Palmyra Palm Shell (Borassus flabellifer) Properties Part 3: Insights Into Its Morphological, Chemical, and Thermal Properties After Alkali Treatment
Funding: The authors received no specific funding for this work.
ABSTRACT
The demand for materials that combine high thermal stability and environmental sustainability is growing in modern engineering. While synthetic fibers are effective, their environmental impact often undermines sustainability goals. This study explores the potential of Borassus flabellifer fruit husk, typically discarded as agricultural waste in Bangladesh, as a bio-fiber alternative for thermal insulation applications. The research investigates the morphological, chemical, and thermal properties of the husk after alkali treatment with sodium hydroxide (NaOH) for varying durations. The results show that alkali treatment significantly enhances the thermal properties of Borassus husk. Notably, char content increased by up to 32%, surpassing other biofibers such as hemp, sisal, jute, and kenaf. The integral process decomposition temperature (IPDT) was found to be up to 30% higher than the untreated husk fiber, indicating improved thermal stability. Additionally, specific heat capacity (Cp) decreased by approximately 37%, correlating with an increase in integral process decomposition heat (IPDH). Scanning electron microscopy (SEM) analysis revealed that treated husks had a rougher and cleaner surface, which may improve thermal insulation properties by creating more voids and enhancing adhesion in composite materials. Fourier Transform Infrared Spectroscopy (FTIR) analysis showed reduced and shifted hemicellulose peaks, consistent with lower moisture absorption, as confirmed by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). Optimal results were observed in samples treated for 0.25 and 0.75 h, suggesting that alkali-treated Borassus husk could serve as an alternative eco-friendly material for energy-efficient and sustainable engineering applications.
1 Introduction
The use of synthetic fiber–reinforced composites has faced growing criticism due to their reliance on non-renewable resources and significant environmental pollution. Rising environmental awareness, increased public concern, the unsustainable consumption of petroleum, and stricter environmental regulations have driven a growing demand for eco-friendly materials [1]. Natural fibers present a promising alternative, offering numerous advantages such as renewability, environmental friendliness, affordability, lightweight properties, and high specific mechanical performance [2-4].
Thermal insulation is an effective technology for achieving the desired thermal comfort while promoting energy saving and environmental sustainability. In modern engineering designs, thermal comfort is a primary driver of energy consumption. For the automotive, construction, and aerospace industries, a key challenge is to minimize the energy usage during manufacturing engineering components while addressing growing social concerns about waste management and the depletion of non-renewable resources [5]. While non-renewable materials like glass fiber and stone wool are highly effective at maintaining thermal comfort, renewable fibers such as flax, jute, and hemp have emerged as viable alternatives to traditional mineral-based insulation [6]. The energy required to produce natural fibers is significantly lower than that for glass fibers. For instance, while Thakur [7] reported that producing glass fiber consumes 5 to 10 times more non-renewable energy than the production of natural fibers, another study [8] suggests that the energy required to produce natural fibers is also approximately 650% more efficient than that of glass fibers. Furthermore, Siciliano and co-researchers [9] stated that adopting bio-based low-carbon goods might reduce CO2 emissions from the materials' production process by more than 80%. It has been discovered that the disposal of synthetic fiber-based composite materials is a serious problem at the conclusion of the life cycle process, and during incineration, roughly 50% of the entire volume remains as a residue (unburned) [10].
Borassus flabellifer, commonly known as the Asian Palmyra palm, is a vital crop supporting local economies across South and Southeast Asia, valued for its fruit and palm sugar production. Archaeological and historical evidence suggests that this species has been present in Southeast Asia for over 1500 years [11, 12]. Borassus fibers are primarily obtained from the fruit of the Borassus flabellifer palm tree, though they can also be extracted from the leaves and bark using mechanical or water retting methods [13]. While other parts of the Borassus flabellifer, such as leaf [11, 14, 15], fruits [11, 16, 17], and trunk [11, 15] have been used for domestic and commercial purposes, husk (fruit shell) has been considered agricultural waste and used as fuel for cooking food in rural Bangladeshs [18-20], which adversely affects the environment.
