Performance Enhancement of Evacuated Tube Solar Collectors Using Fe3O4 Nanoparticle-Enhanced Phase Change Materials for Efficient Thermal Energy Storage
ABSTRACT
One of the most efficient solar thermal systems for water heating and thermal energy storage is the evacuated tube solar collectors (ETSC). Glass tubes are vacuum sealed to minimize heat loss while maximizing solar absorption. This study aims to enhance ETSC performance with the use of iron oxide (Fe3O4) nanoparticles infused phase change materials (PCM). The Fe3O4 concentration was varied at three levels: 0.2 wt.%, 0.4 wt.%, and 0.6 wt.%, and they were tested in terms of fluid temperature, heat absorption, thermal efficiency, and exergy efficiency by experimental analysis. The results show that the inclusion of Fe3O4 nanoparticles greatly increases thermal conductivity and thus improves heat transfer and storage. With growth of 0.6 wt.% Fe3O4, the fluid temperature increased from 58.4°C to 94.5°C, and heat absorption increased from 2516.9 W to 3804.1 W, increasing thermal efficiency from 46.1% to 83.5% and exergy efficiency from 31.2% to 45.2%. To decrease the irreversibility, energy efficiency was improved. The proposed system exhibited enhanced thermal diffusivity, reduced subcooling, and micro-convective currents due to the addition of Fe3O4 nanoparticles. The potential use of Fe3O4 based Ne-PCM was experimentally verified, suggested as a sustainable, energy-efficient thermal storage material for solar thermal applications.
1 Introduction
The evacuated tube solar collectors (ETSC) are high-efficiency solar thermal devices that capture and transport solar energy. Multiple glass tubes with vacuum insulation reduce heat loss and improve thermal performance. ETSCs transform solar radiation into heat for a working fluid [1, 2]. In different weather circumstances, their vacuum design decreases convective and conductive heat losses, making them efficient. These collectors are popular for water, space, and industrial process heat. Integration with PCM or nanofluids boosts efficiency and thermal storage [3]. ETSCs outperform flat plate collectors in colder climates because of their stronger insulation [2]. They are ideal for sustainable energy applications because of their thermal efficiency and durability [4].
Nano-PCM improves thermal characteristics by combining PCM with nanoparticles [5]. Nanoparticles increase thermal conductivity and heat transfer and minimize supercooling [6]. This boosts thermal storage efficiency and energy release. Hence, the Nano-PCM is widely used in solar thermal systems, thermal management, and energy storage applications [7, 8] created a heat storage ETSC using heat storage tubing, achieving 56.9% and 48.46% mean conversion efficiency in serial and parallel combinations. Similarly, the inefficiency of a tiny solar collector using a typical U-pipe ETC and PCM for thermal energy storage was studied [9]. U-pipe ETC is tested un-finned and finned. The two variations are compared to FSWHS under similar operation and weather conditions. Table 1 shows the previous studies using the PCM in a solar collector system.
| References | PCM | Findings |
|---|---|---|
| Xue [10] | Ba(OH)2·8H2O as a PCM | The fluid temperature could affect the system's performance. |
| Abokersh et al. [11] | Paraffin | The annual energy efficiency is about 71.8%. |
| Algarni et al. [12] | Copper based PCM | 0.33 wt% of copper in PCM could increase efficiency by 32%. |
| Kabeel et al. [13] | Hybrid storage materials | Efficiency was enhanced about 72% by using PCM. |
| Olfian et al. [14] | Paraffin | Efficiency was enhanced about 21.55% by using PCM. |
| Olfian et al. [15] | Paraffin wax | The thermal energy process is increased by 20%. |
| Papadimmitratos et al. [16] | Tritriacontane and erythrito | A 26% enhancement of efficiency of PCM. |
| Sekret et al. [17] | Paraffin | The heat absorption was increased by 45%–79%. |
| Sobhansarbandi et al. [18] | Octadecane paraffin wax | The integration of PCM could improve the system's performance. |
| Naghavi et al. [19] | Paraffin wax | The thermal efficiency was found about 38%–42%. |
The literature on ETSC highlights their high efficiency in capturing and transferring solar energy using vacuum-insulated glass tubes. These collectors minimize heat losses and are widely applied in water heating, space heating, and industrial processes. The integration of PCM and nanofluids further enhances their thermal efficiency and energy storage capacity. The Ne-PCM enhances thermal conductivity, heat transport, and storage. Several studies have shown that different PCMs improve thermal efficiency and energy conversion in solar collectors. Researchers found that paraffin, hybrid storage materials, and metal-based PCMs improved heat absorption and system performance.
Comparisons with past studies show that PCM integration in solar thermal systems works. While ETSC systems with PCM integration have been extensively studied, few studies have examined the influence of Fe3O4 nanoparticle-enhanced PCM on thermal and exergy efficiency. Existing literature primarily focuses on different PCM types and compositions but lacks experimental validation of nanoparticle concentration effects on ETSC performance. Additionally, studies comparing the efficiency of Ne-PCM at varying mass fractions remain scarce.
The ETSC device with PCM integration was investigated in this study. The experiment examined the nano-enhanced PCM using iron oxide (Fe3O4) nanoparticles at mass fractions of 0.2 wt.%, 0.4 wt.%, and 0.6 wt.%. Moreover, the paraffin wax acts as PCM material with the heat exchanger system. The thermal and exergy efficiency of ETSC was studied with and without nanoparticles PCM, and the results were compared. The conclusion of the study detailed that the ETSC system with Ne-PCM at 0.6 wt.% shows better results compared to others.
