A Review of Lithium-Ion Battery Empirical and Semi-Empirical Aging Models for Off-Grid Renewable Energy Systems Application
Funding: The authors received no specific funding for this work.
ABSTRACT
Aging of lithium-ion (Li-ion) batteries in off-grid renewable energy systems (RESs) can be monitored and controlled using battery management systems (BMSs) which utilize battery aging models. Empirical and semi-empirical models (EMs) of battery aging are preferred for BMSs due to their simplicity and intuitiveness. This study is unique as it aims at identifying appropriate empirical and semi-EMs, in terms of complexity and current fluctuation representation, for BMS for off-grid RESs. Different EMs of Li-ion battery calendar and cycle aging have been extracted from literature and compared mainly in terms of complexity, current fluctuation representation, and modeling of capacity fade and resistance increase. The extracted models have been put in groups which are based on modeling format used, namely: calendar aging (only) models (CAOM), cycle aging (only) models (CYAOM), calendar and cycle aging (separated) models (CCYAOM), and calendar and cycle aging (combined) models (CCYACM). Results show that three models meet requirements for BMS for off-grid RESs. The three models fall under CYAOM, CCYAOM, and CCYACM. The study further finds that 54% of EMs model current fluctuation as an aging factor, 92% model aging in terms of capacity fade, and 46% model aging as resistance increase. Furthermore, the study recommends comparison of EMs through simulations to further validate the different listed models. It also recommends evaluation of the models to establish an appropriate way of representing Li-ion battery aging, whether in terms of capacity fade or resistance increase.
1 Introduction
Electricity generation using fossil fuels (FFs) is the most substantial source of greenhouse gases, an example of such gases is carbon dioxide. These gases have adverse effects on the environment [1]. On the other side, FFs are inadequate and not fairly distributed in the earth's crust [2]. Furthermore, FF reserves continue to decrease. Therefore, generation of electrical energy by utilizing means that are independent of traditional fuels is the only option available to meet our daily life needs of electricity. Consequently, renewable energy (RE) sources have been regarded as a potential solution to generation of electricity. Examples of commonly available RE sources include wind, solar, hydro, and geothermal [3]. However, the availability of most RE sources is dependent on weather and climate conditions (e.g., solar and wind), which keep on changing. Due to the stochastic nature of these RE sources, they cannot be dispatched [4, 5]; this demands utilization of generated electrical energy as soon as it is generated [6], if not stored. However, deployment of energy storage systems (ESSs) has been recognized as a probable solution to the intermittency of RE sources [7].
Performance of these ESSs in renewable energy systems (RESs) has attracted the attention of researchers from both industry and academia. Among different ESSs that are deployed in RESs, it has been found that lithium-ion (Li-ion) batteries outperform other battery types in terms of energy density, cycle life, and memory effect [8, 9]. Furthermore, the price of Li-ion batteries continues to decline and in general it has been found that Li-ion batteries meet more RES requirements as compared to other battery technologies [10, 11]. Currently, two of the major factors that are hindering the full adoption of Li-ion batteries are cost and aging of the batteries [12]. In terms of aging, Li-ion battery performance reduces as a function of time, which is referred to as calendar aging, and as a function of use, which is referred to as cycle aging.
In general, calendar aging refers to capacity fade or resistance increase (power fade) during the time the battery is not functioning. Calendar aging is mainly a function of temperature and state of charge (SoC). In terms of temperature, the higher the temperature, the higher the aging rate of the Li-ion battery due to increased side reactions. For SoC, high SoC accelerates aging of the battery as well due to the imbalance of potential at the electrode and electrolyte interface [13-15]. Calendar aging can be divided into two groups, namely reversible aging and irreversible aging. Reversible aging can be reversed by charging the battery, while irreversible aging is permanent, that is, it cannot be reversed [16].
Cycle aging, on the other hand, is related to battery usage, that is, charge and discharge cycles. Cycling of the battery causes kinetically induced effects; for example, volume variations when charging and discharging, and concentrations gradients [17]. These effects also result into aging in addition to side reactions, which result from the thermodynamic instability of Li-ion battery construction materials. Cycle aging is mainly influenced by charge and discharge current rate (Crate), initial SoC, and variation of SoC (also called depth of discharge [DoD]).
In off-grid RESs, there are pronounced current (power) fluctuations due to the intermittent nature of RE sources and variation of the load profile. Fluctuations caused by the load profile are due to loads that need high starting currents, for example, motors and welding machines. These fluctuations are intense in off-grid RESs due to the small inertia of the systems in comparison with on-grids [18]. These fluctuations result in spikes in Crate, which then consequently accelerate Li-ion battery aging [19].
To minimize aging of Li-ion battery in off-grid RESs due to current fluctuations, an effective battery management system (BMS) must be deployed. The BMS can also be referred to as a battery storage control strategy, as explained in [20, 21]. The main function of the BMS is to monitor aging stress factors the battery is subjected to and the states of the battery system, such as SoC and state of health (SoH), and take appropriate action to reduce the battery aging rate. These BMSs also ensure safe, reliable, and optimized operations of the battery. Battery states, for example SoC and SoH, cannot be directly measured by any sensor; therefore, for the BMS to monitor them, they are usually inferred, commonly from estimation algorithms that are model-based [22-24].
For correct operation of the BMS, in terms of monitoring and control of the battery aging, accurate aging models are of utmost importance. However, in making sure the models are accurate, it should be noted that there is a need of striking a balance between accuracy and complexity. The balance should make sure that models are flexible for implementation in embedded microprocessors and are able to provide fairly accurate results in real time [25]. From off-grid RESs' economics point of view, BMS together with battery aging models can reduce systems' payback period and increase their profitability. This can be achieved by extending battery system lifetime due to reduced aging rate. Also, the development of aging models, specifically for BMS, is helpful to researchers in terms of identifying paths for improvement in Li-ion battery technology for off-grid RESs' implementation [26].