Like other natural lignocellulosic fibers, like jute, flax, sisal, coir, palm, etc., Borassus flabellifer husk fiber also contains cellulose, hemicellulose, and lignin [20, 21]. In general, cellulose is a primary structure of the cell wall. Cellulose has a crystalline structure that gives high strength to the plant. It has a long polymer chain with a high degree of polymerization and high molecular weight. Cellulose fibers are covered by hemicellulose and lignin. Hemicellulose has a random unshaped structure that gives low strength and a low degree of polymerization. Lignin is a complex branched polymer of phenyl propane and is an important part of the plant's secondary cell walls. Lignin acts as a bonding agent for cellulose fibers that hold neighboring cells together, and its thermal durability is better than that of cellulose and hemicellulose [10, 22-25]. Nevertheless, the variation in the properties of any natural fibers is primarily attributed to their origin, climatic conditions, soil type, and growth period, leading to non-uniformity in physical properties along their length [16, 26, 27].
These fibers are inherently hydrophilic, meaning they absorb moisture, which limits their compatibility with matrices in forming composite materials. This leads to dimensional instability and poor mechanical properties [28]. Additionally, the extracted fibers often contain impurities such as waxes and pectin, making them unsuitable for engineering applications [13, 29, 30]. To enhance compatibility and reduce hydrophilicity by removing the impurities, wax, and chemical compositions, different chemical treatments, such as alkali, saline, potassium permanganate, benzoylation, and acetic acid, have been used in previous studies, and among all, alkali is the most used one because it is economical, convenient, and efficient [29, 30]. Alkali treatment (mercerization) is a widely used chemical treatment in which the structure of the fiber surface undergoes modification by the incorporation of sodium hydroxide (NaOH). As natural fibers tend to absorb moisture in the amorphous regions of cellulose, hemicellulose, and lignin due to the presence of hydroxyl groups, the alkali treatment process modifies this structure. During treatment, the hydroxyl groups in the fiber react with sodium hydroxide (NaOH), leading to the formation of water molecules (H2O) and resulting in the removal of some of the absorbed moisture [31-33]. As a result, it reduces the fibers' hydrophilicity. This process also removes certain amount of hemicellulose, lignin, pectin, wax and oil from the fiber's surface [34]. After alkali treatment, the fibers' surfaces become rougher, resulting in a decline in diameter and an increase in aspect ratio, which improves interfacial bonding along with mechanical and thermal properties [13, 35]. However, a high alkali concentration may result in excessive removal of covering components from the cellulose surface, severely degrading the fiber and reducing its strength [13, 36]. Table 1 summarizes the chemical compositions of alkali treated Borassus fiber as reported in the literature.
Boopathi and co-researchers [37] investigated the chemical and physical properties of treated Borassus fruit fiber with 5%, 10%, and 15% NaOH (alkali) solution, and found that the density (1.32 g/cm3) of the treated fibers was found to be increased with respect to increasing alkali concentration (5%–15%), whereas the fibers mean diameters (226.35–169.46 μm) were reduced with growing alkali concentrations (5%–15%), due to the removal of the hemicellulose. The surface morphology was analyzed using a scanning electron microscope (SEM). The SEM images revealed enhanced surface roughness and more distinct fibril structures on the fiber surface. This improvement increased the surface area by removing binding materials, thereby exposing individual cellular components on the fiber surface. Similar observations have been reported in other studies [38, 40-43].
Fourier-transform infrared spectroscopy (FTIR) has been used in many studies to qualitatively confirm chemical changes caused by chemical reactions [24]. Previous studies [39] carried out FTIR analysis on the treated Borassus fine fibers, mixed with KBr pellets at room temperature, to qualitatively assess their chemical composition. The analysis identified three key components—cellulose, hemicellulose, and lignin—corresponding to functional groups such as hydroxyl (OH), alkyl (C—H), carbonyl (CO), carboxyl (COOH), and ether (C—O—C) at various wavenumbers. These chemical groups are believed to significantly influence the fiber's properties.