2 Materials and Methods
2.1 Nano-Enhanced PCM
The preparation of nano-enhanced phase change material (Ne-PCM) using Fe3O4 nanoparticles and paraffin wax varies depending on the volume fraction of nanoparticles (0.2 wt.%, 0.4 wt.%, and 0.6 wt.%) to optimize thermal performance. Initially, paraffin wax is melted at 70°C–80°C to ensure a uniform liquid phase for effective nanoparticle incorporation. Fe3O4 nanoparticles are first dispersed in ethanol or hexane to prevent agglomeration, with ultrasonication applied for 30 min to achieve uniform dispersion. For 0.2 wt.% Fe3O4, a lower concentration ensures minimal alteration of paraffin's latent heat while enhancing thermal conductivity slightly. For 0.45 wt.% Fe3O4, a balance between thermal conductivity improvement and phase change enthalpy retention is maintained, making it suitable for moderate thermal applications. For 0.6 wt.% Fe3O4, a higher concentration leads to significant thermal conductivity enhancement, but excessive loading may cause particle sedimentation and reduce latent heat storage. Various Fe3O4 (iron oxide) nanoparticle weight percentages amounting to 0.2 wt.%, 0.4 wt.%, and 0.6 wt.% served as the strategic selection basis during this investigation because they meshed scientific principles with practical requirements. Several precise concentrations were chosen because they establish a systematic way to analyze the thermal effects of nano fluids while revealing patterns between thermal performance metrics [20]. The experimental conditions of nanoparticle loading consist of three percentages that generate enough data to determine the best concentration that optimizes Ne-PCM performance without compromising its material stability.
Fe3O4 nanoparticles maintain exceptional thermal conductivity properties with media fluid stability, which makes them appropriate additives for solar thermal energy system improvement. Too many nanoparticles used in combination may create various adverse effects during the process. When the nanoparticle concentration exceeds 0.6 wt.%, van der Waals attractions cause particles to tend to agglutinate. Such nanoparticle aggregation results in non-homogeneous distribution along with reduced surface area, which leads to poor thermal conductivity enhancement and may negatively affect system performance High concentrations of nanoparticles cause PCM viscosity increases that reduce fluid flow efficiency and heat transfer quantity while increasing pump power needs, thus lowering system operational performance.
Operational needs together with economic aspects sail as important factors in the process. The high cost of nanoparticles becomes a major issue when their concentration is elevated because it affects the overall expenses while potentially nullifying the efficiency benefits achievable in practical implementations. The operational stability of higher concentration suspensions can be negatively affected over time by sedimentation and stability issues that emerge under periodic thermal cycling, which happens frequently in solar thermal systems. Although 0.6 wt.% offers excellent thermal capabilities, the system operates within a stable and reliable economic range that ensures Ne-PCM remains functional throughout its operational lifespan. A maximum practical amount of 0.6 wt.% serves as an optimal boundary to enhance nanoparticle benefits in the system because it strikes a balance between performance and system stability and flow characteristics, and cost-effectiveness. The researcher has deliberately manipulated concentrations of Fe3O4 nanoparticles to establish methodical results about performance improvements through nanotechnology in ETSC.
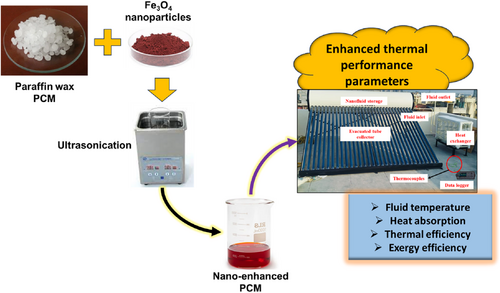
The dispersed Fe3O4 nanoparticles are gradually added to the molten paraffin under continuous stirring for 60 min at 1540 rpm (Sesw 0136 3 L Magnetic Stirrer without Hot Plate with Magnetic Bar). To improve stability, a surfactant such as oleic acid or stearic acid is introduced, especially at higher nanoparticle concentrations, to prevent aggregation. Further ultrasonication is applied to maintain homogeneity, ensuring even nanoparticle distribution. The ultrasonication (Verilux 2 Liter Stainless Steel Ultrasonic Cleaner with Digital Timer & Heater) is done at a temperature of 80°C for 90 min. The solvent is evaporated under vacuum at 60°C to eliminate residuals that may affect thermal properties. The solidified Ne-PCM is characterized using DSC to measure phase transition temperatures and latent heat, revealing that 0.2 wt.% has minimal latent heat reduction, whereas 0.6 wt.% shows a slight decrease due to increased solid content. After the preparation, the PCM was incorporated within the heat exchanger to study the experiment of ETSC. Table 2 shows the properties of PCM. Similarly, Figure 1 shows the Ne-PCM preparation steps.
| Property | Solid phase | Liquid phase |
|---|---|---|
| Latent Heat (kJ/kg) | 143.2 | 143.2 |
| Density (kg/m3) | 675 | 645 |
| Melting Point (°C) | 56 | 56 |
| Specific Heat (kJ/kg °C) | 2.5 | 1.7 |
| Thermal Conductivity (W/m °C) | 0.42 | 0.22 |

Figure 2 shows the Fourier transform infrared (FTIR) spectrum of the Ne-PCM. The broad absorption band observed in the range of 3200–3500 cm−1 corresponds to the OH stretching vibration, indicating the presence of hydroxyl (-OH) groups, which could be due to hydrogen bonding or moisture absorption. Peaks at 2916.5 cm−1 and 2852.9 cm−1 indicate CH stretching vibrations from aliphatic hydrocarbons, indicating the existence of long-chain organic molecules in the PCM. A peak at 1735.1 cm−1 signifies the CO (carbonyl) stretching vibration, typical of ester or carboxyl functional groups, indicating the PCM includes organic substances like fatty acids or esters.