There are different models of Li-ion battery aging behavior. These models are normally grouped into four categories namely: physics-based models (PBMs), equivalent circuit models (ECMs), machine learning models (MLMs), and empirical and semi-empirical models (EMs). A summary of comparison of these models in terms of complexity, accuracy, amount of data, and application areas is given in Table 1. Among these models, PBMs, which are also known as electrochemical models, model the Li-ion battery's electrochemical and physical processes. The first PBM was based on a porous electrode model [27]; thereafter, a pseudo-2-D followed. The pseudo-2-D model extended the first model by including a thermal model [28]. Furthermore, solvent oxidation or layer growth, which are effects of parasitic side reactions, were added to account for aging of the battery. PBMs can achieve high accuracy; however, they are complex since they demand many differential equations to model the physical and chemical properties of the battery [14]. Due to the mentioned challenges, these PBMs are generally mainly used by chemical engineers [29].
| Model type | Complexity | Accuracy | Data amount | Typical applications |
|---|---|---|---|---|
| PBM | High | High | Low | Battery design |
| ECM | Medium | Medium | High | SoH estimation |
| MLM | Medium-high | Medium | High | SoH estimation |
| EM | Low | Low-medium | High | System design and optimization |
ECMs model Li-ion battery by considering their transient response. The models achieve this by using passive circuit components; for example, inductances, resistances, and capacitances. These models can be made more complex and can achieve simulation of internal diffusion and charge transfer processes of the battery, whereby aging can be incorporated based on impedance data using variable components. ECMs are usually used in real-time applications simulation because of their mathematical simplicity [30], and they are combined with state estimators; for example, Kalman filters [14, 30-32].
Machine learning (ML) based models are also commonly used as SoC and SoH estimators. There are different approaches to this modeling technique. Some approaches utilize training algorithms which extract the battery's SoH information from the incremental capacity curve of the battery [33-36]. These models require parameters which can be easily extracted, such as temperature, voltage, and current, which makes the approach advantageous. There are also other techniques that combine ML with other models to train algorithms [37, 38]. For example, ML can be combined with empirical modeling techniques which utilize regression models to estimate SoH of the battery [39]. ML models require large datasets to train the algorithms, and they can achieve high accuracy [14, 33, 40].
EMs model the relationship of various battery aging stress factors using relatively simple analytical formulae. The EMs are used in a wide range of applications because of their simplicity. They are deployed, for example, in BMSs, systems-level design problems, and optimization problems. Due to their simplicity, these models are prone to missing out on some crucial data during battery modeling because of the complex relationship between battery aging stress factors.
In literature, various studies have focused on aging mechanisms and modeling of Li-ion batteries. Reference [41] reviewed factors that influence aging on a cell level as well as battery pack level. In [14, 28, 42], main aging mechanisms were reviewed and estimation techniques based on PBMs, ECMs, EMs, and statistical models were reviewed as well. Comparison of different EMs of battery aging based on a smart grid was done in [43]. Additionally, battery's SoH estimation techniques have been reviewed by many studies of which examples are as in [44-47]. Reference [46] discussed different types of self-adaptive SoH monitoring techniques. The techniques discussed include particle filter method, support vector regression, and neural networks. Furthermore, different types of remaining-useful-life estimation techniques and SoH estimation techniques were broadly reviewed in [44-46]. The techniques reviewed included direct measurement, model-based, and adaptive filtering.
All the studies mentioned focused on giving general reviews of different aging mechanisms or state estimation techniques. In most of these studies, EMs are partially mentioned without diving deep into them. Since EMs are not so complex in implementing in real-time BMS, and they strike a good balance between complexity and battery system representation, they are a better option when it comes to implementing in BMS for off-grid RESs. More complex models indirectly mean high implementation cost for BMS, hence raising the overall cost of off-grid RESs. It is, therefore, paramount to further study EMs to have a deeper understanding in terms of Li-ion battery aging representation and complexity for off-grid RESs. Furthermore, establishing which models best suit implementation in off-grid RESs considering power/current fluctuation representation (Crate), in addition to complexity, is of great importance as well. This is because current fluctuations are eminent in off-grid RESs (as explained in previous paragraphs) leading to accelerated aging of Li-ion battery. In this study, therefore, the research question that is being addressed can be put as follows: what are appropriate EMs for BMS in off-grid RESs considering complexity and current fluctuation representation?
- The study results in the identification of appropriate EMs for implementation in off-grid RESs. These models strike a good balance between complexity and current fluctuation representation for BMS for Li-ion battery aging monitoring and control.
- The study acts as a quick reference material for academia and industry to quickly select Li-ion EMs for implementation during their research depending on their needs in terms of either calendar and cycle life representation or implementation complexity or variables to be measured.
- The study provides an up-to-date research status on EMs' development, which gives insight into areas that need further research. This is helpful to new researchers to know important stress factors of Li-ion battery aging that are mostly considered in current research for informed decisions.
The rest sections of the paper are organized as follows: Section 2 details the methodology used in this study. Methods and materials used in this study are described in this section. Review of Li-ion battery aging mechanisms is done in Section 3, after which Section 4 compares different EMs mainly in terms of complexity as well as current fluctuations representation. After comparison of the different EMs, Section 5 provides a discussion of results by providing key findings and consequently identifying appropriate models for BMS implementation in off-grid RESs. Finally, the study is concluded in Section 6, where also recommendations are provided.
2 Methodology
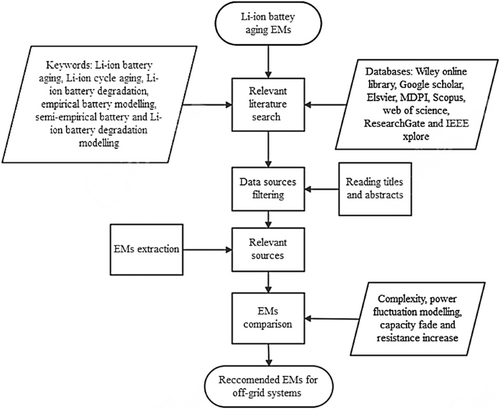
The aim of the study, as mentioned in the introduction section, is to identify appropriate EMs, based on their complexity and current fluctuation representation, for BMS implementation in off-grid RESs. Therefore, in this study, firstly, a review of Li-ion battery aging mechanisms is done to provide general information about battery aging. After which, different studies that have focused on empirical and semi-empirical aging modeling of Li-ion batteries are presented. These studies are split into two groups, that is, those that focused on calendar aging and those that focused on cycle aging (noting that some studies focused on both).
