Hydrothermal Liquefaction of Jatropha curcas (J. curcas) Under Subcritical Water Conditions: Water and Palm Oil Mill Effluent as Solvents
Funding: This work was supported by Faculty of Civil Engineering Technology, Universiti Malaysia Perlis (FRGS/1/2019/STG05/UNIMAP/02/4).
ABSTRACT
In this study, palm oil mill effluent (POME) and water were used as the medium or hydrogen donor solvent in the hydrothermal liquefaction (HTL) of Jatropha curcas. POME, with its high organic compound content, is seen as a promising solvent to be investigated. The POME analysis using GC–MS showed the existence of palmitic and oleic acids. The HTL was performed for both solvents using a batch reactor, and three parameters were varied (biomass-to-solvent ratio, temperature, and reaction time). The results showed that POME could be used as a medium for the HTL of J. curcas. It was found that the HTL of J. curcas with POME produced a higher oil yield (63.4%) than the one using water (43.2%) with a lower biomass-to-solvent ratio (1:2) and lower temperature (300°C) with an optimum reaction time (60 min), and with 30.8% of solid and 5.8% of gas recorded. The results of gas chromatography–mass spectrometry (GC–MS) showed that high ester content and the lowest acid content were obtained at 350°C for both solvents. As for POME, the ester content increased at 350°C while the acid content decreased at the same temperature. High hydrocarbon content was obtained for the experiment at 300°C for both solvents. The highest oil yield under POME conditions recorded a good HHV value of 39.07 MJ/kg with an oxygen content of ~11%, with 81% carbon recovered, indicating high energy recovery. The extra hydrogen generated through the reforming of POME in the liquefaction of J. curcas leads to stabilizing the free radicals and producing high oil yields.
1 Introduction
Over the past decades, research has focused on renewable and sustainable energy sources due to increasing urbanization, which demands more energy due to the exhaustion of fossil fuel sources (e.g., natural gas, petroleum, coal). Besides renewable energy sources such as solar, wind, and hydropower, biomass has gained attention as a promising energy source to be explored, as it is renewable and has less environmental impact of carbon dioxide (CO2) emissions than fossil fuels. Among the examples of biomass and waste feedstock are woods, crops, animal waste, municipal solid waste, and wastewater sludge. Biomass-based liquid hydrocarbon, hydrogen, and biogas can be potentially used as substitutes for fossil fuels [1].
Biomass can be upgraded into biofuels via thermochemical conversion, such as gasification, combustion, pyrolysis, and hydrothermal liquefaction (HTL) [2-6]. HTL has become an important route as compared to pyrolysis due to its easier process, such as avoiding the drying process. The advantage of utilizing wet biomass is that it can be fed directly in HTL, which can reduce the energy used for drying. The process can also be conducted at lower temperatures and heating rates, generally at 250°C and above, and the pressure between 10 and 25 MPa [1, 7, 8]. Besides, HTL allows the formation of hydrogen and hydroxyl ions, which promotes the cleavage of biomass bonds to the products [9]. Also, bio-oil from HTL contains low nitrogen and sulfur concentrations, high energy density, and is CO2 neutral [10]. In HTL, water can function as a reactant, product, and a catalyst.
Moreover, water has a relatively low dielectric constant, low viscosity, and large ion products in subcritical water conditions. According to Weatherley and Rooney [11], the dielectric constant of subcritical water is comparable to that of methanol or acetone under ambient conditions, which makes it an acceptable solvent to dissolve organic compounds. Another distinct advantage of using subcritical water is the capability to attain the same results as a process that utilizes either an alkaline or acidic catalyst [12]. According to Balagurumurthy et al. [13], water can act as a catalyst at high temperatures and pressures by revealing both acidic and basic properties.
Water emerges as a green and environmentally friendly solvent, non-toxic, and non-flammable. Water close to its critical point shows interesting properties. According to Meryemoğlu et al. [14], heated and pressurized solvent has the capability to alter the complex structure of biomass. Many researchers have investigated alternative solvents to substitute the use of organic solvents as potential reaction media. Nevertheless, other solvents like palm oil mill effluent (POME) can be explored as a potential solvent in the HTL of biomass under subcritical conditions. Besides, POME is a potentially good option for hydrogen donor solvents in biodiesel production. According to Isa, Abdullah [15], a hydrogen donor solvent stabilizes free radicals in biomass and leads to higher product conversion. Apart from that, it also shows significant improvements in liquid yield and the quality of bio-oil produced via hydrocracking [15].