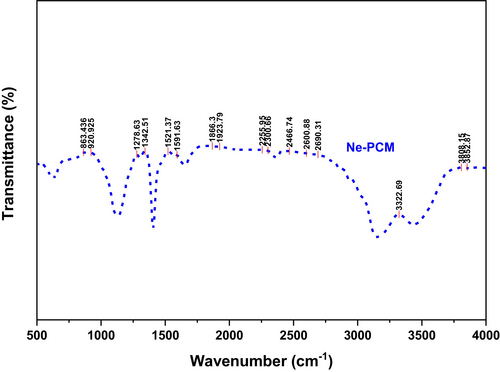
Peaks at 1635.4 cm−1 and 1462.7 cm−1 indicate structural alterations in the PCM, caused by CC stretching vibrations in alkenes or aromatic rings and bending vibrations of –CH2 or –CH3 groups. Further, the peak at 1381.7 cm−1 is associated with the symmetric bending vibration of CH bonds in alkanes, indicating the organic character of the PCM. Strong absorption bands at 1103.4 cm−1 and 1018.7 cm−1 indicate CO stretching vibrations in ether or ester bonds, suggesting Fe3O4 nanoparticles and PCM may interact chemically. The FeO peaks at 580.2 cm−1 and 628.8 cm−1 indicate the existence of Fe3O4 nanoparticles, confirming their effective incorporation into the PCM. Additional peaks (900–1300 cm−1) in the fingerprint region indicate complicated bond bending and stretching vibrations, bolstering the PCM's structural stability. The existence of FeO bonds and organic functional groups indicates that Fe3O4 nanoparticles are evenly distributed throughout the PCM, improving its thermal characteristics. Spectral measurements suggest Fe3O4 nanoparticles efficiently interact with the PCM, potentially enhancing thermal conductivity, phase shift, and energy storage.
Similarly, Figure 3 shows the result of X-ray diffraction (XRD) of Ne-PCM, which provides crucial information about their crystalline structures and the impact of Fe3O4 nanoparticle incorporation. The XRD spectrum of pure PCM exhibits a broad diffraction peak in the range of 15°–30°, indicating its amorphous or semi-crystalline nature. This broad peak is characteristic of organic PCM materials, which generally lack a well-defined crystalline structure. In contrast, the Ne-PCM spectrum shows a significant difference with distinct sharp peaks, particularly at 2θ values of approximately 30.1°, 35.5°, 43.2°, 53.7°, 57.2°, and 62.8°. These peaks correspond to the characteristic diffraction planes (220), (311), (400), (422), (511), and (440) of Fe3O4 nanoparticles, confirming their presence in the modified PCM. The high-intensity peak at around 35.5° indicates strong crystallinity in the Fe3O4 phase, suggesting that the nanoparticles are well dispersed within the PCM matrix.
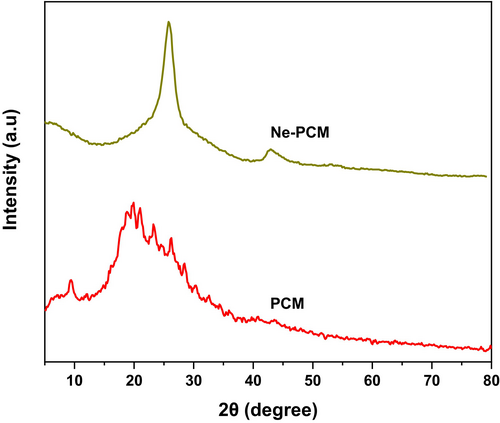
The incorporation of Fe3O4 nanoparticles results in an overall increase in crystallinity, as seen in the Ne-PCM pattern, which exhibits sharper peaks compared to the broad amorphous hump of the pure PCM. This enhanced crystallinity contributes to improved thermal conductivity, as crystalline structures facilitate better heat transfer pathways. Furthermore, the suppression of the amorphous peak in Ne-PCM suggests strong interactions between the PCM molecules and Fe3O4 nanoparticles, leading to structural modifications that enhance phase change behavior.
2.2 Experimental Details
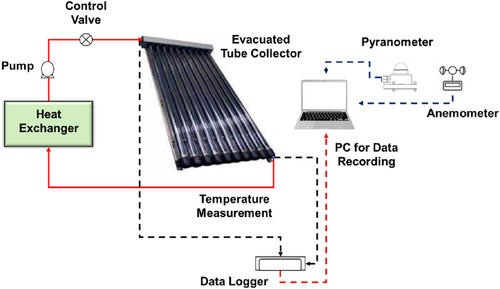
Experimental equipment includes an ETSC, flow meter, control valve, data logger, data recording system, and solar weather station. ETSC has 15 evacuated tubes and a 1.49 m2 collection area. Each tube has a 47-mm inside and 60-mm outside diameter and a length of 1.9 m. Enclosing a heating conduit with an aluminum fin improves tube thermal conductivity and heat transfer. To distribute heat efficiently, a manifold is attached to the heat conduit, which is filled with a high-conductivity liquid. A vacuum barrier between the tubes and the outside glass lowers conduction heat loss, improving thermal efficiency.