The studies have been extracted from literature using research publications (including journal and conference papers and reports from academic databases, for example Scopus and web of science), websites, and datasheets. Steps followed to find the studies were first to search for literature related to Li-ion battery aging using keywords. Examples of keywords used include Li-ion battery aging, Li-ion cycle aging, Li-ion battery degradation, empirical battery modeling, semi-empirical battery modeling, and Li-ion battery degradation modeling. Thereafter, to make a decision as to whether to read the whole article or not, the article's title, abstract, and date of publication (for published works) were read. This screening made sure relevant articles and information for the study are captured.
Second, extraction of models from the filtered studies followed; models were extracted from the studies which explicitly presented their models and validated them. After model extraction, the extracted models have been put into groups based on whether they capture calendar aging or cycle aging, or both, for clarity purposes. Thereafter, they are compared in terms of complexity and current fluctuations representation, because that is the main focus of this study. Model complexity has been evaluated based on the number of variables and parameters and the number of elementary operations the model has. Current fluctuation representation has been based on whether the model captures Crate or not. Furthermore, the models are compared in terms of modeling of capacity fade or resistance increase, which is also referred to as power fade.
Finally, discussion of comparison results follows, which then culminates in recommending appropriate EMs for Li-ion battery aging for BMS in off-grid RESs. The methodology used in this study can be presented in a diagram as shown in Figure 1.
3 Review of Lithium-Ion Battery Aging Mechanism
Li-ion battery is mainly made up of four main components, namely: anode, cathode, electrolyte, and separator. On the anode, the most commonly used material is graphite [14, 48, 49]. Apart from graphite, other used materials include lithium-titanate-oxide (LTO) or silicon [50]. The cathode is made up of a mixture of materials that contains lithium. The most utilized materials are lithium-nickel-cobalt-aluminum (NCA), lithium-nickel-manganese-cobalt (NMC) oxide, lithium-iron-phosphate (LFP), and lithium-manganese oxide (LMO). On the other hand, the electrolyte composes lithium salts and organic solvents [43]. The transfer of ions between the cathode and the anode is achieved by utilizing the electrolyte. Finally, the separator, which is a porous plastic, is used to separate the anode and the cathode to prevent short-circuiting the electrodes. When the Li-ion battery is charging, Li-ions are released, a process called deintercalation, from the cathode and move through the electrolyte and separator and finally intercalate at the anode. An electrical current results from the flow of these ions from the cathode to the anode. During Li-ion battery charging, the cathode, which is the lithium-containing part of the cell, is the limiting factor as its maximum voltage determines the end of battery charge. On the other side, during discharge mode, the anode is the limiting factor because of its minimum discharge voltage limit [49].
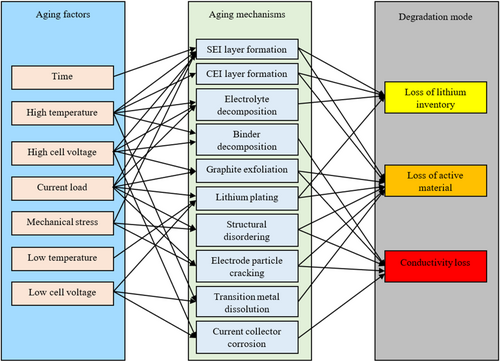
- Loss of Active Material (LAM): this is anode or cathode material structural degradation. It is normally caused by electrode surface layer growth or cycling-induced cracks or exfoliation. The LAM can result in both capacity fade and resistance increase.
- Loss of Lithium Inventory (LLI): this is the loss of active lithium ions. It makes the ions no longer available for cycling. LLI is mainly caused by parasitic side reactions which include lithium plating, decomposition reactions, and surface film formation. This LLI results in capacity fade, which is generally defined as the loss of effective milli-ampere-hour (mAh) of a cell.
- Conductivity Loss (CL): This is the degradation of electrical parts, for example, corrosion of the current collector and decomposition of the binder [48]. CL results in resistance increase.
Another aging mode could be mentioned as loss of electrolytes, which results in LAM and CL after a certain point. These listed degradation modes interact, although they are mentioned separately in literature as listed. For example, a single degradation mode, such as surface layer formation, can trigger several degradation modes. Figure 2 shows aging factors, the corresponding aging mechanisms, and their effect on Li-ion battery aging. Aging occurs at the anode and cathode; therefore, aging is normally described as anode aging and cathode aging, which are detailed in the next subsections.
3.1 Anode Aging
This subsection focuses on anode aging of graphite-based Li-ion batteries. The focus is on graphite-based Li-ion batteries because the anode material that is used most is graphite [42, 48, 53]. Mainly, the aging of these anodes is grouped into five categories. These categories are listed as follows: solid electrolyte interphase (SEI) layer formation, lithium plating, mechanical stress at the anode, transition metal dissolution, and other aging mechanisms. These aging modes are detailed in the following subsections.
3.1.1 Solid Electrolyte Interphase Layer Formation
Organic electrolytes used in Li-ion batteries have a stability window in the range of 1–4.5 Volts (V). Various Li-ion batteries' electrode materials have different operating voltage ranges in relation to the stability window of the electrolytes. For graphite-based anodes, their voltage range is between 0.05 and 1 V [41, 54]. This range is outside the stability window of the electrolyte, making the batteries thermodynamically unstable, which then results in a reductive electrolyte decomposition reaction. This reaction causes a layer to form on the surface of the anode and consumes Li-ions as well, which then results in LLI and LAM [14, 41, 48]. During the initial few cycles of Li-ion batteries, the decomposition reaction rate is high, and a surface layer permeable to Li-ions but less permeable to electrolyte components is created. Due to this process, the decomposition rate decreases [48, 55, 56]. Regardless of the reduced decomposition rate, solvated lithium and other electrolyte components continue to pass through this semipermeable layer throughout the lifetime of the battery. This decomposition reaction occurs mainly at the electrolyte and anode interphase and is referred to as the solid SEI layer. The growth of the SEI layer is regarded as one of the main aging factors of graphite-based Li-ion batteries. The layer affects the capability of the battery in terms of energy as well as power capacities. In addition to this layer, there is also another layer that grows on the basal plane surface and is mostly referred to as the non-SEI layer [57]. This layer is impermeable to Li-ions. In general, both of these layers are commonly called the SEI layer [48]. The formation of the SEI layer continues throughout the lifetime of the battery during both cycling and idle states.