The liquefaction using hydrogen donor solvents was carried out previously; the utilization of cyclohexanol as the solvent for the hydrogenation of wood lignin was reported by Duan et al. [16]. The method conducted was to suggest a minimum degree of processing to obtain a high-quality liquid yield. Two mechanisms were proposed and reported: (1) The bonding-cleavage by heating up the biomass. (2) Hydrogen atoms prevent repolymerization due to biomass decomposition. Many solvents were reported to act as hydrogen donors as long as they have mobile carbon–hydrogen bonds with the ability to donate hydrogen to stabilize the unstable biomass fragments [15]. Other hydrogen donor solvents, such as ethanol, were found to improve bio-oil yield and quality compared to water [17]. They found that the condition with an ethanol–water solvent system, with a solvent-to-lignin mass ratio of 10, a temperature of 250°C, and a reaction time of 2 h, produced maximum bio-oil yield with high antioxidant compounds compared to water. In the HTL stage, the act of hydrogen donation from the solvent was more efficiently employed compared to gaseous hydrogen to produce bio-oil [18].
POME is a form of environmental pollution where the wastewater generated alongside crude palm oil production causes issues in the palm oil industry due to its polluting characteristics [10]. As mentioned by [11], this thick brownish liquid formed by POME gives a very high reading for its chemical oxygen demand (COD), biochemical oxygen demand (BOD), concentration of suspended solids, and pH reading, which is acidic; thus, POME is not suitable to be released or discharged into watercourses directly. The release of effluent into either a watercourse or a land area needs to comply with the rules and regulations set by the Department of Environment (DOE) under the Environmental Quality Act 1974. These regulations are known as the Environmental Quality (Prescribed Premises) (Crude Palm Oil) Regulations 1977. In addition, the release of effluent also needs to adhere to the BOD and COD reading levels for disposal emphasized by the DOE. Furthermore, POME has a high organic content, which makes it a good source in gas production, such as hydrogen. Therefore, using POME as an HTL solvent could generate hydrogen in the reaction vessel. A previous study by Sarawut et al. [17] reported that hydrogen decreased char formation and increased bio-oil yield compared to using water as a solvent. Hydrogen promoted the breakdown of the structure of the raw material and prevented condensation, cyclization, and repolymerization reactions of the intermediates to decrease char formation [17].
POME is generally treated using a biological treatment system, which is also known as large shallow ponds [19]. The pond depth cannot be too deep; hence, the surface area is increased to achieve large reaction volumes. The limitation on depth mostly arises from the problem of oxygen penetrating the bottom of a deep pond [20, 21]. Consequently, the successful treatment of POME requires the utilization of large land areas and extended hydraulic retention times (HRTs) of about 40 days through the implementation of a series of aeration ponds. However, according to Loh [22], this approach is regarded as obsolete, and hence, POME treatment is not effective. The failure of the treatment system can be attributed to the sensitivity of the microorganisms in the treatment ponds to climate changes, particularly temperature changes and pH variations [23]. In addition, it should be noted that shallow ponds necessitate a large land area to facilitate the release of noxious gases, such as hydrogen sulfide and methane, into the surrounding environment. This process requires an extended HRT. The progress of the POME treatment system is assisted by the widespread distribution of information concerning the hazards linked to POME.
There is no study on POME as a solvent in the liquefaction process. Therefore, the extra hydrogen generated through reforming or cracking of the organic compounds of POME in the liquefaction of biomass is interesting to explore. The reason J. curcas is chosen in this study is because of its potential as an oil seed and its high lipid content. The previous study reported the lipid content in J. curcas seed as high as 66.4% [24]. Researchers selected oil seed biomass as feedstock in thermochemical conversion due to its high lipid content compared to other biomass [25]. It is also suggested that lipid content in raw material plays an important role in accomplishing a higher bio-oil yield. This work provides information on the advantages of utilizing hydrogen donors as a solvent.
2 Methods
2.1 Biomass
In this study, J. curcas seeds were used as feedstock in the liquefaction process, which were collected in Johor, Malaysia. The seeds were washed to remove impurities and oven-dried at 105°C for 24 h. Upon completion, the seeds were ground using a mortar and pestle to separate the kernel and shell. The kernel (inner part) was used as feedstock in the liquefaction process. The selected seed sample was carefully stored in an air-tight container to prevent moisture ingress.
