Emerging Energy Applications of Multi-Principal Element Alloys: A Mini Review
Funding: This work was supported by the National Research Foundation (CSUR230430100268).
ABSTRACT
Multi-principal element alloys (MPEAs) have emerged as a transformative class of materials with exceptional mechanical, thermal, and chemical properties, making them promising candidates for energy applications. This mini-review explores the role of MPEAs in energy systems, focusing on their potential for high-temperature energy applications, hydrogen storage, fuel cells, thermoelectric energy conversion, and advanced battery technologies. The unique combination of configurational entropy, phase stability, and corrosion resistance in MPEAs offers significant advantages over conventional materials in extreme environments. Despite their promising properties, challenges such as compositional optimization, processing scalability, and cost remain key areas for future research. This short communication provides insights into the advancements, opportunities, and future directions for the utilization of MPEAs in next-generation energy systems.
1 Introduction
In recent years, the search for advanced materials capable of withstanding extreme conditions while offering superior performance has led to the development of multi-principal element alloys (MPEAs). Unlike conventional alloys, which are primarily based on one or two major elements with small amounts of additional alloying elements, MPEAs are composed of multiple elements, often in near-equimolar or high-concentration ratios [1]. This distinct compositional design allows for the emergence of unique atomic interactions and microstructures that exhibit enhanced properties compared to traditional metallic systems. MPEAs are a broad class of materials that include both high-entropy alloys (HEAs) and medium-entropy alloys (MEAs). HEAs typically consist of five or more principal elements with high configurational entropy, which stabilizes single-phase solid solutions [2]. In contrast, MEAs contain fewer principal elements but still demonstrate remarkable mechanical and functional properties [3]. The collective effects of multiple elements in MPEAs (Figure 1) result in unique attributes, including the high-entropy effect, sluggish diffusion, severe lattice distortion, and the cocktail effect, which contribute to their outstanding strength, toughness, oxidation resistance, and thermal stability [4]. Since their conceptualization in the early 2000s, MPEAs have been widely explored for applications in aerospace, defense, biomedical implants, and structural components. However, one of their most promising and rapidly growing areas of interest is energy systems, where materials are required to function under extreme temperatures, aggressive chemical environments, and prolonged operational conditions.
The transition toward sustainable and high-efficiency energy technologies necessitates the development of novel materials that offer superior thermal, mechanical, and electrochemical properties. Conventional materials such as nickel-based superalloys, stainless steels, and titanium alloys often suffer from limitations such as phase instability, creep, oxidation, and corrosion at high temperatures [5], which restrict their long-term performance in energy-related applications. MPEAs, due to their high thermal stability, corrosion resistance, and tunable electronic properties, have emerged as promising candidates for revolutionizing energy systems [6]. One of the most attractive features of MPEAs is their ability to operate under extreme environments, making them ideal for high-temperature applications such as nuclear reactors, gas turbines, and concentrated solar power systems. Refractory MPEAs containing elements such as Nb, Mo, Ta, and W exhibit exceptional creep resistance and oxidation stability, outperforming many conventional high-temperature alloys.
Beyond structural applications, MPEAs have also demonstrated great potential in electrochemical energy storage and conversion technologies, including batteries, supercapacitors, fuel cells, and thermoelectric materials [6]. In hydrogen storage systems, MPEAs provide adjustable lattice structures that facilitate efficient hydrogen absorption and desorption, overcoming challenges faced by traditional metal hydrides [7]. Similarly, their ability to exhibit low thermal conductivity while maintaining high electrical conductivity makes them excellent candidates for thermoelectric power generation, where waste heat can be converted into electricity. Another key advantage of MPEAs is their design flexibility, which allows researchers to tailor their compositions to meet specific performance requirements. With the integration of computational modeling, machine learning, and advanced manufacturing techniques such as additive manufacturing and spark plasma sintering, the development of next-generation MPEAs for energy applications is becoming increasingly feasible.
This short communication provides a concise review of the potential applications of MPEAs in energy systems, focusing on four key areas: high-temperature energy applications, hydrogen storage and fuel cells, thermoelectric energy conversion, and batteries and supercapacitors as depicted in Figure 2. It highlights the advantages of MPEAs, including their exceptional thermal stability, corrosion resistance, and tunable electronic properties, while also addressing challenges related to scalability, cost, and long-term stability. Finally, the paper outlines future research directions to advance the development of MPEAs for next-generation energy technologies, contributing to the pursuit of more sustainable and efficient energy solutions.
