Chemical and Biochemical Engineering at Missouri University of Science and Technology. He obtained his Ph.D. degrees in Catalysis from University Putra Malaysia (UPM) in 2008. Following his postdoctoral research at Georgia Tech, he began his academic career at Missouri S&T in 2014. Research in his group is focused on creating, understanding, and rationally engineering advanced materials for catalysis, membrane, and barrier applications through innovative and scalable processing strategies.
Biographical Information
Dr. Rezaei is an assistant professor of Chemical and Biochemical Engineering at Missouri University of Science and Technology. She obtained her Ph.D. degrees in Chemical Engineering from Monash University in Australia and Luleå University of Technology (LTU) in Sweden in 2011. She worked as a postdoctoral fellow at Georgia Tech before she joined Missouri S&T in 2014. Her research focus broadly lies at the interface of chemical, materials science, and environmental engineering, where the general goal of her work is to develop advanced materials and processes for clean energy and sustainable chemical processes.