2.2 POME Solvent
Raw POME, which was used as a solvent, was collected from Setiawan Sdn. Bhd., a palm oil mill company in Kulim, Malaysia. Later, POME was stored in huge bottles and kept inside cold storage.
2.3 Feedstock Characterization
2.3.1 Proximate Analysis
Proximate analysis was carried out using a thermogravimetric analyzer (Perkin Elmer STA 8000) from the Institute of Hydrogen Economy, University Teknologi Malaysia (UTM), Kuala Lumpur, Malaysia. The moisture content, volatile matter, fixed carbon, and ash content of the J. curcas sample can be obtained using the proximate analysis method. During the analysis, a standard weight of J. curcas sample was maintained. The pyrolysis and combustion of J. curcas were performed under inert nitrogen gas and purified air with a constant flow rate of 100 mL/min and a heating rate of 20°C/min. The sample (16 mg) was weighed directly into the alumina crucible, and the temperature was kept isothermal for 0.5 min until a steady condition was obtained before ramping to the desired temperature [26]. The experiments were replicated at least twice to obtain reproducibility.
2.3.2 Ultimate Analysis
An elemental analyzer (Series II CHNS/O 2400) from Centralized Analytical Laboratory, University Teknologi Petronas, Banda Seri Iskandar, Perak, Malaysia was used to conduct the ultimate analysis of J. curcas, and helium was employed as the carrier gas. The percentages of carbon and hydrogen of the J. curcas sample were measured, followed by calculating the oxygen percentage by subtracting the combined percentages of carbon, hydrogen, nitrogen, and sulfur. For the analysis using the elemental analyzer, 2 mg of the sample was inserted into the tin capsule and then introduced into the sample chamber.
2.3.3 Heating Value
2.4 POME Characterization
2.4.1 pH
In this research, the pH of the POME samples was checked using a pH meter and recorded. Buffer solutions with pH values of 4 and 7 were used to calibrate the pH meter to verify its efficacy and accuracy.
2.4.2 Total Solids (TS)
2.4.3 Total Volatile Solids (TVS)
2.4.4 Mixed Liquor Suspended Solids (MLSS)
A is the weight of dried residue + fiber disk + crucible (post-weight), mg and B is the weight of fiber disk + crucible (pre-weight), mg.
2.4.5 Mixed Liquor Volatile Suspended Solids (MLVSS)
The volatile solids concentration in a mixed liquor sample primarily comprises microorganisms and organic matter. Consequently, this concentration serves as a proxy for the quantity of microorganisms present in the water and is useful for assessing microbial abundance. Following the completion of MLSS determination, the same crucibles that were initially used for MLSS analysis were repurposed for the MLVSS experiment.
A is the weight of dried residue + fiber disk + crucible (post-weight), mg and B is the weight of fiber disk + crucible (pre-weight), mg.
2.5 Hydrothermal Liquefaction
Prior to commencing the experiment, the reactor's interior was purged with nitrogen to create an inert environment. In the liquefaction procedure, 5 g of J. curcas was introduced into a 0.1-L CJF stainless steel reactor, and a predetermined amount of either POME or water was added. The liquefaction procedure adhered to specific parameters, including the set temperature, biomass-to-solvent ratio, and reaction time. The pressure started to rise with the temperature from 10 MPa until it reached 20 MPa. After the reaction was finished, the reactor was cooled to room temperature.
The resulting products, which included both solid and liquid components, underwent a washing step with approximately 150 mL of dichloromethane (DCM). These washed products were subsequently transferred into a 250-mL conical flask.
The solid–liquid mixture was subsequently separated using filter paper. The filtered liquid product was transferred to a fume hood to accelerate the evaporation of DCM. The resulting crude oil was carefully measured and its properties were recorded for further analysis.
2.5.1 Biomass-To-Solvent Ratio
The experimental design involved setting the mass ratio of biomass to solvent at three different ratios (1:2, 1:4, and 1:6). These experiments were carried out at a constant temperature of 300°C with a fixed reaction time of 60 min while maintaining a stirring rate of 540 rpm. In order to guarantee the dependability of the findings, each experiment was repeated three times, and the results were documented.