Reddy with co-researchers [38] conducted a TGA experiment on alkali-treated Borassus fibers and revealed that alkali treatment enhances the thermal stability of the fibers compared to untreated ones. This improvement is attributed to the removal of amorphous hemicellulose during the alkali treatment. The findings confirm that these fibers are well-suited for use as reinforcement in thermoplastic matrix materials, especially those processed at temperatures under 270°C. Chilukoti et al. [43] treated the Borassus fiber with a 2%–5% NaOH (alkali) solution and subjected it to thermal (TGA) analysis under nitrogen gas, and the results were consistent with previously published work [41], which treated the fibers with 2%–20% NaOH (alkali) solutions and, through thermogravimetric analysis (TGA), observed notable changes in thermal stability. Mercerized fibers demonstrated improved thermal stability compared to untreated fibers, with residual char content increasing from 16.5% to 24.1% following alkali treatment. This enhancement is primarily due to a substantial reduction in hemicellulose, resulting in the formation of a lignin-cellulose complex. However, treatment with 20% NaOH adversely affected thermal stability, as excessive cellulose degradation occurred at this concentration.
Most previous studies have primarily concentrated on the fine and coarse fibers of Borassus, with relatively little research exploring the properties of Borassus husk fibers. However, the authors have already conducted preliminary investigations into the physical, chemical, thermal, and mechanical characteristics of Borassus husk fibers [19, 20]. This paper presents an experimental investigation into the thermal properties of alkali-treated Borassus flabellifer husk fibers, including onset decomposition temperature, maximum decomposition temperature, integral process decomposition temperature (IPDT), integral process decomposition heat flow (IPDH) and specific heat capacity. These properties were evaluated using Thermogravimetric Analysis (TGA) (ASTM E2550) and Differential Scanning Calorimetry (DSC) (ASTM E1269-11), with varying treatment durations from 0.25 to 2 h. The study also explores the chemical properties of the fibers qualitatively through Fourier Transform Infrared Spectroscopy (FTIR) (ASTM E168-06 and ASTM E1252-98) and examines their morphological characteristics using Scanning Electron Microscopy (SEM).
2 Materials and Methods
2.1 Raw Materials
Fruit shells of the Palmyra Palm (Borassus flabellifer) were collected from rural areas in Bogura, Bangladesh. The shells were cut and then thoroughly cleaned using a water-retting process. Afterwards, the shells were sun-dried for several days before being chopped and crushed into powder using an electric blender, followed by sieve analysis. For alkali treatment, hydrochloric acid (HCl), litmus paper, and sodium hydroxide (NaOH) were obtained from Sigma-Aldrich, UK.
2.2 Alkali Treatment
The husk powder was treated with a 5% (wt.) NaOH (alkali) solution, maintaining a fiber-to-solution ratio of 1:20 [44], for varying durations of 0.25, 0.5, 0.75, 1, and 2 h at room temperature. After the treatment, the material was neutralized with 37% concentrated hydrochloric acid (HCl) using litmus paper as an indicator. It was then thoroughly rinsed with tap water to remove any residual chemical traces, ensuring a clean and neutralized sample. From the previous studies [37, 38, 45], it has been found that the optimum properties of the fibers were observed at a concentration of 5% NaOH, and the alkali treatment did not affect the microfibrils of cellulose. The fibers were washed and then conditioned at 80°C for 24 h in a hot air oven before being stored in desiccator containers for morphological, chemical, and thermal experiments.
2.3 Scanning Electron Microscopy (SEM)
The surface morphology of the shell was examined using a Hitachi S-3400 N scanning electron microscope (SEM). Treated samples were mounted on aluminum posts with carbon conductive adhesive tape and sputter-coated with a conductive gold layer using a Quorum Technologies SC7620 coater prior to SEM analysis. The imaging was conducted at a low electron beam energy range of 5 keV to 10 keV.
2.4 Fourier Transform Infrared Spectroscopy (FTIR)
Pellets (discs) were prepared using alkali-treated dried fibers and potassium bromide (KBr) at a 2% concentration. The FTIR spectra of the untreated samples were recorded in the 4000–400 cm−1 range, with 36 scans at a resolution of 4 cm−1. A Thermo Scientific Nicolet iS10 FTIR spectrometer was used, adhering to ASTM E168-06 and ASTM E1252-98 standards and previous works [39, 46, 47].