Growth rate of SEI layer is a function of operating conditions of the Li-ion cell [58]. During idle state, the rate is mainly dependent on temperature and SoC. On temperature, the higher the temperature, the higher the reaction rate, which then results in less stable organic SEI components to transform into more stable products, consequently decreasing the conductivity of the SEI layer. Moreover, the cell can catch fire or explode if temperatures go above 60°C due to thermal runaway. On SoC, at higher SoC, there is more intercalation of Li-ions into the anode material, which decreases the anode potential. Decreased anode potential results in increased reductive reaction rate [54]. In addition to temperature and SoC, there are also mechanical stresses on the electrodes during cycling, which result from volume changes as a result of (de) intercalation of Li-ions. These volume changes may result in the formation of cracks in the SEI layer, thereby allowing new reactions to take place, which then makes SEI grow further grow causing additional LLI and LAM [48]. Additionally, cycling Li-ion battery with high Crates results in a more porous SEI layer compared to idle state [58]. This increased porosity creates room for more reductive reactions to occur as compared to a denser layer. Electrode balance and electrolyte composition are other factors that influence reaction rate [56].
3.1.2 Lithium Plating
Temperatures below 20°C cause the diffusion rate of Li-ions into the electrolyte or anode to reduce. This reduction in diffusion rate results in the intercalation potential of the anode material to approach that of a metallic lithium, causing lithium plating. In addition to low temperatures, low SoC and high Crates also cause lithium plating. Lithium plating results in LLI and also causes dendrites to grow, which then reduces the safety of the cell since these dendrites can cause internal short circuits [48].
3.1.3 Mechanical Stress at Anode
During intercalation of Li-ions into the anode, there are rapid volume changes due to particles undergoing phase transition [48]. During this phase transition, different geometrical and electrical properties are realized because the orientation of molecules changes as a result of more molecules of Li-ion being inserted. The resulting geometrical and electrical property changes cause mechanical stress, which leads to surface layer cracking (LLI and LAM), anode structural damage (LAM), and contact loss with the composite electrode (CL). The changes in active material volume can also decrease electrode porosity, thereby blocking the electrolyte from reaching most of the electrode [48].
3.1.4 Transition Metal Dissolution
Cathodes and electrolytes of Li-ion batteries contain impurities. In cathodes, there are metals, for example, manganese, nickel, cobalt, iron, and vanadium, which are referred to as transition metals. Electrolytes, on the other hand, contain a salt called lithium hexafluorophosphate (LiPF6). When lithium cells are cycled or stored at high voltages or temperatures, hydrogen fluoride is formed from the impurities in the electrolytes, which results in increased electrolyte acidity [59]. The formed hydrogen fluoride corrodes cathodes and also dissolves the transition metals. This phenomenon results in many degradation mechanisms at both the anode and cathode, which results in capacity fade or resistance increase of the battery. The dissolved metals can cause structural degradation of the cathode (LAM) which results in reduced insertion capability of the lithium ions [60]. Additionally, these dissolved metals move through the electrolyte to the anode, where they are deposited, and they influence the growth of the SEI layer by acting as catalyzers for the reductive decomposition reaction [61]. Also, when transition metals are deposited on the anode, they induce the formation of lithium dendrites (LLI and LAM), which can cause short circuits inside the battery.
3.1.5 Other Anode Aging Mechanisms
In addition to the described degradation mechanisms, there are also other mechanisms including graphite exfoliation, particle cracking, and binder decomposition. Graphite exfoliation (LAM) results from high current densities or solvent co-intercalation, and particle cracking (LAM) results from high current load or mechanical stress. Binder decomposition (CL) is mainly caused by high cell voltages and high temperatures. Other examples include loss of electrical contact (CL) and corrosion of current collectors (CL). More information about these can be found in literature including in [17, 48, 49].
3.2 Cathode Aging
There are different cathode materials for Li-ion batteries. These materials vary in terms of safety, cost, energy density, power density, toxicity, and natural abundance, to mention a few. Cathode materials that are commonly used are NMC, LFP, LCO, NCA, and LMO. Regarding the aging of Li-ion batteries, cathode aging is considered less significant [62] as compared to anode aging. Transition metal dissolution, surface film formation, and mechanical stress are the main aging mechanisms of the cathode materials. Furthermore, current collector corrosion, binder decomposition, and conducting agent oxidation are listed as other aging mechanisms of the inactive components [48].
3.2.1 Cathode Surface Layer Formation
Due to electrolyte oxidation and salt deposition at the cathode, surface layer films referred to as cathode electrolyte interphase (CEI) layer form [48, 63]. The CEI layer is similar to the SEI layer at the anode. The layer reduces the conductivity of the Li-ion, hence increasing cathode resistance. Furthermore, the organic products that result from the reduction process at the anode can also be transported through the electrolyte to the cathode. At the cathode, they can be oxidized and then get deposited on its surface, resulting in LAM and LLI [17].
3.2.2 Mechanical Stress at Cathode
During intercalation and deintercalation of Li-ions at the cathode, the cathode also goes through phase transitions, similar to that at the anode, resulting in CL and LAM. When transitions are taking place, the cathode material is the determining factor for volume changes. Some materials experience huge changes compared to others, resulting in different aging rates [48].
3.2.3 Transition Metal Dissolution
Similar to anode aging, structural degradation of the cathode (LAM) also takes place at the cathode due to transition metal in the cathode dissolution [59]. Transition metal dissolution effects are similar to the ones at the anode.
4 Comparison of Empirical and Semi-Empirical Aging Models
Several studies have been done on aging of Li-ion batteries. These studies either focus on calendar aging or cycle aging or both. In this review study, the studies which focused on calendar aging have been presented separately from the ones which focused on cycle aging. Even though some studies focused on both, they have been separately presented for clarity's sake under calendar aging studies and cycle aging studies, for example studies done in [64, 65].