The working fluid is accurately guided to the absorber by a flow meter and control valve. A 1 HP pump maintains fluid circulation pressure. J-type thermocouples with a broad operating range of −300°C–1500°C are mounted at the inlet and egress to assess thermal efficacy and detect fluid temperature variations. In addition, a pyranometer and anemometer are employed to continuously monitor wind speed and solar radiation to ascertain the extent to which environmental conditions affect the system's performance. The Keysight 34970A data gathering system facilitates precise thermal analysis by collecting real-time temperature data. The experimental structure of the ETSC system is illustrated in Figure 4, which illustrates the integration of the solar collector with the measuring devices. Additionally, the ETSC specification is delineated in Table 3.
| Parameters | Values |
|---|---|
| Area | 1.73 m2 |
| Glass transmissivity | 0.92 |
| Absorptivity | 0.91 |
| Glass emissivity | 0.8 |
| Heat pipe material | Copper |
| Heat exchanger dimension | 50 cm × 75 cm × 8 cm |
| PCM type | Paraffin |
| Nanoparticles | Fe3O4 |
| Volume fraction | 0.2 wt.%, 0.4 wt.%, and 0.6 wt.% |
Additionally, the PCM material was retained in the heat exchanger to ensure a consistent heat supply to the air, thereby mitigating the temperature fluctuations caused by passing clouds. This, in turn, led to intermittent heat transmission to the operating fluid. Paraffin wax was selected as the PCM for this investigation due to its thermal stability, high latent heat storage capacity, and non-corrosive characteristics. The heat exchanger contained 3.8 kg of paraffin wax, which occupied 75% of the available capacity. The remaining 25% was left unoccupied to accommodate the volumetric expansion of PCM during its transition from solid to liquid.
To maximize heat transfer, the heat exchanger used a cylindrical copper coil. Copper tubes with 10 mm diameters and 2.8 m lengths transmitted heat between the ETSC's working fluid and the stored PCM. ETSC heat was stored in paraffin wax during peak sun radiation. For consistent air heating, this heat was gradually released. Copper's strong thermal conductivity improves heat exchange efficacy to reduce heat losses and improve system performance. Due to its thermal buffering effect, this setup maintained heat output regardless of solar radiation, improving the system's thermal performance.
2.3 Experimental Procedure
To assess the thermal efficiency of the ETSC, the experiment was conducted with the Nee-PCM with varying mass fractions of nanoparticles 0.2 wt%, 0.4 wt%, and 0.6 wt%. The experimentation was conducted at Saveetha University in Chennai (13.0802°N, 80.2011°E), where the ambient temperature ranged from 30°C–38°C and solar irradiance oscillated between 50 and 950 W/m2. The ETSC was initially evaluated using water without PCM conditions, and its efficacy was compared to water with PCM and Ne-PCM conditions. For precise thermal analysis, J-type thermocouples were employed in conjunction with a data-logging system to collect temperature measurements. The study examined critical performance parameters, such as the fluid temperature profile, heat accumulation, thermal efficiency, and exergy efficiency. The experimental setup's photocopy is illustrated in Figure 5.
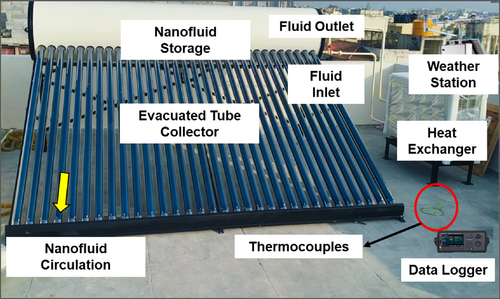
Figure 5 displays an experimental procedure that measures the performance outcomes of an ETSC system along with its Ne-PCM Fe3O4 nanoparticle-enhanced PCMs. As a highly efficient solar thermal system, the ETSC operates mainly for water heating purposes and thermal energy storage needs. The system has parallel vacuum-sealed glass tubes that work as individual solar collectors. The vacuum-sealed tubes achieve solar heat absorption through a reduction in conductive and convective heat losses. Each tube represents 1800 mm in length while measuring 58 mm around its outside and 47 mm around its inside diameter, is constructed using borosilicate glass with selective coating applied to the inner surface to boost solar absorption.
A nanofluid storage tank operated by Fe3O4 nanoparticles and water solutions exists at the top portion of the ETSC. The system uses the nanofluid as it travels from the storage tank through the fluid inlet and then sends it into the evacuated tubes for solar energy absorption. The nanofluid circulation flow run is marked as “Nanofluid Circulation” in the representation. After heat absorption from solar energy, the nanofluid exits the system through the fluid outlet to reach the heat exchanger. The closed-loop pattern of circulation enables continuous heat absorption as well as heat transfer activities.
The thermocouple sensors track temperatures along the nanofluid route starting from the collector inlet and extending to the outlet and potentially extending to the storage device and heat exchanger units. The sensors interface with a data logger system which maintains continuous temperature measurement for thermal measurement purposes. Data analysis consisting of heat absorption and thermal and exergy efficiency calculations depends on the measurements obtained under changing nanoparticle concentrations. The results of the reaction depend on data obtained when the nanoparticle concentration levels change. The setup contains a weather monitoring station that collects measurements of solar irradiance together with wind speed and temperature levels because these environmental elements impact the system output. These measurement variables enable precise connection of system production data to external inputs.