2 MPEAs in Energy Systems
MPEAs have demonstrated remarkable potential for energy applications due to their superior thermal stability, corrosion resistance, and tunable electronic properties. This section explores their applications in high-temperature energy systems, hydrogen storage, and fuel cells, thermoelectric energy conversion, and electrochemical energy storage.
2.1 High-Temperature Energy Applications
Energy systems such as nuclear reactors, fossil fuel power plants, and gas turbines require materials that can withstand extreme temperatures, radiation exposure, and aggressive chemical environments. Traditional materials such as Ni-based superalloys and stainless steels suffer from phase instability, oxidation, and creep at elevated temperatures. MPEAs, due to their high configurational entropy and sluggish diffusion, exhibit excellent high-temperature mechanical strength and oxidation resistance, making them promising candidates for structural components in these energy systems. MPEAs with refractory elements (e.g., Nb, Mo, Ta, Ti, Hf, and W) have shown outstanding high-temperature performance. As noted by Ouyang et al. [8], the first two refractory MPEA systems—MoNbTaW and MoNbTaVW—exhibit high-temperature strength that significantly exceeds that of conventional metal alloys. These equiatomic solid solutions achieve impressive compressive yield strengths between 400 and 700 MPa at 1600°C. In comparison, typical nickel-based superalloys like Inconel 718 see their yield strength diminish to nearly zero at approximately 1200°C. Additionally, the formation of stable oxide layers in Cr-containing MPEAs enhances their resistance to oxidation and thermal degradation, making them suitable for long-term applications in extreme environments. This aligns with findings by Feng et al. [9], who emphasize that refractory MPEAs like CrMoNbV demonstrate remarkable high-temperature strengths—over 1000 MPa at 1273 K—due to intrinsic material characteristics. Key factors include large atomic-size mismatches, elastic-modulus mismatches, and the predominance of non-screw character dislocations. These design principles can inform the development of other alloys aimed at achieving exceptional high-temperature performance. Figure 3 shows the comparison of the yield strength of several refractory MPEAs at room and high temperatures (RT and HTs), highlighting their suitability for high-temperature energy applications. The strain rate of 0.001 s−1 was used for all alloys except CrMoNbV, for which the referenced study did not explicitly specify the strain rate. While this consistency improves comparability, variations in testing environments, such as atmosphere, may have also influenced the reported results.
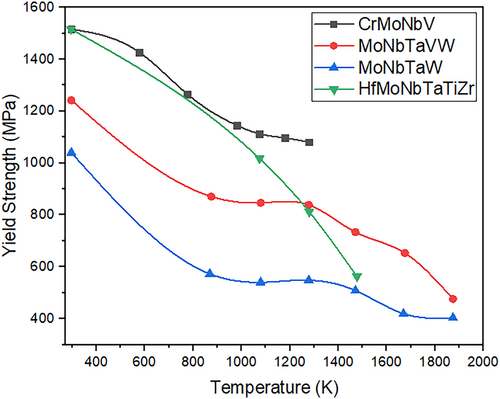
Although MPEAs exhibit outstanding thermal and oxidation resistance, their long-term phase stability in extreme conditions remains a concern. High-temperature refractory MPEAs (e.g., MoNbTaW, MoNbTaVW) have demonstrated yield strengths exceeding 400 MPa at 1600°C, significantly outperforming Ni-based superalloys, which experience severe degradation above 1200°C. However, their complex phase structures make them susceptible to phase separation under cyclic thermal loading, an issue that is relatively well-controlled in traditional superalloys through decades of alloy development.