4.1 Calendar and Cycle Aging Studies
Studies which focused on calendar aging of Li-ion battery using empirical and semi-EMs are shown in Table 2. Shown in the table are the chemistry of the battery, aging stress factors, aging conditions, capacity fade (cap. fade), resistance increase (res. increase), and the respective reference (ref.).
| Aging conditions | ||||||
|---|---|---|---|---|---|---|
| Battery chemistry | Aging stress factors | Temp. (°C) | SoC (%) | Cap. fade | Res. increase | References |
| LFP | SoC, T, time | 40–55 | 10–90 | X | √ | [66] |
| LFP | SoC, T, time | 40–70 | 60–80 | √ | X | [67] |
| LFP | SoC, T, time | 25–55 | 20–80 | √ | √ | [68] |
| LFP | SoC, T, time | 30–60 | 30–100 | √ | X | [69] |
| LFP | SoC, T, time | 25–55 | 10–100 | √ | √ | [70] |
| LFP | SoC, T, time | 30–60 | 30–90 | √ | √ | [71] |
| NMC | SoC, T, time | 0–45 | 0–100 | √ | √ | [15] |
| NMC | SoC, T, time | 25–45 | 20–100 | √ | X | [72] |
| NMC-LMO | SoC, T, time | 10–46 | 10–90 | √ | X | [64] |
| NMC-LMO & NCA | SoC, T, time | 30–60 | 30–100 | √ | √ | [73] |
| NMC | SoC, T, time | 30–60 | 30–100 | √ | √ | [74] |
| NMC | SoC, T, time | 35–50 | 0–100 | √ | √ | [29] |
| NMC | SoC, T, time | 0–60 | 5–100 | √ | X | [75] |
| NMC | SoC, T, time | 25–60 | 20–100 | √ | √ | [76] |
| NMC | SoC, T, time | 35–50 | 0–100 | √ | √ | [77] |
| NMC | SoC, T, time | 40–70 | 60–80 | X | √ | [78] |
| LFP | SoC, T, time | 0–55 | 0–100 | √ | X | [65] |
| NMC | SoC, T, time | 10–55 | 10–100 | √ | X | [79] |
| LFP | SoC, T, time | 40–70 | 20–100 | √ | √ | [80] |
From the data presented in Table 2, it is noted that all the captured studies considered SoC, temperature (T) and time as calendar aging stress factors. Also, it has been noted that 17 out of the 19 studies (89.47%) presented considered capacity fade as a result of calendar aging. On the other hand, 12 out of the 19 studies (63.16%) presented considered resistance increase as a result of calendar aging. Furthermore, 10 out of the 19 studies (52.63%) considered both capacity fade and resistance increase as results of calendar aging of Li-ion batteries. Therefore, it can be concluded, based on the data in the table, that most studies about calendar aging of Li-ion battery consider capacity fade as a result of aging. In addition to that, capacity fade is a function of SoC, T, and time.
Studies that focused on cycle aging of Li-ion battery using empirical and semi-EMs are shown in Table 3. Shown in the table are the chemistry of the battery, aging stress factors, aging conditions, capacity fade, resistance increase, and respective reference.
| Aging conditions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Battery chemistry | Aging stress factors | Temp. (°C) | SoC (%) | DoD (%) | Crate (C) | Cap. fade | Res. increase | References |
| NMC | SoC, C, T | 40–70 | 50–70 | — | 1–8 | X | √ | [78] |
| LFP | SoC, DoD, C, T | 25 | 53.5–100 | 12.5–75 | 0.22–0.67 | √ | X | [81] |
| NMC | SoC, DoD, C, T | 35 | 0–100 | 10–100 | 1 | √ | √ | [77] |
| NMC | SoC, DoD, C, T | 25–60 | 20–100 | 10–50 | 1 | √ | X | [76] |
| NMC | SoC, DoD, C, T | 0–60 | 0–100 | 3–80 | C/2 | √ | X | [75] |
| NMC-LMO | SoC, DoD, C, T | 10–46 | 10–90 | 10–90 | 0.5–6.5 | √ | X | [64] |
| NMC/LMO & NCA | ΔSoC, C, T | 40–50 | 20–40 | — | C/20-C | √ | √ | [73] |
| LFP | SoC, DoD, C, T | 40–70 | 60–80 | 10–60 | C | √ | √ | [67] |
| LFP | SoC, C, T | 25–55 | 20–80 | — | 4C | √ | √ | [68] |
| LFP | SoC, C, T | 0–55 | 0–100 | 0.25–1 | √ | X | [65] | |
| LFP | DoD, C, T | −30 to 60 | — | 10–90 | C/2–10 | √ | √ | [82] |
| NMC-LMO | SoC, C, T | 10–45 | 25–45 | — | C/3-3C/2 | √ | √ | [83] |
| NMC | SoC, DoD, C, T | 10–46 | 100 (start) | 50 | 0.5–6.5 | √ | X | [79] |
| NMC | SoC, DoD, C, T | 10–50 | 20–80 | 10–100 | C/3-2C | √ | X | [72] |
| LFP | DoD, C, T | 30 | — | 20–80 | 1–6 | √ | √ | [80] |
| NMC | SoC, DoD, C, T | 35 | 10–95 (avg) | 5–100 | 1 | √ | √ | [29] |
| NMC | SoC, DoD, C, T | −10 to 50 | 20–80 (avg) | 10–100 | 1/3–2 | √ | X | [75] |
| NCA | SoC, DoD, T, C | 40–50 | 60–85 (avg) | 20–65 | 18 | √ | X | [80] |
| LFP | SoC, DoD, T, C | 30 | 50 | 5–100 | 1–3.5 | √ | X | [84] |
| LFP & NMC | DoD, T, C | 23–45 | — | 25–100 | 1–8 | √ | √ | [85] |
From the data presented in Table 3, it is noted that all the captured studies (100%) considered temperature and Crate as cycle aging stress factors. It is further noted that 17 out of the 20 studies (85.00%) capture SoC as an aging stress factor. Considering that the change of SoC (ΔSoC) is also referred to as DoD, as previously indicated in this study, it can be stated that all the studies capture SoC as a cycle aging stress factor. Also, it is noted that 19 out of the 20 studies (95.00%) presented considered capacity fade as a result of cycle aging. On the other hand, 10 out of the 20 studies (50.00%) presented considered resistance increase as a result of cycle aging. Furthermore, 9 out of the 20 studies (45.00%) considered both capacity fade and resistance increase as results of cycle aging of Li-ion batteries. Therefore, it can be concluded, based on the data in the table, that most studies about the cycle aging of Li-ion battery consider capacity fade as a result of aging. Furthermore, this aging is mainly influenced by temperature, Crate, and SoC as stress factors.