The system contains a heat exchanger that serves as a crucial device for moving thermal energy from nanofluid to stored or used water. The system uses spiral coil heat exchangers because they provide both compactness and efficient heat transfer performance for this application. For this application, the heat exchanger has its copper tubes approximately 2 to 3 m long, with an outer coil diameter of 300 mm, an inner diameter of 200 mm, and a tube diameter of 10 mm Such a system design creates high heat transfer surface capacity from limited space while properly functioning for solar thermal applications.
The experimental framework enables thorough analysis of fluid temperature changes, heat transfer behavior, along with thermal and exergy performance at 0.2 wt.%, 0.4 wt.%, and 0.6 wt.% Fe3O4 nanoparticle dosages for validating PCM-based improvement in solar power applications.
2.4 Thermal Modeling
The equations provided for analyzing the thermal and exergy performance of an ETSC are based on a set of underlying assumptions that ensure the validity and applicability of the models. Firstly, it is assumed that the solar radiation IT is uniformly distributed across the collector surface and that the collector area Ac is effectively exposed to this radiation. The transmittance–absorptance product (τα) is considered constant, reflecting stable optical properties of the collector during operation. The heat loss coefficient UL is treated as constant over the range of operating temperatures, implying that the thermal losses through convection, conduction, and radiation are linear with respect to the temperature difference between the absorber surface and ambient air.
In the derivation of the heat removal factor FR, the fluid properties such as specific heat Cp and mass flow rate m are considered steady and uniform, ensuring a consistent heat transfer throughout the collector. The temperature distribution within the working fluid is assumed to be one-dimensional, neglecting any radial gradients, and the flow is considered to be fully developed and steady-state. It is also assumed that there are no heat losses along the flow path inside the tubes apart from the predefined losses accounted for by UL.
For the thermal efficiency equation, the assumption is that the system operates under steady-state conditions with no energy accumulation, and all absorbed energy is either transferred to the fluid or lost through heat transfer to the surroundings. The exergy efficiency equation assumes that the irreversibility in the system can be quantified through entropy generation Sgen, and that the average surface temperature Ts of the collector is well-defined and stable. The ambient temperature Ta is also considered constant during the period of analysis. Additionally, the second law efficiency calculation presumes that the energy conversion processes follow ideal thermodynamic behavior where exergy destruction is solely due to heat transfer across finite temperature differences.
These assumptions simplify the real system into a tractable analytical model, allowing for performance evaluation and comparison, while recognizing that deviations may occur under dynamic or transient operational conditions.
2.5 Uncertainty Analysis
The instrument's uncertainty in Table 4. Moreover, Figure 6 shows the graphical flow of the current study. Uncertainty in temperature measurements (±1%) had a negligible impact on efficiency calculations, as confirmed by error propagation analysis (Equation 5). In general, all uncertainties measured and the sum of them were less than 5%. This confirms a 95% confidence level. The uncertainties were mentioned in the results at 95% CI, ±1 SD with the use of error bars (Figures 7-11).
| Device | Thermocouples | Anemometer | Pyranometer | Data logger |
|---|---|---|---|---|
| Model | THERMONIC | AM4208 | RS-TBQ-N01-AL | Keysight 34970A |
| Range | −0°C–700°C | 0.1–50 m/s | 0–3000 W/m2 | −600°C–1800°C |
| Uncertainty | ±1% | ±0.5% | ±1.5% | ±2.2% |
3 Results and Discussion
3.1 Fluid Temperature
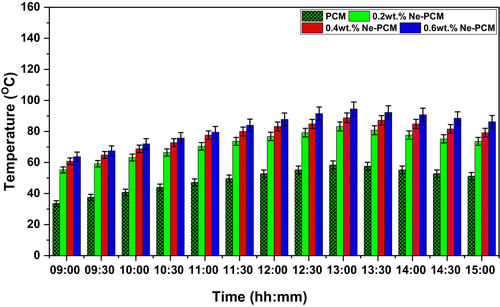
The thermocouple measured the fluid outlet temperature, which is illustrated in Figure 7. It clearly shows that the fluid temperature increases with the solar radiation increasing and decreases with solar radiation decreasing. Also, the maximum fluid temperature was recorded at noon due to peak radiation availability. The fluid temperature of water under the PVCM (pure PCM) condition is recorded as the lowest, at approximately 58.4°C. This is primarily due to the limited thermal conductivity of conventional PCM materials, which results in slower heat absorption and release rates. In contrast, the addition of Fe3O4 nanoparticles into the PCM matrix (Ne-PCM) significantly enhances thermal conductivity, thereby improving heat transfer efficiency and raising fluid temperature. The increase in fluid temperature with varying concentrations of Fe3O4, Ne-PCM is evident, with values of 83.2°C, 88.8°C, and 94.5°C for 0.2 wt.%, 0.4 wt.%, and 0.6 wt.% nanoparticle concentrations, respectively. The uncertainties were mentioned in the results at 95% CI, ±1 SD in error bars on the graph in Figure 7.