2.2 Hydrogen Storage and Fuel Cells
Hydrogen is a key energy carrier for future clean energy systems, but efficient hydrogen storage remains a major challenge [12]. MPEAs offer tunable lattice structures that can facilitate reversible hydrogen absorption and desorption, making them potential candidates for solid-state hydrogen storage materials. Recent studies [13, 14] have demonstrated that MPEAs, particularly body-centered cubic (BCC) structured alloys, offer significant potential for hydrogen storage due to their high hydrogen absorption capacity, tunable thermodynamics, and fast absorption/desorption kinetics. The unique atomic structure of MPEAs provides ample interstitial sites for hydrogen accommodation, while the sluggish diffusion effect promotes nanostructuring, further enhancing hydrogen uptake and release rates. Kao et al. [15] conducted one of the first studies on MPEAs for hydrogen storage, synthesizing CoFeMnTi x VZr, CoFeMnTiV y Zr, and CoFeMnTiVZr z HEAs. Their findings confirmed the formation of a stable C-14 Laves phase after hydrogen cycling. By adjusting Ti (x), V (y), and Zr (z) concentrations, they optimized hydrogen storage capacity, with the highest performance observed for x = 0.5–2.0 and z = 0.4–2.3. However, excessive Ti (x ≥ 2.5) and Zr (z ≥ 2.3) led to phase segregation, reducing storage efficiency. Notably is the work of Sahlberg et al. [16] TiVZrNbHf BCC MPEA has achieved an exceptional H/M ratio of 2.5, outperforming conventional hydrogen storage alloys. Additionally, the ability to tailor element composition in MPEAs offers greater flexibility in optimizing storage properties, making them promising candidates for next-generation hydrogen storage materials with improved efficiency and stability. Table 1 shows some MPEAs and their hydrogen storage properties. Liu et al. [26] recently investigated the microstructure and hydrogen storage properties of Ti-V-Cr-based BCC-type MPEAs, identifying high storage capacities in selected compositions. They synthesized V35Ti30Cr25Fe10, V35Ti30Cr25Mn10, V30Ti30Cr25Fe10Nb5, and V35Ti30Cr25Fe5Mn5 alloys, finding that V35Ti30Cr25Mn10 exhibited the highest absorption capacity, while V35Ti30Cr25Fe5Mn5 showed the highest reversible capacity. All alloys were produced via melting. Hydrogen storage MPEAs with BCC structures have achieved hydrogen-to-metal ratios (˜2.5 H/M) exceeding those of conventional metal hydrides (˜1.8 H/M). However, while MPEAs offer greater flexibility in compositional tuning, they often suffer from higher susceptibility to embrittlement and phase segregation after repeated hydrogen cycles, unlike well-established systems such as LaNi5, which retain high cycle stability. Research into compositional modifications and nano-structuring techniques could address this limitation.
| Author(s) | MPEA composition | Processing method | Phase | Hydrogen- to-metal ratio (H/M) | Decomposition temperature (K) | Absorption kinetics | Hydrogen absorption capacity (wt%) | Reversible hydrogen capacity |
|---|---|---|---|---|---|---|---|---|
| Shen et al. [17] | Ti0.3Zr0.2Nb0.2Hf0.2 | Arc Melting | BCC | — | 656 | — | 1.12 | — |
| Ek et al. [18] | Ti0.25V0.25Zr0.25Nb0.25 | Arc Melting | BCC | 1.98 (293 K) | Approx. 573 | — | — | — |
| Shen et al. [19] | Ti0.2Zr0.2Nb0.2Mo0.2Hf0.2 | Arc Melting | BCC | — | Approx. 540–575 | — | 1.18 | — |
| Sleiman and Huot [20] | Ti0.2V0.2Zr0.2Nb0.2Hf0.2 | Arc Melting | BCC | 1.94 (573 K), 2 (473 K) | 623 | 1.7 wt% in 300 s (573 K, 2 MPa H2) | 2.1 (573 K), 2.2 (473 K) | — |
| Silva et al. [21] | Ti0.2V0.2Zr0.2Nb0.2Mo0.2 | LENS | BCC, NbTi4 (minor) | — | — | 2.3 wt% in 1380s (303 K, 8.5 MPa H2) | 2.3 (323 K), 1.78 (after activation 673 K) | — |
| Montero et al. [22] | Ti0.3V0.25Zr0.1Nb0.25Ta0.1 | Arc Melting | BCC | 1.73 (298 K) | Approx. 500–590 | Approx. 2.4 wt% in 120 s (373 K, 3.3 MPa H2) | 2.2 (298 K) | — |
| Nygård et al. [23] | Ti0.2V0.2Cr0.2Nb0.2Mo0.2 | Arc Melting | BCC | Approx. 0.3 | Approx. 523 | — | — | — |
| Ti0.22V0.22Zr0.11Nb0.22Hf0.22 | Arc Melting | BCC | 1.99 (293 K) | Approx. 593 | — | — | — | |
| Ti0.25V0.25Cr0.25Nb0.25 | Arc Melting | BCC | ∼2 | 473–556 | — | — | 1.96 wt% (293 K, vac/2.3 MPa H2), stable for 10 cycles | |
| Strozi et al. [24] | Mg0.20Al0.20V0.20Cr0.20Ni0.20 | Reactive Milling (3.0 MPa H2) | BCC, TiH2 (minor), Mg2FeH6 (minor) | — | 558–598 | 1.0 wt% in 3600 s (598 K, 15 MPa) | 1.0 (598 K) | — |
| Montero et al. [25] | Ti0.325V0.275Zr0.125Nb0.275 | Reactive Milling (H2) | BCC | 1.8 | Approx. 458–523 | — | 2.5 (293 K, 4 MPa) | 2.0 wt% |
- Note: Data marked with “—” were not reported in the cited references.