2.5 Thermogravimetric and Differential Thermal Analysis (TGA/DTA)
TGA/DTA was conducted using a TA Instruments SDT-Q600 Simultaneous TGA/DTA instrument. Treated samples of varying durations, weighing between 5 and 11 mg, were analyzed at heating rates of 10°C/min and 20°C/min under a nitrogen flow of 110 mL/min. Each sample was heated from room temperature to 800°C to observe the shifts in the dehydration peak, hemicellulose peak, cellulose peak, and char content. The experiments were conducted in accordance with the ASTM E2550 standard [19, 48, 49].
2.6 Differential Scanning Calorimetry (DSC)
3 Results and Discussion
3.1 Scanning Electron Microscopy (SEM)
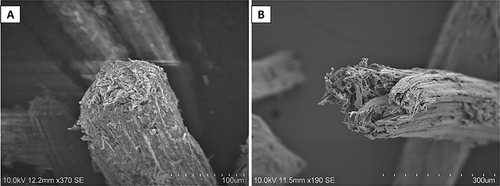
SEM analysis presents the surface morphology of the untreated fiber Figure 4A that appears to be a tubular smooth fiber covered with impurities, whereas alkali-treated fiber in Figure 4B shows a clear and rough surface revealing internal porosity. The outcomes were found to be consistent with the previous studies [40, 42, 43, 56]. It was observed that the treated fiber exhibited a porous, rough yet clean texture due to contaminants being dissolved in the solution because of the alkali solution. Siciliano et al. [9] suggest after SEM analysis on wood particles that with chemical treatment, additional pores (voids) introduced that were covered under the surface, which can increase the tortuosity of the heat transfer pathway, thus decreasing the overall thermal conductivity. Similar findings were also observed by Zhao et al. [57], who looked into the viability of wood to be incorporated in sound absorption and thermal insulation purposes. They state that natural woods have abundant pores; their sound-absorption performance is poor due to the hard and dense surface and the low number of continuous pores. Nevertheless, after chemical treatment, the hemicellulose is removed, thus improving thermal (insulation) stability. Voids act as scattering centers for phonons and absorb a small portion of the heat conduction volume of the material, resulting in lower thermal conductivity [58]. This process also eliminated binding materials, such as wax and pectin, exposing cellular components on the surface. The removal of hemicellulose and other impurities resulted in a roughened texture, which could enhance the fiber's adhesion property [23, 32, 33].
3.2 Fourier Transform Infrared Spectroscopy (FTIR)
The spectra of the alkali-treated samples in Figure 5 revealed a significant absorption band for inter-molecularly linked hydroxyl (O—H) groups at around 3419 cm−1 corresponding to cellulose. This observation provided evidence that the hydroxyl groups were engaged in hydrogen bonding. The C—H stretching vibrations of the methylene/methylene units of all three ingredients were detected as a shoulder peak at 2906 cm−1 owing to aliphatic saturated molecules, such as cellulose and hemicellulose.
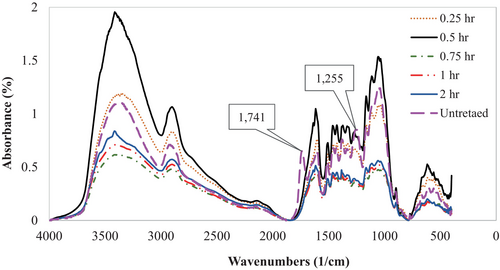
Peaks at 1610 is attributed to bending hydroxyl (O—H) bonds that account for water absorption. The bands at 1509 and 1462 cm−1 correspond to aromatic CC ring stretching and C—H deformation in the methyl, methylene, and methoxyl groups of lignin. The bands at 1426, 1377, and 1320 cm−1 correspond to the —CH2 scissoring, —OH bending vibration, and C—H asymmetric deformation of cellulose. The bands at 1162, 1109, and 1045 cm−1 correspond to the C—O—C, C—O, and C—C stretching of cellulose. The analysis has been found to be consistent with previous research works [20, 39, 41, 59].