4.2 Empirical and Semi-Empirical Aging Models
- Calendar aging (only) models (CAOM).
- Cycle aging (only) models (CYAOM).
- Calendar and cycle aging (separated) models (CCYASM).
- Calendar and cycle aging (combined) models (CCYACM).
The CAOM are models that capture calendar aging only in their formulae, while CYAOM are models that capture cycle aging only in their formulae. On the other hand, CCYASM are models that capture both calendar aging and cycle aging, but in separated formulae for each, while CCYACM are models that capture both calendar aging and cycle aging in a single formula.
Models under each group have been assigned codes to ease reference. These codes have a number at the end which represents the model number in the specific group, for example CAOM1 and CAOM2 meaning calendar aging (only) model numbers 1 and 2, respectively. The named and listed four groups of models and models falling under each group, including their codes, have been presented in Table 4.
| Aging models | Cap. fade | Res. increase | No. of vars. and pars. | No. of ele. opers. | Crate | Model code | References | |
|---|---|---|---|---|---|---|---|---|
| Calendar aging only models (CAOM) | ||||||||
where is internal resistance increase (%), is time (months), is temperature (degrees kelvin) and is in percent (%). |
X | √ | 9 | 9 | X | CAOM1 | [66] | |
| Cycle aging only models (CYAOM) | ||||||||
, , Where is capacity loss, is depth of discharge (%), is ampere-hour (charge) throughput and , and are fitting parameters for the model. |
√ | X | 10 | 16 | X | CYAOM1 | [84] | |
, Where is charge/discharge current value, is a constant and its optimal value for specific was found to be as indicated in the following brackets against each current: C/2 (31,630); 2C (21,681); 6C (12,934), and 10C (15,512). is universal gas constant, is temperature in degrees Celsius (°C). |
√ | X | 9 | 8 | √ | CYAOM2 | [82] | |
, where is capacity severity factor function given by the following equation: . where is resistance severity factor function given by the following equation: . Where is ratio of discharging time to sum of discharging time and charge sustainability time. is a function of , and is eqaul to 0 if is 0, and is equal to if is greater than 0. The constant coefficients for the model are as follows: = 137, = 420, = 9610, = 0.34, = 3, = 0.48, = 0.25, = 3.2exp5, = 1.37exp9, = 3.6exp3, = 5.45, = 0.9179, = 1.8277, = 5, = 22,406 (J mol−1) and = 8.314 (J K−1 mol−1). |
√ | √ | 23 | 33 | √ | CYAOM3 | [83] | |
, , Where and are battery capacity and internal resistance, respectively, at cycle number n, and are the battery capacity at beginning of life () and end of life () respectively, and are battery resistance at and respectively, and are the aging exponents for the battery capacity and resistance respectively, and is aging index after each cycle. Aging index is given by the following equation: , , . Where is number of cycles, is equivalent number of cycles, is reference number of cycles, is total stress factor, is reference depth of discharge, and are average charge and discharge currents with their references and respectively, is reference temperature, is ambient temperature, is Arrhenius rate and are stress exponents for DoD, discharge and charge currents respectively. Estimated values for are 9175, 0.8, 3.7e3, 0.8, 2.34, and 0.9708, respectively. |
√ | √ | 33 | 34 | √ | CYAOM4 | [85] | |
|
Calendar and cycle aging separated models (CCYASM) |
||||||||
| Calendar model | Cycle model | |||||||
|
. Where and are constant coefficients and is time in days. |
. Where B is a constant. |
√ | X | 14 | 12 | √ | CCYASM1 | [79] |
Where and are cycle depth and cycle number respectively. |
√ | √ | 32 | 38 | X | CCYASM2 | [68] | |
With Where is life time in years. |
Where the coefficients are given as follows: 8.61E−6 1/Ah-K2, −5.13E−3 1/Ah-K, = 7.63E−1 1/Ah, −6.7E−3 1/K, 2.35 1/(Crate), 14,876 1/day1/2 and 24.5 kJ mol−1. |
√ | X | 26 | 35 | √ | CCYASM3 | [64] |
Where V is battery voltage (volts). Parameters of model are given as follows: = 1.14e−3, = 258, = 2.01, = −3.91e−3, = 310.9, = −124.36, = 2.357e−14 and = 4.471e−10. |
∆EOL is relative variation necessary to reach EOL criterion. = 0.2 and = 1. NEFC represents the number of equivalent full cycles and is given by the following relationship: , where = 27453.96, = −0.711, = 16619.58 and = −0.4898, are aging coefficients. |
√ | √ | 19 | 27 | X | CCYASM4 | [80] |
, , Where and are calendar aging factors for capacity and resistance respectively. |
, , and Where and are cycle aging factors for capacity fade and resistance increase respectively, is quadratic average voltage and is linear cycle depth. |
√ | √ | 34 | 35 | X | CCYASM5 | [29] |
|
Calendar and cycle aging combined models (CCYACM) |
||||||||
and . where is capacity degradation rate, and values for are 8.314, 72,580, 0.092 and 170.22 J mol−1 K−1 respectively for capacity degradation. is resistor increase rate, and the values for values are 8.314, 57,520, 0.35 and 112.92, respectively. |
√ | √ | 17 | 25 | √ | CCYACM1 | [73] | |
Where is an event, is charge processed during event , is total number of events, is SoC standard deviation, is average SoC, is temperature during event , is reference temperature, −4.092E−4, −2.167, 1.408E−5 and 6.130. |
√ | X | 12 | 16 | X | CCYACM2 | [81] | |
, where , , , and . is calendar stress factor, is calendar reference stress factor, is parameter activation energy, is reference temperature, anode open circuit potential, anode open circuit reference, α = 0.384, = 0.142, is Faraday's constant, is cycle stress factor, is high temperature, is low temperature, is charge current, is charge current reference, is nominal cell capacity, is 2.64 h, is time in days, is total charge throughput, charge throughput in charge direction and is 7.8 h. |
√ | X | 31 | 52 | √ | CCYACM3 | [65] | |
- Note: Shown in the table are aging models, that is, calendar aging (only) models (CAOM), cycle aging (only) models (CYAOM), calendar and cycle aging (separated) models (CCYASM), and calendar and cycle aging (combined) models (CCYACM).