Recent studies have demonstrated that Pt-MPEAs (PtCoV, PtCuFe, and PtCoxCu1−x ) are promising electrocatalysts for hydrogen fuel cells due to their high surface activity, corrosion resistance, and tunable electronic structures [27-29]. According to Chen et al. [30], Pt-based MPEAs, such as PtCuSn and PtCuW, exhibit superior oxygen reduction reaction (ORR) activity. These alloys leverage the high-entropy effect and element synergy to enhance catalytic performance, structural stability, and electrochemical efficiency. For example, PtCuSn achieves an onset potential of 1.09 V and a half-wave potential of 0.86 V, outperforming conventional Pt-based catalysts. The ability to tailor composition in MPEAs offers a unique advantage in optimizing catalytic activity for fuel cell applications. These alloys form a Pt-skin structure with atomic segregation of W or Sn in the subsurface, enhancing intrinsic activity and structural stability. Additionally, the compressive strain effect and d-band center modulation in MPEAs improve catalytic performance by optimizing oxygen adsorption and reducing the overpotential required for ORR. The PtBiNiCoSn/C MPEA catalyst recently developed by Miao et al. [31] achieved a current density of 1.406 A mg−1 Pt, 6.39 times higher than commercial Pt/C, due to strong synergistic effects that optimize Pt's electronic structure. It also retained 46.5% of its initial current after 3000 s, outperforming Pt/C (34.9%), demonstrating enhanced stability and anti-CO poisoning properties. Also, Sumanta et al. reported that an equiatomic MPEA composed of CoCuFeNiTi, featuring both BCC and FCC structures, exhibits optimal workability at elevated temperatures, making it suitable for high-temperature electrochemical applications, including solid oxide electrolyzer cells (SOECs). This underscores the versatility of MPEAs in various fuel cell technologies, providing cost-effective and durable alternatives for next-generation proton exchange membrane fuel cells (PEMFCs) and SOECs. In particular, Pt-based MPEAs have demonstrated exceptional electrocatalytic performance, making them highly promising for hydrogen fuel cells with improved efficiency and longevity.
2.3 Thermoelectric and Energy Conversion Applications
MPEAs have emerged as promising candidates for thermoelectric and energy conversion applications due to their ability to balance electrical conductivity and thermal insulation. Their high configurational entropy enhances phonon scattering, reducing lattice thermal conductivity while maintaining favorable Seebeck coefficient and electrical transport properties. Studies on CoCrFeNiNb0.45 have demonstrated a high thermoelectric figure of merit, (zT) at elevated temperatures, making it suitable for waste heat recovery in industrial applications [32]. Recent research highlights the role of entropy engineering in enhancing thermoelectric performance. Jiang et al. [33, 34] have demonstrated that high-entropy chalcogenides, such as PbSe- and GeTe-based materials, exhibit enhanced thermoelectric performance due to entropy stabilization. For instance, Pb0.975Na0.025Se0.5S0.25Te0.25 has been reported to achieve a high zT value of 2.0 at 900 K, owing to band convergence and hierarchical microstructures, which improve phonon scattering and reduce lattice thermal conductivity. Additionally, half-Heusler (hH) alloys have shown promise as mid-to-high-temperature thermoelectric materials, with high entropy Nb1−x MxFeSb (M = Zr, V, Mo, Ti, Hf) achieving a zT of 0.88 at 873 K due to reduced thermal conductivity [35]. However, challenges such as phase segregation continue to impact electrical transport properties, limiting the overall zT improvement. Ren et al. emphasized the significance of band structure engineering, showing that modulating carrier concentration significantly boosts thermoelectric efficiency [36]. MPEAs have shown promise in thermoelectric applications due to their ability to balance electrical conductivity and thermal insulation. Some compositions have achieved zT values exceeding 2.0, comparable to high-performance thermoelectric materials like Bi2Te3. However, challenges such as phase segregation and limited long-term stability under thermal cycling need further investigation to match the well-characterized performance of conventional thermoelectric materials.