The intensity of these bands was significantly reduced in the extracted cellulose because of the removal of most of the hemicellulose and lignin by alkali treatment, which has also been found in the literature [46, 60]. The hemicellulose content of Borassus husk fibers (indicated by peaks at 1741 cm−1 and 1255 cm−1 in the untreated sample) significantly decreased after alkali treatment with varying durations. This treatment resulted in a marked increase in the percentage of α-cellulose and lignin content. With the longer treatment duration, the absorbance of the fiber reduced in previously published works [39, 44], whereas Marathe and co-researchers [40] found that the treated fiber shows less transmittance than the untreated one, which indicates higher absorbance of the alkali-treated Borassus fruit fibers. Research carried out on Coccinia grandis L. [61], hemp fiber [23, 62] and sisal [63] reported similar findings. In this analysis, the least absorbance was found to be in the 0.75 h treated fiber and the highest being in the 0.5 h treated one, which can be attributed to the sample collection and preparation process that may also influence its thermal and mechanical properties [62, 63]. However, Zhao et al. [57] found that with the longer chemical treatment on wood, the removal of hemicellulose as well as lignin also considerably decreases the mechanical strength of the wood cell walls, which allows sound waves to be converted into mechanical and thermal energy through resonances of the wood cell walls.
3.3 Thermogravimetric Analysis (TGA)
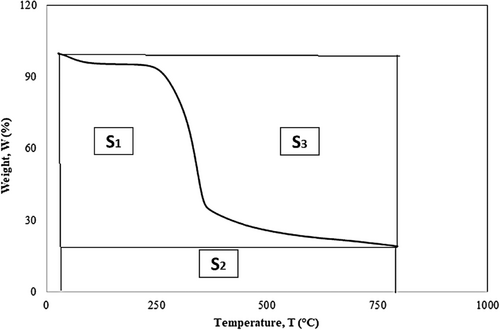
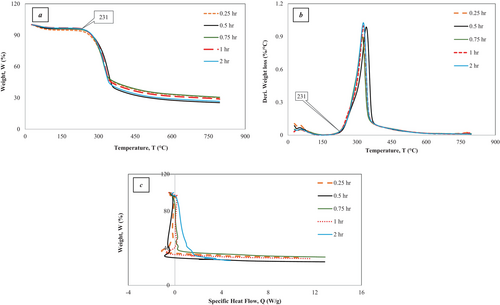
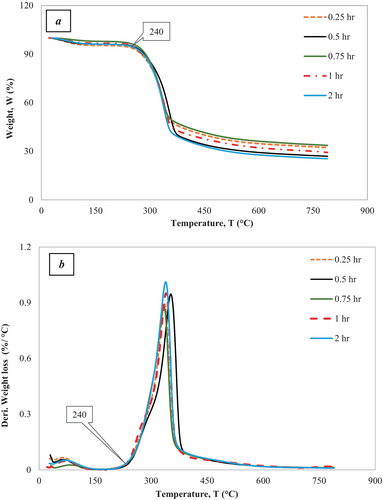
The presented graphs in Figure 6 and Figure 7 depict thermogravimetric analyses (TGA) of the alkali-treated Borassus husk powder samples under pyrolysis conditions at 10 C/min–20°C/min. They demonstrate significant events like moisture loss and potential decomposition of hemicellulose, cellulose, lignin, or other organic compounds over the studied temperature range. The entire process can be divided into two phases. There is an initial sharp decline in small weight loss, with about 5.5%, which is indicative of the moisture content in the sample being evaporated, followed by a significant drop in weight, about 27%, observed around the temperature range from 150°C to 400°C. This corresponds to the decomposition nominal portion of hemicellulose, cellulose, and some other organic components. The onset decomposition temperature for the chemical constituent, hemicellulose (remaining) followed by cellulose, was found to be around 231°C and 240°C in terms of heating rates of 10°C/min and 20°C/min, respectively, for all treated samples, which was a bit higher than its untreated value reported by the authors in their previous work [19]. Nevertheless, depending on the samples, changes of onset temperatures can be observed in Figure 6A,B and Figure 7a,b, which are not significant. The weight steadily decreases, with a loss of rest by around 790°C, which could be due to the slow degradation of complex cellulose and lignin constituents. The most prominent peak for derivative weight loss occurs around 322 