This improvement can be attributed to the superior thermal conductivity of Fe3O4 nanoparticles, which facilitate rapid heat absorption and storage within the PCM, leading to more effective thermal energy exchange with the fluid. Dispersed nanoparticles improve convective heat transmission, resulting in more uniform and efficient energy distribution. Higher nanoparticle concentrations raise fluid temperature due to better thermal diffusivity and reduced thermal resistance. Additionally, Fe3O4 nanoparticles maintain stable dispersion in PCM, minimizing agglomeration and maintaining heat transfer performance. Nanomaterials and PCM synergistically reduce subcooling, ensuring a more constant phase transition and heat storage. Due to its higher specific heat capacity, the hybrid Ne-PCM retains energy and releases heat slowly, maintaining high fluid temperatures for longer. The magnetic characteristics of Fe3O4 nanoparticles may enhance thermal performance by promoting micro-convective currents. The pattern shows that nanoparticles and PCM optimize heat transfer fluid thermal regulation. This helps solar thermal systems, where heat storage and retrieval are crucial to performance. Incorporating Fe3O4 Ne-PCM shows promise in improving thermal behavior and heat transmission between PCM and water [27].
3.2 Heat Absorption
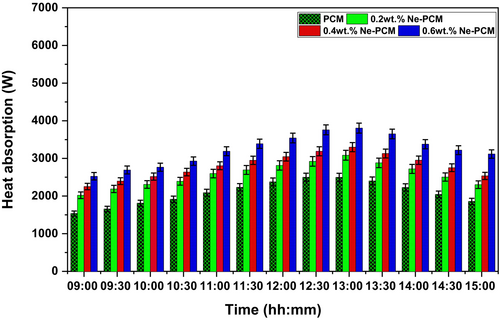
The temperature differential between the fluid inlet and outlet is measured to compute the heat absorption rate. Figure 8 depicts the heat absorption value for the different PCM integration conditions. The uncertainties were mentioned in the results at 95% CI, ±1 SD in error bars on the graph in Figure 8. The maximum heat absorption capacity of the PCM and Ne-PCM composites varies significantly depending on the concentration of Fe3O4 nanoparticles. The pure PCM exhibits the lowest heat absorption, at approximately 2516.9 W, due to its inherently low thermal conductivity, which restricts the rate of heat transfer. However, the introduction of Fe3O4 nanoparticles into the PCM matrix enhances its thermal performance, leading to higher heat absorption values. At 0.2 wt.% of Ne-PCM, the heat absorption increases to 3084.8 W, indicating an improvement in thermal conductivity due to the dispersion of nanoparticles, which facilitates better heat distribution. As the concentration of Fe3O4 nanoparticles rises to 0.4 wt.%, the heat absorption further increases to 3298.5 W, demonstrating the effectiveness of the nanoparticle-enhanced PCM in improving energy storage and transfer efficiency. The highest recorded heat absorption, at 3804.1 W, corresponds to the Ne-PCM with 0.6 wt.% Fe3O4, highlighting the substantial role of nanoparticles in promoting thermal conductivity and reducing thermal resistance within the PCM. This progressive increase in heat absorption capacity can be attributed to the enhanced specific heat and improved latent heat storage properties of the nanocomposite PCM [27].
Fe3O4 nanoparticles induce micro-convective currents, enhancing heat dispersion and thermal management. Nanoparticles also reduce subcooling, improving phase transition and heat absorption. Ne-PCM is ideal for thermal energy storage applications, especially solar thermal systems, where heat absorption and storage are important to system performance. Ne-PCM can be used in sophisticated methods of storing energy since it absorbs and stores more heat than pure PCM [12]. Applications demanding high energy density and effective temperature regulation need this increase. Integrating Fe3O4 nanomaterials into PCM enhances heat absorption, thermal energy usage, and ETSC system efficiency.
3.3 Thermal Efficiency
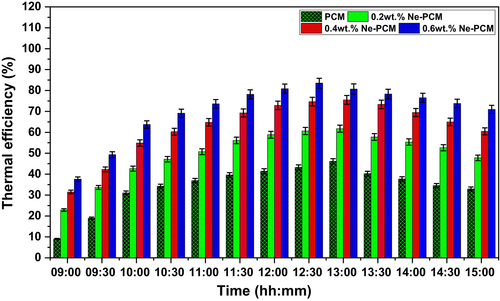
The heat input and output ratio gives the thermal efficiency profile of the ETSC system, and it is illustrated in Figure 9. It also follows the same trend of heat absorption profile. The peak thermal efficiency was recorded at peak fluid temperature and heat absorption values. The uncertainties were mentioned in the results at 95% CI, ±1 SD in error bars on the graph in Figure 9. The thermal efficiency of the system exhibits a significant improvement with the integration of Fe3O4 nanoparticles into the PCM. Pure PCM demonstrates the lowest thermal efficiency at approximately 46.1%, primarily due to its limited thermal conductivity, which restricts heat transfer rates and results in suboptimal energy utilization. However, the addition of Fe3O4 nanoparticles enhances the thermal properties of PCM, leading to a notable increase in efficiency. At 0.2 wt.% Ne-PCM, the efficiency rises to 61.7%, indicating improved heat absorption and distribution due to enhanced thermal conductivity.