2.5.2 Temperature
The effect of temperature at 300°C, 330°C, 350°C, and 365°C with stirring at 540 rpm was investigated for 60 min. A constant ratio of 5 g of biomass to 20 g of solvent was used for this set of experiments. The experiments were repeated three times and recorded.
2.5.3 Reaction Time
A series of tests were carried out using reaction times of 30, 60, and 90 min to investigate the effect of reaction time on the production of HTL compounds. The temperature and biomass-to-solvent ratio from the previous experiment, which gave the highest yield of oil product, were chosen for this set of experiments. The experiments were repeated three times and recorded.
2.6 Products Yield
2.7 Characterization of Oil/POME
2.7.1 GC–MS Analysis
The oils were subjected to gas chromatography–mass spectrometry (GC–MS) analysis using a Shimadzu QP2010 Ultra instrument. About 1 μL of the oils dissolved in DCM was used for the analysis with the source temperature of 280°C. The separation procedure was conducted utilizing a fused silica capillary column BPX5 (length = 30 m, ID = 0.25 mm, film thickness = 0.25 μm). The carrier gas used in this experiment was helium. The temperature program consisted of a starting temperature of 35°C for a duration of 2 min, followed by a linear increase to 250°C at a rate of 20°C/min, and the final temperature was maintained for 20 min. The full scan mode was selected with a split ratio of 1:30. The experiments were replicated three times to assess their repeatability. The GC–MS analysis for POME was outsourced to the accredited ISO17025 Laboratory (Bio Synergy Laboratories Sdn Bhd).
2.7.2 Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The elemental analyses and FTIR were conducted using the methodology outlined by previous researchers [16]. The heating value was calculated using Dulong's equation.
3 Results and Discussion
3.1 Characterization of J. curcas
The properties of J. curcas were examined and summarized in Table 1. In the proximate analysis, it was found that J. curcas seeds contained a maximum volatile matter content of 77.9%. This high volatile matter content is significant because it gives a high possibility of a greater yield of liquid and gaseous fuel during HTL. Additionally, the fixed carbon content of J. curcas seeds was measured at 21.83%, while the moisture content was found to be 3.66%. These properties are crucial factors to consider when evaluating the potential for fuel production via HTL.
| wt. (%) | |
|---|---|
| Proximate analysisa | |
| Moisture content | 3.66 |
| Volatile matter | 77.92 |
| Fixed carbon | 21.83 |
| Ash content | 0.24 |
| Ultimate analysisa | |
| Carbon (C) | 58.64 |
| Hydrogen (H) | 9.33 |
| Nitrogen (N) | 5.53 |
| Sulfur (S) | 0.46 |
| Oxygenb (O) | 26.04 |
| Heating value | 28.56 MJ/kg |
- a As received.
- b By difference.
The composition of J. curcas seeds was found to consist of 58.6% carbon, 26.0% oxygen, 9.3% hydrogen, 5.5% nitrogen, and 0.5% sulfur. The seeds possess low sulfur content, rendering them well-suited for HTL processes for producing a high-quality liquid output. The calculated heating value of J. curcas seeds in this study was determined to be 28.56 MJ/kg using the Dulong equation. The high heating value (HHV) in this study is reasonably higher compared to other researchers, indicating that the seeds are much more suitable as feedstock for HTL.
3.2 Characterization of POME
The POME samples were characterized for pH, temperature, TS, TVS, MLSS, MLVSS, and COD. The results of the experiment are presented in Table 2. It shows the comparison between the characteristics of fresh POME used in this study and the published data in the literature. For the present study, all the characteristics of the collected POME are in the range of POME as previously reported by Loh [22], Nasrullah et al. [28], and Ng et al. [29], except for TVS. This is caused by the higher organic content of the collected samples. Apart from that, the TVS value can be estimated as the biogas potential [28]. The biogas, which has methane as the main component, that was produced from the volatilization of TSs can be used for heat in the HTL process. Thus, the advantage of high TVS produces high biogas, which serves as a heat source for the reaction system.