C–352°C, where the rate is 0.9%/ C–1%/°C, depending on different treated samples (Table 2 and Table 3) with respect to heating rates. By the end of the analyses at approximately 800°C, the weight percentage of 24%–30% was found at the heating rate of 10°C/min, whereas the weight percentage of 26%–33% was found at the heating rate of 20°C/min, signifying the amount of char residue that the degradation of lignin resulted in char formation and decreased volatile gas generation [64]. The outcomes of the experiments demonstrate that all treated fibers got less moisture content and superior thermal stability to the untreated Borassus husk powder, which shows good agreement with a number of works carried out previously [19, 41, 65]. Nevertheless, Reddy et al. [65] found the char contents of alkali-treated Borassus coarse and fine fibers to be around 19% and 20% while carrying out the TGA analysis at 10°C/min from room temperature to 600°C. Rath et al. [66] found the char content of spruce wood to be around 20% and 19.5% when nitrogen flow rates were 80 mL/min and 160 mL/min, respectively, for a heating rate of 10°C/min. Furthermore, Senthamaraikannan with co-worker [61] found the residue of alkali-treated Coccinia grandis L. to be around 15% at 600°C, which was higher than its raw fiber. Ray et al. [67] found that char residue was around 22% for the alkali-treated (2 h—8 h) jute fiber. Rachini et al. [62] found the char content to be around 21% of 48-h alkali-treated hemp fiber under argon gas flow. Sahu and Gupta [63] found the char residue to be around 30% of alkali-treated (0.5 h) sisal fiber at 600°C, and the char residue of 6% alkali-treated (1-h) was found to be around 22% up to 650°C at a 20 C/min heating rate [68].
| Alkali treated samples | Decomposition stages [peak temperature (°C)] | Max. dev. Weight loss rate (%/°C) | Char Residue (%) | Integral process decomposition temperature, IPDT (°C) | Integral process decomposition heat, IPDH (W/g) | |
|---|---|---|---|---|---|---|
| Dehydration | Cellulose | |||||
| 0.25 h | 53.0 | 323.7 | 0.9 | 29.8 | 1047.4 | 12.0 |
| 0.50 h | 51.8 | 339.8 | 1.0 | 24.4 | 904.7 | 13.0 |
| 0.75 h | 53.2 | 322.0 | 0.9 | 29.4 | 1037.9 | 16.0 |
| 1 h | 52.2 | 326.9 | 1.0 | 28.7 | 1020.5 | 12.8 |
| 2 h | 53.5 | 327.3 | 1.0 | 25.4 | 928.4 | 5.3 |
| Alkali treated samples | Decomposition stages [peak temperature (°C)] | Max. dev. weight loss rate (%/°C) | Char residue (%) | |
|---|---|---|---|---|
| Dehydration | Cellulose | |||
| 0.25 h | 75.9 | 336.0 | 0.9 | 32.03 |
| 0.50 h | 62.7 | 352.4 | 0.9 | 26.16 |
| 0.75 h | 68.3 | 333.9 | 0.9 | 32.34 |
| 1 h | 65.1 | 339.9 | 1.0 | 29.29 |
| 2 h | 69.8 | 339.0 | 1.0 | 26.52 |
After the alkali treatment, the distinct hemicellulose peak was not visible, which can be attributed to the removal of hemicellulose and impurities that were confirmed from the earlier FTIR chemical analysis and previous work [40]. In principle, heat degrades hemicellulose and cellulose from lignocellulosic fibers before lignin [22, 23, 25]. The complex lignin component leads to char production, where the charred layer protects the fibers from further deterioration and enhances thermal insulation property [1].