As the concentration increases to 0.4 wt.%, the efficiency further improves to 75.5%, highlighting the role of nanoparticles in facilitating better heat transfer and reducing thermal resistance within the material. The highest recorded thermal efficiency, at 83.5%, corresponds to the Ne-PCM with 0.6 wt.% Fe3O4, emphasizing the synergistic effect of nanoparticles in enhancing both sensible and latent heat storage capabilities. This progressive increase in efficiency can be attributed to several factors, including improved thermal diffusivity, reduced subcooling effects, and better phase transition characteristics. The Fe3O4 nanoparticles act as thermal conductivity enhancers, promoting faster and more uniform heat distribution throughout the PCM matrix [28]. Additionally, their presence induces micro-convective currents, further optimizing the heat transfer process. Another contributing factor is the increased specific heat capacity of Ne-PCM, which allows for greater energy storage and a more sustained heat release. The observed trend suggests that an optimized concentration of nanoparticles significantly improves the overall thermal performance of PCM, making it more effective for thermal energy storage applications. Higher thermal efficiency improves energy conversion and system reliability in solar thermal systems. Ne-PCM's higher performance over traditional PCM shows its promise for enhanced energy storage technologies, maximizing heat source usage. Adding Fe3O4 nanoparticles to PCM can improve thermal efficiency, leading to more sustainable thermal management systems.
3.4 Exergy Efficiency
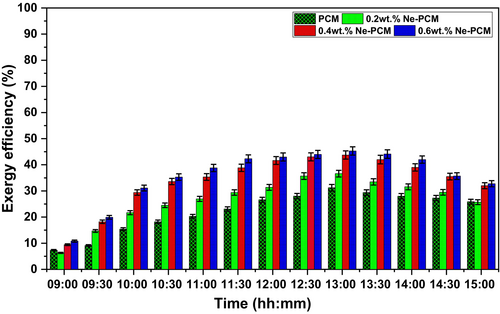
Figure 10 shows time-dependent exergy efficiency for different PCM settings. The uncertainties were mentioned in the results at 95% CI, ±1 SD in error bars on the graph in Figure 10. The addition of Fe3O4 nanoparticles to the PCM improves energy usage and reduces irreversibility, resulting in increased exergy efficiency. Due to its limited thermal conductivity and heat transfer rate constraints, pure PCM has the lowest exergy efficiency of 31.2%. Thermal resistance and poor phase transitions waste much of the absorbed energy. The inclusion of Fe3O4 nanoparticles significantly enhances exergy efficiency. Nanoparticles boost thermal conductivity, which improves heat absorption and utilization, increasing exergy efficiency to 36.6% at 0.2 wt.% Ne-PCM. As the concentration climbs to 0.4 wt.%, thermal diffusivity, subcooling, and phase transition kinetics improve, resulting in 43.7% exergy efficiency. The Ne-PCM with 0.6 wt.% Fe3O4 had the highest exergy efficiency of 45.2%, highlighting the synergistic benefits of nanoparticles in reducing entropy and optimizing thermal performance. Good heat distribution, lower thermal resistance, and increased latent heat storage capacity increase exergy efficiency. Fe3O4 nanoparticles improve heat transmission by generating micro-convective currents in the PCM matrix, resulting in better thermal energy conversion. Additionally, Ne-PCM's increased specific heat capacity retains energy, reducing exergy destruction. The trend shows that PCM-based thermal storage systems with adequate nanoparticle concentration improve exergy efficiency, making them more practicable for solar thermal energy systems [28]. Higher exergy efficiency converts thermal energy into productive work, maximizing heat source use. Solar collectors, heat exchangers, and thermal energy storage devices need this innovation for efficient thermal management. Adding Fe3O4 nanoparticles to PCM can improve energy efficiency, reduce losses, and improve thermal storage and conversion processes [29].
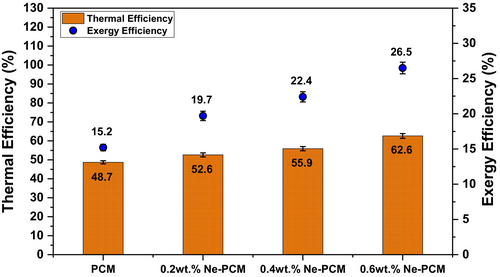
Figure 11 displays the average thermal and exergy efficiency curve for different PCM settings. As nanoparticle concentration increases, PCM and Ne-PCM composite thermal and exergy efficiency improve. Due to phase change heat storage and release, the base PCM has 48.7% thermal efficiency and 15.2% exergy efficiency. Lower exergy efficiency indicates higher system irreversibility. With the addition of 0.2 wt.% nanoparticles, thermal efficiency increases to 52.6% as nanoparticles enhance thermal conductivity, leading to better heat absorption and release. The exergy efficiency also rises to 19.7% due to reduced thermal resistance and improved energy utilization [30]. At the Ne-PCM concentration of 0.4 wt.%, thermal efficiency further improves to 55.9% because of enhanced thermal diffusivity, allowing quicker heat transfer. Correspondingly, exergy efficiency reaches 22.4% as the system becomes more thermodynamically efficient with reduced entropy generation. The highest performance is observed at 0.6 wt.% Ne-PCM, where thermal efficiency reaches 62.6% due to superior heat transfer characteristics, ensuring rapid energy storage and retrieval. Exergy efficiency peaks at 26.5% as heat utilization is optimized, minimizing exergy destruction [31]. The uncertainties were mentioned in the results at 95% CI, ±1 SD in error bars on the graph in Figure 11. Table 5 shows the comparative analysis of previous studies with current research.