The raw POME has a lower pH. As the pH of raw POME is below pH 4.3 (i.e., pH 4.063), the total alkalinity test cannot be conducted as the test requires the sample to reach pH 4.3 of the end titration value. The pH result is similar to Loh et al.'s previous study. Raw POME is a thick brown effluent containing mixtures of oil, water, and suspended solids known to have high organic acid content, which eventually leads to its acidic pH value. Raw POME has a higher TS content of 35,980 mg/L and 35,162.67 mg/L of COD. Table 3 shows the main compounds of POME, which were palmitic acid and oleic acid via GC–MS analysis.
| Organic contents | Area (%) |
|---|---|
| Heptanoic acid | 2.18 |
| Cyclohexanecarboxylic acid | 0.89 |
| Octanoic acid | 1.01 |
| 1 s,4R,7R,11R-1,3,4,7-Tetramethyltricyclo[5.3.1.0(4,11)]undec-2-en-8- one | 3.02 |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 1.51 |
| Palmitic Acid, TMS derivative | 54.87 |
| n-Hexadecanoic acid | 1.78 |
| Oleic acid | 28.45 |
| Octadecanoic acid, 4-hydroxy-, methyl ester | 0.64 |
| Hexadecanoic acid, 2-(octadecyloxy)ethyl ester | 0.81 |
| Ethyl iso-allocholate | 0.83 |
| Dodecyl cis-9,10-epoxyoctadecanoate | 2.12 |
| 17-Pentatriacontene | 1.89 |
3.3 Effect of HTL Parameters on Oil Yield
Different types of parameters (i.e., biomass-to-solvent ratio, temperature, and reaction time) were studied in this work, which aims to obtain higher oil yield under optimized one-factor-at-a-time conditions.
3.3.1 Effect of Biomass-To-Solvent Ratio on Oil Yield
The effect of biomass-to-solvent ratio on the amount of oil produced for both water and POME is illustrated in Figure 1. The experiments were performed by keeping the reaction time at 60 min and temperature at 300°C. As can be seen from Figure 1, the percentage of oil yield using water solvent increased by 2.4% from the ratio of 1:2 to 1:4 due to enhanced extraction by water [30]. The addition of water has the potential to enhance the rate of the hydrolysis reaction and increase the depolymerization of biomass [30, 31]. Nevertheless, the increase in ratio resulted in a reduction of oil yield by 16.4%. At high biomass-to-solvent ratios, the interactions between biomass and solvent molecules diminish, hence preventing the degradation of biomass fragments [31].
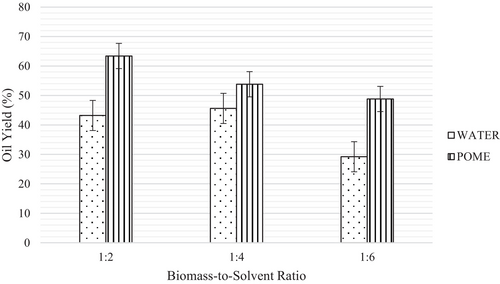
In a previous study conducted by Wang et al. [25], they performed HTL on Litsea cubeba seeds using various solvent loadings ranging from 0.5 to 4.5 g, with water as the solvent. Their findings indicated that the bio-oil yield exhibited an increase from 42.4% to 56.9% by weight as the reactor loading increased from 0.5 to 2.5 g. However, it was observed that as the solvent loading was further increased, the bio-oil yield began to decrease in this study. This information underscores the influence of solvent loading on bio-oil production during the HTL process.
In contrast to water, POME gives the highest percentage at a lower ratio, which was 63.4% of oil yield. However, the oil yield started to decrease as the amount of POME increased. According to Ahamed Kameel et al. [32], an adequate amount of solvent allows intermolecular hydrogen transfer during the reaction of the product. POME contains organic compounds where hydrogen is potentially generated through reforming or cracking of the organic. Therefore, extra hydrogen stabilized the free radicals leading to the formation of high bio-oil yield.
POME can act as a hydrogen donor to stabilize free radicals derived from this biomass, as well as inhibit condensation, cyclization, and repolymerization reactions [33]. The presence of hydrogen donors in POME promotes the hydrogenation of organic particles generated from the hydrothermal degradation of the biomass [34]. Thus, the act of hydrogen donation from POME indicates that the ratio of 1:2 is sufficient for POME to degrade and stabilize the free radicals of biomass fragments into bio-oil. Hence, the production efficiency of bio-oil is relatively high at this ratio. An increase in biomass-to-solvent ratio favors char formation using POME as a solvent due to the higher MLVSS for the tested POME.