Table 2 and Table 3 summarize the thermogravimetric data for all treated samples at heating rates of 10°C/min and 20°C/min, respectively, which show that the 0.25 h and 0.75 h alkali-treated Borassus husk fibers have higher char contents compared to the other samples. In contrast, the 0.5 h treated fiber exhibits the lowest char content under both heating conditions. Furthermore, to analytically represent intrinsic thermal stability at a heating rate of 10°C/min of the different alkali-treated samples, IPDT values of the 0.25 h and 0.75 h treated samples show 104 C and 1038°C, respectively, which have been higher than other treated samples reported in a previous study [65], where they found the IPDT values of treated Borassus coarse and fine fibers to be 227°C and 221°C, respectively. Nevertheless, the IPDT value increased up to about 30% compared to untreated Borassus husk fiber [19].
Moreover, the IPDH values found to be 12.0 W/g, 13.0 W/g, 16.0 W/g, 12.8 W/g and 5.3 W/g for the all the treated fibers: 0.25 h, 0.5 h, 0.75 h, 1 h and 2 h, respectively that are illustrated in Figure 6c which can be attributed to the heat capacity of the samples. It has been also been found that with the increasing of heating rates (i.e., from 10 C/min to 20 C/min), char contents increased for every sample and this pattern is similar to previous researchers' work [19, 41]. Nevertheless, the variation in char residues of treated samples can be associated with treatment duration, sample heterogeneity (biomass) and preparation that also found from the earlier FTIR analysis in this work, which has been suggested by [66], while carrying out thermal analysis on beech and spruce woods.
3.4 Specific Heat Capacity, Cp
The specific heat capacity (Cp) of a substance refers to the amount of heat required to change its temperature by 1°C per unit mass. Although there is no universally fixed method for determining the Cp of biomass as a function of temperature, the American Society for Testing and Materials (ASTM) has outlined a standard approach in ASTM E1269-11. Nevertheless, some researchers [54, 69] investigated Cp at low temperatures (below 150°C) to mitigate mass loss events during pyrolysis.
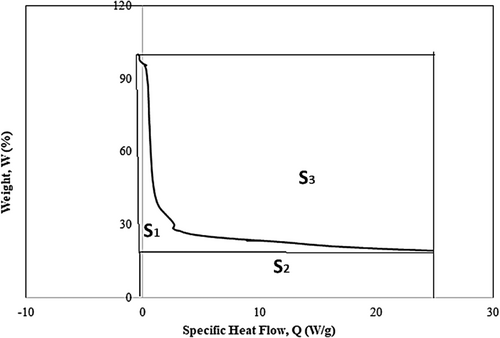
The specific heat capacity of the alkali treated Borassus fruit shell powder was found to be around 9–11 J/g °C at around 100 C–117°C due to dehydration (endothermic heat flow) depending on different samples. Figure 8 below shows a peak between 368°C and 385°C that corresponds to the decomposition of cellulose depending on the samples. Nevertheless, the similar last peak was also observed from raw coconut fiber [53], seaweed biomass [51], raw Borassus husk fiber [19] and untreated hemp fiber [23]. Because of the alkali treatment, the distinct hemicellulose peak was eliminated, which was also confirmed from the earlier TGA thermograms and FTIR analysis in this study.
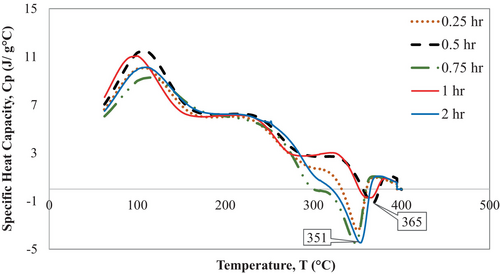
The specific heat capacity of the Borassus fruit husk powder gradually dropped with rising temperature. This pattern of dropping value is associated with the decomposition of chemical compositions, such as hemicellulose, cellulose, and lignin in the biomass consecutively. Interestingly, from 340°C to 365°C, there is a sharp drop in heat capacity being observed in Figure 8, which can be attributed to the removal of hemicellulose and other volatile components from the samples through alkali treatment, and this pattern was found by [23] for hemp fiber. From the earlier TGA experiment, it has been found that biomass produces char residue via pyrolysis process, and the specific heat capacity of char is found to be less [51, 53, 69, 70] compared to hemicellulose and cellulose components. Table 4 summarizes the notable events along with the specific heat capacity of cellulose for all treated samples of Borassus husk fiber. It is noticeable that the specific heat capacity of untreated Borassus husk fiber found (1.6 J/g °C) in the author's previous work [19] was higher, while the IPDH value was negative (−15.36 W/g), which suggests that the material experiences larger endothermic heat flow because of the presence of hemicellulose (hydrophilic constituent). Nevertheless, after alkali treatment, all samples showed positive IPDH values, as indicated by the TGA analysis, which suggests samples experienced higher exothermic heat flow. This heat release is likely due to the removal of weaker components, such as hemicellulose, as identified in the FTIR analysis [64, 71]. As a result, the material becomes more heat-resistant due to the increased presence of cellulose and lignin [66, 70], which contribute to the observed decrease in specific heat capacity values in the DSC analysis.