2.4 Batteries and Supercapacitors
The rapid advancement of renewable energy systems and electric vehicles has significantly increased the demand for high-performance energy storage materials. MPEAs have emerged as next-generation electrode materials for batteries and supercapacitors due to their excellent electrochemical stability, high electrical conductivity, and resistance to degradation [37]. The high-entropy effect in these alloys enhances structural stability, preventing phase segregation and ensuring long-term cycling performance during repeated charge–discharge cycles.
MPEA-based anodes and cathodes have shown significant potential in advanced battery technologies, particularly in lithium-ion (Li-ion), sodium-ion (Na-ion), and zinc-ion (Zn-ion) batteries. They demonstrate remarkable improvements in cycling stability, capacity retention, and charge storage efficiency, attributed to their excellent electrochemical stability, tunable compositions, and enhanced ion transport properties [38]. In Li-ion batteries, MPEAs improve charge–discharge efficiency and extend battery lifespan by mitigating phase degradation and enhancing Li-ion diffusion kinetics. For Na-ion batteries, MPEAs offer a cost-effective alternative to Li-ion systems, leveraging high configurational entropy and lattice distortion effects to improve ion storage capability, electrode stability, and long-term cycling performance. Meanwhile, in Zn-ion batteries, MPEAs provide corrosion-resistant electrode materials with superior electrical conductivity and redox activity, ensuring stable cycling and high charge-storage efficiency for large-scale energy storage applications. By tailoring elemental compositions, researchers have optimized the electrochemical performance of MPEA-based electrodes across these battery technologies, making them promising candidates for next-generation energy storage systems.
Additionally, the tunability of MPEA compositions allows for the optimization of redox activity, further improving electrochemical performance. In supercapacitors, MPEA-based materials provide a high surface area and rapid ion transport, enabling fast energy storage and release. The combination of transition metals in MPEAs contributes to superior capacitance, rate performance, and durability compared to conventional electrode materials. Furthermore, MPEAs are being explored for redox-flow batteries and solid-state electrolytes, offering a potential route to enhanced safety, scalability, and efficiency in large-scale energy storage applications. Table 2 highlights the synthesis methods, capacitive performance, and stability of some MPEA-based electrodes for supercapacitor applications.
| MPEA composition | Synthesis method | Electrolyte | Capacitance (F/g) | Stability (%) | Cycle count | Ref. |
|---|---|---|---|---|---|---|
| K(MgMnFeNiCu)Fe(CN)6 | High-entropy Prussian blue analogs | 1 M Na2SO4 | 175 | — | — | [37] |
| FeCoNiCuSn/HCPCs | In situ reduction on hypercrosslinked polymer carbon | 1 M KOH | 495.4 | 94.70 | 15,000 | [39] |
| FeNiCoMnMg | Nanoparticles on carbon nanofibers | 6 M KOH | 203 | > 85 | 2000 | [40] |
| (FeCoCrMnZn)3O4 | Facile solid-state reaction route | 1 M KOH | ⁓340 | 82.8 | _ | [38] |
| AlCoCrFeNi-CNTs | Sol–gel auto-combustion + CVD | polyvinyl alcohol (PVA) /H2SO4 | 286.6 | 100 | 15,000 | [41] |
- Note: Data marked with “—” were not reported in the cited references.