3.3.2 Effect of Temperature on Oil Yield
Figure 2 shows the results of the effect of temperature on bio-oil yield using water and POME. A biomass-to-solvent ratio of 1:2 and a reaction time of 60 min were applied for this series of experimental work. The ratio was chosen considering the best conditions for POME from the previous experiment. It was observed that there was no significant difference in bio-oil yield using water at the tested temperatures. This is because water requires a higher amount of biomass-to-solvent ratio for a greater dissolution effect. Kaur and Gera [35] conducted a study on the HTL of castor seed in the presence of water. The researchers noticed that the highest yield of bio-oil from castor oil (15.8 wt.%) was achieved at a temperature of 300°C for a duration of 60 min. The observed reduction in bio-oil production at a high temperature (365°C) can be primarily attributed to the increased generation of condensable gases inside the reaction system [36]. The results show the increase in temperature had an important effect on oil yields, where light oils were further broken down to form gas products, and it was evidenced by a reduction trend of oil yields as temperature increased [15].

However, as can be seen from the percentage of oil yield using POME as a solvent, the oil yield was recorded as high as 67.1% at the lowest temperature (300°C), decreased to 33.1%, and decreased continuously as the temperature increased. The hydrogen free radical by POME stabilized the fragmented biomass and yielded more bio-oil yield at 300°C. As the temperature further increased, repolymerization occurred, leading to higher char formation. Also, the solubility of J. curcas and the ability to disseminate oxygen-containing intermediates are much stronger in POME, which promotes the mass transfer of the reaction, resulting in high oil yield at low temperatures [37]. The yield of heavy oil strongly depends on the reaction temperature at 250°C and above, as a result of the competition between the two reactions involved in the liquefaction, that is, hydrolysis and repolymerization [15].
3.3.3 Effect of Reaction Time on Oil Yield
Figure 3 shows the effect of reaction time on oil yield. The reaction time was varied from 30 to 90 min. The temperature and biomass-to-solvent ratio were set constant at 300°C and 1:2, respectively. Although it is less significant than temperature in influencing the thermochemical process [21], further cracking and repolymerization of intermediates can be controlled by managing the reaction time. The bio-oil yield was found to increase from 30 to 60 min for both solvents and started to decrease for 90 min. This phenomenon could perhaps be attributed to achieving equilibrium in biomass degradation [21].
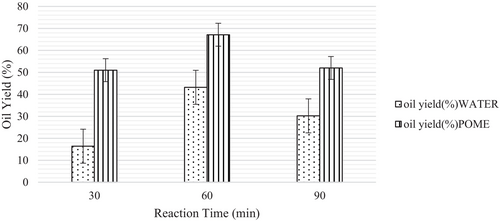
A cracking of bio-oil occurred by prolonging the reaction time to 90 min, resulting in reduced bio-oil yield. At a prolonged reaction time of 90 min, the bio-oil decreased for both water and POME, where the secondary decomposition of bio-oil results in the formation of solid and gaseous products by various reactions, including gasification, repolymerization, and recondensation [21]. Nevertheless, longer reaction times may be economically disadvantageous for the commercial production of bio-oil due to energy consumption [18].
3.4 Characterization of Liquid Products and Hydrochar
3.4.1 Elemental Analysis
The bio-oils obtained from the HTL of J. curcas seeds were characterized as brown to black in color and exhibited a viscous consistency. These bio-oils were noted for their aromatic and pungent odors. In Table 3, the elemental composition, calorific value, and ash content of the representative bio-oil samples are presented. This particular bio-oil was obtained under the conditions of 300°C temperature, 60 min reaction time, and a biomass-to-solvent ratio of 1:2 (under POME condition). For comparison purposes, the characteristics of the bio-oils obtained from the pyrolysis of different biomass feedstocks and HTL of microalgae biomass were compared [18]. We compare the results obtained from our work with those of previous researchers as a comparison.