As highlighted earlier that the study focuses mainly on models' complexity and their ability to capture current fluctuations (Crate); these factors have been indicated against each extracted model as shown in the same Table 4. Model complexity has been presented using the total number of variables and parameters (no. of vars. and pars.) and the number of elementary operations (no. of ele. opers) for each model [86]. Furthermore, information regarding whether the model presents Li-ion battery aging as either capacity fade (cap. fade) or resistance increase (res. increase) or both has been presented in the same Table 4 as well.
5 Discussion of Results
Extracted data from the listed empirical and semi-EMs for Li-ion battery calendar aging and cycle aging presented in Table 4 has been summarized and presented in Table 5 to ease readability. Specifically, shown in Table 5 are assigned model code, number of variables and parameters (no. of vars. and pars.), number of elementary operations (no. of ele. opers.), current fluctuation modeling (Crate), capacity fade (cap. fade), and resistance increase (res. increase). Additionally, the total number of variables, parameters, and operations has been calculated and included as well for analysis purposes.
| Assigned model code | No. of vars. and pars. | No. of ele. opers. | Total number of vars, pars. and opers. | Crate | Cap. fade | Res. increase |
|---|---|---|---|---|---|---|
| CAOM1 | 9 | 9 | 18 | X | X | √ |
| CYAOM1 | 10 | 16 | 26 | X | √ | X |
| CYAOM2 | 9 | 8 | 17 | √ | √ | X |
| CYAOM3 | 23 | 33 | 56 | √ | √ | √ |
| CYAOM4 | 33 | 34 | 67 | √ | √ | √ |
| CCYASM1 | 14 | 12 | 26 | √ | √ | X |
| CCYASM2 | 32 | 38 | 70 | X | √ | √ |
| CCYASM3 | 26 | 35 | 61 | √ | √ | X |
| CCYASM4 | 19 | 27 | 46 | X | √ | √ |
| CCYASM5 | 34 | 35 | 69 | X | √ | √ |
| CCYACM1 | 17 | 25 | 42 | √ | √ | √ |
| CCYACM2 | 12 | 16 | 28 | X | √ | X |
| CCYACM3 | 31 | 52 | 83 | √ | √ | X |
- Only one model was found under CAOM. Furthermore, it has been noted that most researchers model calendar aging using Arrhenius law [15, 76] for temperature dependency, power laws for time dependency, and exponential models for SoC dependency. An example of the model equation of which is presented under calendar aging of Table 4 (CAOM1).
- Four models under CYAOM were extracted. Of these four, CYAOM1 does not consider current fluctuation in its model. Furthermore, CYAOM1 models capacity fade only, not resistance increase. Model CYAOM2 considers current fluctuations and models capacity fade as well; however, it does not model resistance increase. Models CYAOM3 and CYAOM4 model current fluctuation, capacity fade as well as resistance increase. In terms of model complexity, it is noted that model CYAOM4 has the highest total number of variables, parameters, and operations (67), making it more complex in that category. Model CYAOM2 has minimum complexity (17). Therefore, in general, it can be stated that models in this category which capture current fluctuations, capacity fade, and resistance increase are more complex than the ones which do not capture all three considered factors.
- Five models under CCYASM were extracted as shown in the summary table (Table 5). It is noted that all these models model capacity fade of the batteries under consideration. However, out of these five models, two further capture current fluctuations (CCYASM1 and CCYASM3) but not resistance increase, and vice versa for the remaining three models (CCYASM2, CCYASM4, and CCYASM5). On complexity, it is noted that models which capture current fluctuations are less complex as compared to those which do not capture them, with the exception of model CCYASM4, which has 46 total number of variables, parameters, and operations. There is no model in this group that captures all three factors considered. Furthermore, it is also noted that models that capture both capacity fade and resistance increase are more complex, on average, as compared to those that capture current fluctuations and capacity fade only.
- Three models under CCYACM were extracted. Out of these models, one model (CCYACM1) captures all three factors, namely current fluctuations, capacity fade, and resistance increase. For the remaining two models, CCYACM2 captures capacity fade only, while CCYACM3 captures capacity fade as well as current fluctuations. In terms of complexity, it is noted that model CCYACM3 is more complex (83) than the other two models, CCYACM1 (42) and CCYACM2 (28).
5.1 Key Findings
The extracted data regarding current fluctuation modeling, capacity fade, and resistance increase modeling of the models summarized in Table 5 has been further summed up for all the models considered. After which, a graph showing how current fluctuation, capacity fade, and resistance increase modeling have been represented in the considered models has been plotted as shown in Figure 3.
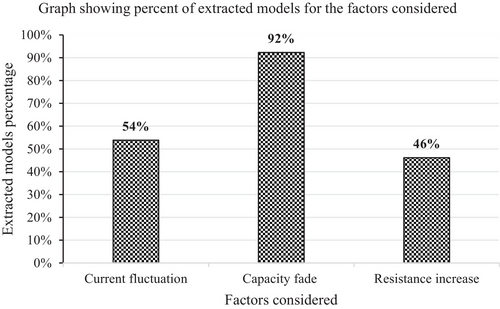
- During modeling aging of Li-ion batteries, much focus is on capacity fade as compared to resistance increase (power fade). This is evidenced in Table 5 as almost all studies, 12 out of 13 (92.30%) considered capacity fade during aging modeling of the battery. Of these 12 studies, only 6 (50.00%) considered resistance increase as well during aging modeling. The remaining one study that did not consider capacity fade considered resistance increase only, and it focused on calendar aging only of the battery.
- Of the 13 extracted cycle and calendar aging models, 7 models (53.85%) considered current fluctuations as one of the input variables to aging models. This shows that Crate of Li-ion batteries is an important factor when it comes to the aging of the Li-ion battery.
- There are 7 models (53.85%) that model capacity fade as a function of current fluctuation, while there are only 3 models (23.07%) that model resistance increase as a function of current fluctuation. This shows that current fluctuation is much more considered when modeling capacity fade of Li-ion battery as compared to when modeling resistance increase.