| References | Nanoparticles | PCM | Findings |
|---|---|---|---|
| Colla et al. [32] | Al2O3 and black carbon (CB) | Paraffin RT20 and RT25 | 1 wt.% of CB nanoparticles leads to 355 thermal conductivity enhancement. |
| Li et al. [33] | Expanded Graphite (EG) | Stearic acid (SA) | The melting time was reduced by about 63.3% by nanoparticles. |
| SunilRaj and Eswaramoorthy [34] | Al2O3 | Paraffin | The thermal efficiency was increased by about 12%. |
| Ebadi et al. [35] | CuO | Bio-based coconut oil | The addition of nanoparticles leads to increased thermal properties and system performance. |
| Chaichan and Kazem [36] | Al2O3 | Paraffin | The thermal efficiency was enhanced by about 60.53% by nanoparticle addition. |
| Rufuss et al. [37] | CuO | Paraffin | Efficiency was reached about 88.46% by nanoparticle addition. |
| Krishna et al. [38] | Al2O3 | Tricosane | The addition of nanoparticles leads to increased thermal properties up to 32% and system performance. |
| Mandal et al. [39] | CuO | Paraffinn | The nanoparticles with PCM improve the system's thermal performance. |
| Singh et al. [39] | Cu, Al, Ni, Ag CuO, Al2O3, TiO2, SiO2 | Eutectic salt (LiNO3-KCl; 50:50) | The graphene nanoparticles show higher thermal conductivity. |
| Singh et al. [40] | Graphene nanoplates | Eutectic PCM | The PCM with 5% graphene nano-plates reduces the melting time by 43%. |
| The current study | Fe3O4 | Paraffin | The maximum thermal and exergy efficiency was about 62.6%, and 26.5. |
4 Conclusions
This current study explored an experimental investigation on an ETSC under PCM integration. The nano-enhanced PCM was explored with the iron oxide (Fe3O4) nanoparticles and the experiment was conducted at different mass fractions of about 0.2 wt.%, 0.4 wt.%, and 0.6 wt.%. Moreover, the paraffin wax acts as PCM material with the heat exchanger system. The incorporation of Fe3O4 nanoparticles into PCM significantly enhances fluid temperature, reaching a maximum of 94.5°C at 0.6 wt.% concentration. This improvement is attributed to increased thermal conductivity, better heat absorption, and more efficient energy distribution. As a result, the system experiences improved heat transfer, ensuring higher thermal performance. Similarly, heat absorption increases with Fe3O4 nanoparticle concentration, peaking at 3804.1 W for 0.6 wt.% Ne-PCM.
Ne-PCM is a great heat storage and transmission medium due to its improved thermal characteristics, phase transition, and thermal resistance. Thermal efficiency increases from 46.1% in pure PCM to 83.5% with 0.6 wt.% Ne-PCM. Nanoparticles promote heat transport, subcooling, and energy storage, causing this increase. Exergy efficiency also increases from 31.2% in pure PCM to 45.2% at the greatest nanoparticle concentration. Better energy use, reduced entropy formation, and optimized thermal energy conversion reduce energy losses and improve system performance. These thermal and exergy efficiency improvements make Ne-PCM suitable for solar thermal applications, where heat retention and energy conversion are essential for system dependability and sustainability.
Nomenclature
-
-
- specific heat (J/kg K)
-
-
- exergy input
-
-
- exergy output
-
- IT
-
- solar radiation (W/m2)
-
-
- flow rate (kg/s)
-
-
- exergy efficiency (%)
-
-
- thermal efficiency (%)
-
-
- energy input (W)
-
-
- heat gain (W)
-
- Sgen
-
- entropy generation due to irreversibility in the system (W/K)
-
-
- ambient temperature (K)
-
-
- inlet temperature (K)
-
-
- outlet temperature (K)
-
-
- sun temperature (5770 K)
-
- UL
-
- heat loss coefficient
-
-
- overall uncertainty
Abbreviations
-
- Ac
-
- area of ETSC (m2)
-
- Al2O3
-
- aluminum oxide
-
- CuO
-
- copper oxide
-
- ETSC
-
- evacuated tube solar collector
-
- FR
-
- heat removal factor
-
- FTIR
-
- Fourier transform infrared
-
- MgO
-
- magnesium oxide
-
- MWCNT
-
- multiwalled carbon nanotubes
-
- PCM
-
- phase change material
-
- TiO2
-
- titanium dioxide
-
- XRD
-
- X-ray diffraction
Greek Symbol
-
- τ
-
- transmissivity
-
-
- absorptivity
Author Contributions
T. Sathish: conceptualization, investigation, funding acquisition, writing – original draft, writing – review and editing, visualization, validation, methodology, software, formal analysis, project administration, resources, supervision, data curation. R. Saravanan: conceptualization, investigation, validation, methodology, writing – review and editing, formal analysis, software, data curation. Jayant Giri: funding acquisition, visualization, project administration, resources, writing – review and editing, validation, conceptualization, formal analysis. Rasan Sarbast Faisal: supervision, data curation, software, project administration, methodology, investigation, writing – review and editing, conceptualization. Tariq Alkhrissat: writing – review and editing, funding acquisition, investigation, conceptualization, methodology, validation, formal analysis, software, supervision, resources. Nidhal Becheikh: writing – review and editing, funding acquisition, investigation, conceptualization, visualization, project administration, data curation, supervision. Boutheyna Belhaj Bettaieb: writing – review and editing, conceptualization, validation, methodology, software, data curation, project administration. A. Johnson Santhosh: writing – original draft, funding acquisition, investigation, conceptualization, methodology, validation, visualization, writing – review and editing, project administration, formal analysis, software, data curation, supervision, resources.
Acknowledgments
The authors extend their appreciation to Northern Border University, Saudi Arabia, for supporting this work through project number (NBU-CRP-2025-2933).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