Table 4 lists the ultimate analysis and heating value of the oil yield from the HTL of J. curcas with POME. The carbon content in the oil was high (i.e., 74.6%), indicating the good quality of the oil. This result is parallel with the GC–MS result for oil obtained using POME at 300°C, which gives the highest percentage of hydrocarbon content. Besides, nitrogen content is a little higher in this oil although the ash content is lower. The nitrogen content in bio-oil is attributed to the nature of POME and J. curcas seed, as reported the conversion of amine groups into ammonium ions and their subsequent transfer to the aqueous phase [39]. The references indicated that the nitrogen content in bio-oil derived from POME [40] and J. curcas seed [41] was high due to the presence of nitrogen-containing compounds in the feedstock and POME. On the other hand, the results of low oxygen content (10.97%), high hydrogen content (11.10%), and heating value (39.07 MJ/kg) in the bio-oil suggested that this oil has the potential to be a good biofuel.
| Experimental conditions | C% | H% | O% | N% | H/C Ratio | HHV (MJ/kg) | Ash % |
|---|---|---|---|---|---|---|---|
| Bio-oil from J. curcas | 74.6 | 11.1 | 10.9 | 3.27 | 1.8 | 39.07 | < 0.05 |
| Bio-oil from L. cubeba seeds [25] | 76.2 | 11.9 | 10.4 | 1.6 | 1.9 | 40.8 | 0.1 |
| Bio-oil from microalgae (Chlorella sp.) [18] | 75.1 | 9.9 | 7.8 | 7.3 | 1.6 | 38.1 | 2.6 |
| Pyrolysis oil [38] | 50–67 | 7–8 | 15–25 | 8–10 | 1.7–1.4 | 21.1–24.7 | 0.4–10 |
As shown in Table 3, the bio-oil from oil seeds has higher carbon and hydrogen content than that of microalgae and pyrolysis oil. Therefore, the bio-oil derived from J. curcas has a higher HHV than other bio-oils produced from non-oil seed types. According to Wang and Chang [25], the bio-oil obtained from oil seeds exhibits a high HHV that is comparable to that of petroleum-derived fuels, around 42 MJ/kg. This finding suggests that this particular oil possesses considerable potential as a viable fuel source. The hydrogen-to-carbon molar ratio in the bio-oil is comparatively higher than that observed in pyrolysis oil and algal oil, suggesting its potential as a viable substitute for fossil fuels.
3.4.2 GC–MS Analysis
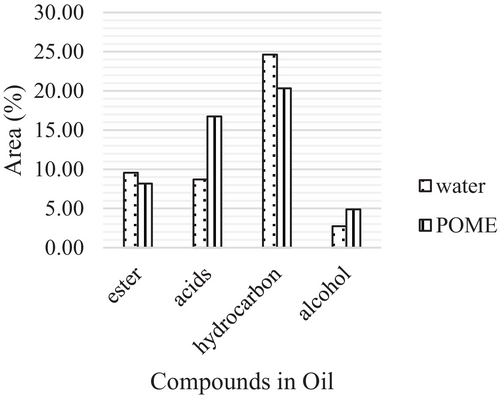
GC–MS analysis was performed to identify the chemical compounds present in the oil product obtained from the HTL of J. curcas seeds with water and POME. The compounds identified using GC–MS can be classified into six different groups: esters, carboxylic acids, hydrocarbons, and alcohols. Figure 4 showed the chemical compound of bio-oil at a temperature of 300°C, while the chemical compound of bio-oil at a temperature of 350°C was illustrated in Figure 5. The acid conversion was found to be higher at 350°C for the bio-oil obtained from POME as a solvent, leading to higher ester content.

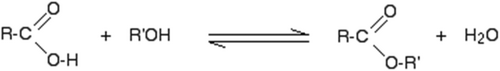
The mechanism of formation of ester from carboxylic acids is presented in Figure 6.
As the reaction temperature increased to subcritical water, the alcohol and hydrocarbon contents were reduced. It might be due to the heavy hydrocarbon content that decomposed into light hydrocarbons at this temperature. The formation of hydrocarbons is mainly due to the decarboxylation of fatty acids produced by the hydrolysis of lipids at temperatures higher than 300°C [43]. Figure 5 also shows that the composition of alcohol decreased at 350°C for both solvents. This is due to the reaction of alcohol with carboxylic acids to form esters.
Furthermore, the reaction of hydrocarbons with the oxygen bond in the hydrothermal reaction produces CO2 and water [44]. The result at 350°C indicated that a cracking process occurred at a higher temperature.