| Alkali treated samples | Decomposition stages [peak temperature (°C)] | Specific heat capacity, Cp (J/g °C) | |
|---|---|---|---|
| Dehydration | Cellulose | ||
| 0.25 h | 108.1 | 369.2 | 1.0 |
| 0.50 h | 108.1 | 384.3 | 1.1 |
| 0.75 h | 116.3 | 369.2 | 1.0 |
| 1 h | 100.2 | 381.8 | 0.9 |
| 2 h | 109.9 | 374.0 | 1.1 |
4 Conclusions
- TGA analysis demonstrated a significant char residue of up to 32%, which was higher than that of fine and coarse Borassus fibers, as well as other materials such as jute, hemp, sisal, Coccinia grandis, kenaf, flax, and spruce wood when subjected to pyrolysis. The char content increased as the heating rate was raised from 10 C to 20°C/min, and 0.25-h and 0.75-h treated samples show higher values than the rest.
- The onset temperature of cellulose was observed to be between 231°C and 240°C across different heating rates (10 C/min–20°C/min). However, only minimal variation was noted among the alkali-treated samples.
- The IPDT values for all alkali-treated fibers were approximately four times higher than those of the coarse (227°C) and fine (221°C) fibers. Specifically, fibers treated for 0.25 h and 0.75 h exhibited the highest IPDT values, at 1047°C and 1038°C, respectively, indicating significantly enhanced thermal stability.
- The IPDH values for all alkali-treated fibers are found to be higher than their untreated one (−15.36 W/g), and 0.75-h shows the highest value (16 W/g), which may have an impact on material-specific heat capacity, Cp.
- The specific heat capacity (Cp) of all treated fibers was found to be approximately 1 J/g °C, lower than that of the untreated fiber, which had a Cp of 1.6 J/g °C. This decrease in heat capacity is likely attributed to the removal of hemicellulose and lignin, as indicated by the sharp decline observed in the heat capacity plot.
- FTIR plots indicate a significant removal of hemicellulose from the Borassus husk fibers following alkali treatment. Similar results have been observed for both fine and coarse Borassus fibers, as well as other biofibres, such as sisal, hemp, kenaf, and jute.
- SEM analysis revealed that alkali-treated fibers have a more porous and rougher surface compared to the smooth and covered surface of untreated fibers. The additional voids created in the treated fibers increase the tortuosity of the heat transfer pathway, thereby enhancing their thermal insulation properties.
Nomenclature
-
- Cp
-
- Specific heat capacity (J/g °C)
-
- IPDH
-
- Integral process decomposition heat flow (W/g)
-
- IPDT
-
- Integral process decomposition temperature (°C)
-
- Q
-
- Specific heat flow (W/g)
-
- q(DSC)
-
- Heat flow (mW)
-
- W
-
- Weight of the material (mg)
Subscripts
-
- r
-
- Reference material
-
- s
-
- Specimen
-
- T
-
- Temperature (°C)
Author Contributions
Md Atiqur Rahman: conceptualization, investigation, funding acquisition, writing – original draft, writing – review and editing, methodology, validation, formal analysis, software, data curation, resources. Mamadou Ndiaye: writing – review and editing, visualization, project administration, supervision. Bartosz Weclawski: writing – review and editing, visualization, methodology, validation, project administration, supervision. Peter Farrell: visualization, project administration, supervision.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