Although MPEAs offer promising advantages in energy applications, including superior thermal stability, tunable electronic properties, and enhanced mechanical strength, several challenges must be addressed before their widespread adoption. The successful integration of MPEAs into practical energy systems requires overcoming key obstacles such as phase stability, processing scalability, and cost considerations. Section 3 explores these challenges and also highlights strategies to enhance MPEA performance and manufacturability.
3 Challenges and Future Perspectives
Despite MPEAs' prospective uses in energy systems, several difficulties must be overcome before they can be widely adopted. These challenges include compositional optimization, processing scalability, cost considerations, and long-term stability. This section highlights the key barriers to implementation and potential future research directions.
3.1 Compositional Optimization and Alloy Design
One of the key issues in MPEA development is determining the best composition for various energy applications. The wide compositional space of MPEAs makes it challenging to precisely predict the material properties [42]. Although high-throughput computational methods, such as density functional theory and machine learning, have been employed to guide alloy design, experimental validation remains time-consuming and resource-intensive [43]. Several predictions done on MPEAs have made use of algorithms like Gaussian process classification, artificial neural networks, neural networks, deep neural networks, and kernel-based extreme learning machine, with accuracy ranging from 81% to 95%, with neural networks (NN) achieving the highest accuracy [44-48]. Future research should focus on integrating computational modeling with experimental techniques to accelerate MPEA discovery. Machine learning algorithms can help identify promising compositions with tailored properties for high-temperature applications, hydrogen storage, and thermoelectric performance. Additionally, the development of property databases for MPEAs will enable better predictive modeling, reducing trial-and-error approaches in alloy design. Computational approaches and machine learning-driven alloy design have significantly accelerated MPEA discovery by predicting phase stability, mechanical properties, and oxidation resistance. However, translating computational predictions into practical synthesis remains a challenge due to factors such as compositional inhomogeneity, phase segregation, and fabrication limitations. To bridge the gap between computational predictions and practical synthesis, experimental validation should involve high-throughput alloy screening, processing optimization, and iterative refinement using AI-assisted feedback loops to enhance prediction accuracy. A systematic approach that integrates computational efficiency with experimental rigor is essential for achieving reliable and scalable MPEA development. To bridge this gap, experimental validation must be integrated at multiple stages.
3.2 Processing and Manufacturing Challenges
MPEAs' complicated chemistry and tendency for phase segregation present yet another formidable obstacle to their fabrication. Mechanical and electrochemical performance is impacted by compositional inhomogeneity, which is a common consequence of conventional casting techniques [49]. Although scalability is still an issue, advanced processing methods like high-energy ball milling, additive manufacturing, and spark plasma sintering have been investigated to produce homogeneous microstructures [50]. More investigation is required to improve processing techniques that preserve homogeneity while guaranteeing cost-effectiveness to address this. Techniques like combinatorial synthesis and rapid solidification could be used to improve energy-related attributes and fine-tune microstructures. Furthermore, incorporating MPEA-based electrodes and catalysts into energy storage and conversion devices will require the development of coating methods.
3.3 Cost Considerations and Material Availability
Although MPEAs offer superior performance in energy applications, the cost of raw materials and complex processing can limit their industrial viability. Many high-performance MPEAs contain expensive refractory elements (e.g., Mo, Nb, Ta, W) and noble metals (e.g., Pt and Ir), which may not be feasible for large-scale production [51]. The reliance on rare elements also raises concerns about supply chain stability and environmental impact [52]. A potential solution is to explore cost-effective, lightweight MPEAs that replace expensive elements with more abundant alternatives while maintaining desirable properties. For instance, Fe-based and Al-containing MPEAs have shown promising properties at a lower cost. AlCoCrFeNi MPEA exemplifies this approach, replacing noble metals like Pt and Ir while delivering exceptional catalytic activity for alkaline water electrolysis [53]. With Fe and Al constituting 20% of its composition, this alloy significantly reduces costs without compromising efficiency. Its synthesis via spark plasma sintering (SPS) and rapid 5-min anodization provides a scalable, energy-efficient alternative to high-temperature, resource-intensive methods. Enhanced by the formation of Cr-rich nanoparticles and oxyhydroxide layers (e.g., FeOOH, NiOOH), this MPEA achieves hydrogen evolution reaction (HER)/oxygen evolution reaction (OER) overpotentials of 880 mV and 845 mV at 500 mA cm−2, rivaling noble-metal catalysts while ensuring over 100 h of stability. Its self-supporting structure eliminates the need for costly substrates, further improving economic viability. Additionally, recycling strategies and sustainable material sourcing should be investigated to improve the economic feasibility of MPEAs for energy applications.