Based on these findings and the fact that the study focused on Li-ion battery aging modeling for BMS for off-grid RESs, mainly considering current fluctuation representation and complexity (considering minimum complexity), it is noted that there are three models that meet these requirements. These three models fall under CYAOM, CCYAOM, and CCYACM. The three model codes are CYAOM2, CCYASM1, and CCYACM1, and they have been extracted from Table 5 and presented in Table 6. Other extracted information is the total number of variables, parameters, and operations (renamed to complexity), current fluctuation modeling, capacity fade modeling, and resistance increase modeling.
| Assigned model code | Complexity | Crate | Cap. fade | Res. increase |
|---|---|---|---|---|
| CYAOM2 | 17 | √ | √ | X |
| CCYASM1 | 26 | √ | √ | X |
| CCYACM1 | 42 | √ | √ | √ |
It is noted from Table 6 that CYAOM2 has minimum complexity among the three models; however, it only models cycle aging, disregarding calendar aging. Among the three, CCYACM1 has the highest complexity; this is due to the inclusion of resistance increase in the model. The other two models do not include resistance increase, as seen in the table. All these three models presented in Table 6 could be used depending on users' needs regarding aging monitoring and control of Li-ion batteries in off-grid RESs. However, if minimum complexity and current fluctuation representation are among the requirements for BMS for off-grid RESs, then the study recommends selecting either CYAOM2 or CCYASM1, as they have lower complexity compared to CCYACM1. Finally, the choice between CYAOM2 and CCYASM1 is dependent on whether the target is to capture cycle aging only or cycle and calendar. If cycle aging only is needed, then CYAOM2 is the option; otherwise, CCYASM1 is the option.
6 Conclusions and Recommendations
In this research paper, empirical and semi-empirical Li-ion battery aging models have been studied to identify appropriate models in terms of complexity and current fluctuation representation for BMS application in off-grid RESs. In this regard, different studies that focused on calendar aging and cycle aging of Li-ion battery and modeling of the same using empirical and semi-EMs were listed from the literature. After which, aging models were extracted and evaluated in terms of complexity, current fluctuation representation, capacity fade modeling, and resistance increase modeling. These evaluations eventually resulted in identifying appropriate Li-ion battery aging models for BMS application in off-grid RESs.
- When modeling aging of Li-ion batteries, capacity fade is mostly utilized compared to resistance increase. This capacity fade is influenced by SoC, T, and time during calendar aging, and mainly by temperature, Crate, and SoC during cycle aging.
- Empirical and semi-EMs of Li-ion battery aging that capture both capacity fade and resistance increase are more complex than those that capture only one of these parameters.
- Three models meet BMS requirements for off-grid RESs considered in this study, which are current fluctuation representation and complexity. These three models fall under CYAOM, CCYAOM, and CCYACM. The three model codes are CYAOM2, CCYASM1, and CCYACM1. Any of these models can be implemented in Li-ion battery BMS for aging monitoring and control depending on the specific needs of the user.
- Compare empirical and semi-EMs through both simulation and experimental studies using selected commonly available Li-ion battery chemistries. This would assist in validating the models using similar conditions, hence improving confidence levels for implementation in BMS for off-grid RESs. This would also make it possible to establish accuracy levels of the models when deployed for different Li-ion battery chemistries.
- Evaluate the empirical and semi-EM using case studies to establish the appropriate way of representing Li-ion battery aging, whether in terms of capacity fade or resistance increase. This could be vital in further improving the models in terms of complexity and accuracy for different applications, including in off-grid RESs.
- Characterize model-based BMS aiming at matching with appropriate empirical and semi-EMs for off-grid RESs implementation for reduced complexity and current fluctuation representation. This would facilitate deployment of efficient model-based BMS resulting in reduced overall Li-ion battery aging rate.
Nomenclature
-
- Ah
-
- Ampere-hour
-
- BMS
-
- battery management system
-
- CAOM
-
- calendar aging (only) models
-
- CCYACM
-
- calendar and cycle aging (combined) models
-
- CCYASM
-
- calendar and cycle aging (separated) models
-
- CEI
-
- cathode electrolyte interphase
-
- CL
-
- conductivity loss
-
- Closs
-
- capacity loss
-
- Crate
-
- current rate
-
- CYAOM
-
- cycle aging (only) models
-
- DoD
-
- depth of discharge
-
- Ea
-
- activation energy
-
- ECM
-
- equivalent circuit models
-
- EM
-
- empirical model
-
- ESS
-
- energy storage system
-
- FF
-
- fossil fuel
-
- LAM
-
- loss of active material
-
- LCO
-
- lithium cobalt oxide
-
- LFP
-
- lithium-iron-phosphate
-
- Li-ion
-
- lithium-ion
-
- LLI
-
- loss of lithium inventory
-
- LMO
-
- lithium-manganese oxide
-
- LTO
-
- lithium-titanate-oxide
-
- ML
-
- machine learning
-
- MLM
-
- machine learning model
-
- NCA
-
- nickel-cobalt-aluminum
-
- NMC
-
- nickel-manganese-cobalt
-
- PBM
-
- physics-based model
-
- R
-
- universal gas constant
-
- RE
-
- renewable energy
-
- RES
-
- renewable energy system
-
- Ri
-
- internal resistance increase
-
- SEI
-
- solid electrolyte interphase
-
- SoC
-
- state of charge
-
- SoH
-
- state of health
-
- T
-
- temperature in degrees Celsius
-
- td
-
- time in days
-
- Tk
-
- temperature in degrees Kelvins
-
- tm
-
- time in months
-
- ΔSoC
-
- change of SoC
Author Contributions
Isaac Gwayi: conceptualization, investigation, writing – original draft, methodology, validation, visualization, writing – review and editing, data curation, formal analysis. Sarah Paul Ayeng'o: conceptualization, writing – review and editing, supervision, methodology. Cuthbert Z. M. Kimambo: conceptualization, methodology, writing – review and editing, supervision.
Acknowledgments
The authors acknowledge support from Malawi University of Business and Applied Sciences (MUBAS) and from the Department of Mechanical and Industrial Engineering, University of Dar es Salaam (UDSM).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