3.4.3 FTIR Analysis of Bio-Oil
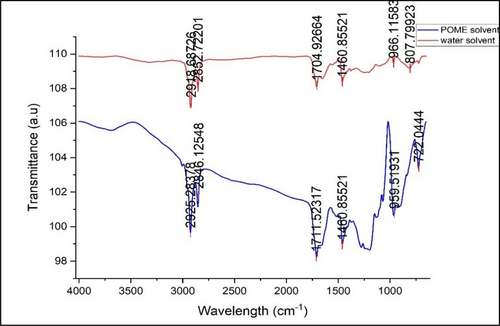
Figure 7 shows the FTIR spectra for the bio-oil produced at 300°C, 60 min, and a 1:2 ratio of J. curcas to POME. A sharp and strong absorbance at around 2925 cm−1 was observed for the bio-oil, which represents the =C-H and C-H vibrations and indicates a high content of alkanes. The C-H stretching vibrations also appeared at the wavenumber of 2854 and 1460 cm−1, indicating the presence of alkanes. Two large bands were presented in this region. Wang and Chang [25] also reported that in their study of the HTL of L. cubeba seeds, their bio-oil also appeared at the wavenumber of 2800–3000 cm−1, representing the C-H stretching bond. A wide and strong C=O stretching bond also appeared at the wavenumber of 1711 cm−1, indicating the presence of an ester group in the bio-oil. Similar to a previous study by Gundupalli and Bhattacharyya [45], they observed strong stretching vibration at the peak of 1731 cm−1 for untreated samples, representing the ester group. The O-H band in the -COOH group appeared between the wavenumber of 960 cm−1, showing the presence of carboxylic acids. The C-H rock bond also appeared at the wavenumber of 722 cm−1, indicating the presence of alkanes in the bio-oil.
3.5 Carbon Balance
Table 5 gives the carbon conversion for the recovered products obtained at 300°C, with a biomass to solvent ratio of 1:2 and a 60 min reaction time, to highlight the key differences between the yield expressed on a mass and a carbon basis. About 81% carbon recovered for the produced oil indicated high energy recovery, and product loss was minimal.
| C (wt.%) | Yield (%) | Mass sample (g) | C (g) | |
|---|---|---|---|---|
| Initial sample (J. curcas seed) | 58.64 | 5.0 | 2.95 | |
| Oil | 74.59 | 63.4 | 3.17 | 2.38 (81%) |
| Char | 12.67 | 4.7 | 0.24 | 0.03 (1%) |
| CO2 (Model) | 31.9 | 1.60 | 0.43 (15%) |
4 Conclusion
HTL was employed in subcritical conditions to convert J. curcas seeds into an alternative energy product, which is bio-oil. A thorough analysis of the bio-oil from the HTL of J. curcas with water and POME showed that a high yield was obtained at the optimum temperature of 300°C, biomass-to-solvent ratio of 1:2, and reaction time of 60 min using POME as a solvent, whereas a high oil yield was obtained at subcritical water conditions at the temperature of 350°C, biomass-to-solvent ratio of 1:2, and reaction time of 60 min using water as a solvent. However, the highest ester content and lower acid content were obtained at the temperature of 350°C for both solvents. POME could be an alternative source for a hydrogen donor solvent as it successfully increased the oil yield at a lower mass-to-solvent ratio, and is economically advantageous for commercialization. Furthermore, using POME as a solvent can produce bio-oil with high carbon content and heating value.
Author Contributions
Rohazriny Rohim: investigation, writing – original draft, methodology, writing – review and editing, software, formal analysis, data curation. Khairuddin M. Isa: conceptualization, investigation, funding acquisition, writing – original draft, validation, visualization, writing – review and editing, supervision. Umi Fazara Md Ali: supervision, formal analysis, validation, funding acquisition. Mohd. Aizudin Abd. Aziz: validation, formal analysis, supervision, software. Naimah Ibrahim: validation, formal analysis, supervision, project administration. Muhammad Auni Hairunnaja: project administration. Saiful Azhar Saad: supervision, project administration, data curation, validation. Nur Amira Fatihah Bashari: writing – review and editing, project administration, writing – original draft.
Acknowledgments
The authors express their gratitude for the financial support received from the Fundamental Research Grant Scheme (FRGS) with a grant number FRGS/1/2019/STG05/UNIMAP/02/4, as well as the facilities used in this research by the Faculty of Civil Engineering Technology, Universiti Malaysia Perlis.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