3.4 Long-Term Stability and Degradation Mechanisms
For MPEAs to be successfully implemented in energy systems, their long-term stability under real-world conditions must be thoroughly assessed. In hydrogen storage applications, repeated absorption/desorption cycles may lead to structural degradation, affecting efficiency [54]. Similarly, thermal cycling could induce phase transformations in thermoelectric applications, reducing performance over time [54]. MPEAs have shown promise in thermoelectric applications due to their ability to balance electrical conductivity and thermal insulation. Some compositions have achieved zT values exceeding 2.0, comparable to high-performance thermoelectric materials like Bi2Te3. However, challenges such as phase segregation and limited long-term stability under thermal cycling need further investigation to match the well-characterized performance of conventional thermoelectric materials. To enhance the long-term stability of thermoelectric MPEAs, strategies such as grain boundary engineering, nanostructuring, dopant stabilization, and optimized processing routes can be employed to minimize phase segregation and improve durability. Additionally, surface engineering techniques, including oxide coatings and in situ characterization methods like transmission electron microscopy and atom probe tomography, can provide valuable insights into degradation mechanisms, enabling the development of more stable and efficient energy materials.
3.5 Future Research Directions
- Advanced computational design: Using high-throughput simulations and machine learning to optimize compositions for particular energy applications.
- Scalable processing methods: creating economical manufacturing processes for producing MPEA-based components on a wide scale.
- Hybrid materials: Investigating MPEA composites that contain graphene, ceramics, or other nanomaterials to improve their electrochemical and mechanical properties. Hybrid materials such as MPEA-graphene nanocomposites (e.g., FeCoNiCrMn embedded in graphene aerogels for supercapacitors with > 600 F/g capacitance [39]) and MPEA-carbon core-shell nanostructures (e.g., CoNiCuMnAl HEA nanoparticles derived from MOFs, wrapped in ultrathin carbon shells for OER with an ultralow overpotential of 215 mV at 10 mA cm−2 and 30-h stability at 200 mA cm−2 [55]) exemplify how integrating MPEAs with advanced nanomaterials can synergistically boost both electrochemical and mechanical performance.
- Performance testing: Evaluating MPEA stability in energy devices such as fuel cells, batteries, and thermoelectric generators using practical durability tests.
4 Conclusions
MPEAs have emerged as potential materials for energy applications due to their high thermal stability, mechanical strength, corrosion resistance, and tunable electronic properties. Their distinct structural properties, resulting from high configurational entropy, sluggish diffusion, and lattice distortion effects, make them suitable for a wide range of energy-related technologies, including high-temperature power systems, hydrogen storage, fuel cells, thermoelectric energy conversion, and electrochemical energy storage. Despite its benefits, MPEAs are not widely adopted in energy systems due to some obstacles. Key challenges include the requirement for compositional optimization, scalable processing approaches, cost reduction, and long-term stability under operational conditions. Advances in computational modeling, additive manufacturing, and surface engineering will be critical to overcome these obstacles. Future research should concentrate on producing low-cost, lightweight MPEAs and hybrid materials to boost efficiency and sustainability. To summarize, MPEAs are a transformational class of materials with the potential to change energy technologies. Continued interdisciplinary research will be required to realize their full potential, opening the way for next-generation energy systems that are more efficient, long-lasting, and ecologically friendly.
Author Contributions
Samson Olaitan Jeje: conceptualization, writing – original draft, writing – review and editing, methodology, investigation. Mxolisi Brendon Shongwe: writing – review and editing, conceptualization.
Acknowledgments
This study is based on the research supported in part by the National Research Foundation of South Africa (Ref Number: CSUR230430100268). The authors acknowledge the support from the Faculty of Engineering and Built Environment, Tshwane University of Technology, Pretoria, South Africa, without which this study would not have been possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




