Waste Tire Oil Fueled CI Engine Combustion Argumentation for Emission Reduction by Butylated Hydroxy Anisole (BHA) Antioxidant
ABSTRACT
In the world, transport vehicle usage is increasing every day. Corresponding wastes of their used tire become a challenge to the environment as non-biodegradable waste. This waste tire can be used to derive the waste tire oil with distillation for engine application. This oil cannot used as neat fuel for the engine. They were blended with diesel support to reduce the total diesel usage. The base fuel is a 20% waste tire oil blend with 80% diesel (D80WTO20). Butylated hydroxy anisole (BHA) antioxidants of 250–1000 ppm with a 250 ppm deviation were added with a D80WTO20 blend for testing in a 5.2 kW CI engine. The D80WTO20 blend has lesser performance and higher emissions than diesel. The increase of BHA concentration in the fuel enhances the results. The maximum concentration of BHA (1000 ppm) used in D80WTO20 is obtained BTE of 32.92%, which is higher than diesel and the blend. The same combination has lesser BSFC (0.247 kg/kW-h), NOx (1406 ppm), smoke (51.68%), HC (61 ppm), and CO (0.224%) emission D80WTO20 blend than because of the better oxidation, the catalytic reaction produced improved combustion. Therefore, the 1000 ppm of BHA antioxidant mixed D80WTO20 blend can be the alternate fuel for the CI engine with lesser emission and improved performance.
1 Introduction
It is well-known that diesel engines outperform spark-ignited engines in terms of durability, efficiency, and lifespan. Diesel engines emit less harmful gases due to the lean burn combustion process. But this engine produced soot and nitrogen oxide emissions, which are the challenges. These are widely used in transport, industrial, power generation, and agricultural applications [1]. Scholars are researching diesel engines to make them more efficient and eco-friendlier. Conventional energy sources are becoming less viable, so there is a push for renewable energy sources like solar radiation, hydrological systems, wind patterns, and organic matter. These sources offer a sustainable alternative to traditional energy generation methods [2].
Waste-based fuels are eco-friendly alternatives to diesel engines, but we need multiple sources to meet energy demands [3]. Engine fuels from waste oil and other non-consumable materials are becoming more common. This approach helps reduce costs and minimize environmental harm. India is the world's second most populous country, with over 1.3 billion people. Population growth leads to an increase in waste generation and negative environmental impacts. According to projections, India is expected to become the most populous country in the world by 2024. The proliferation of trade and industry, coupled with urbanization and modernization, has led to significant alterations in climate patterns and the destruction of ecosystems [4].
Energy consumption and population growth are linked. The study analyzes energy usage in developed nations like the US, Germany, and Japan and developing countries like India and Bangladesh. The research also looks at China's growing energy consumption and the impact of fossil fuel availability on energy supply and demand [5]. Managing waste involves minimizing its impact on people and the environment. Scientists are researching methods to transform waste into energy or useful commodities [6].
“Special Waste” refers to regulated materials like clinical, asbestos, and used tire waste that require proper environmental and human health management [7]. Solid waste can be classified as biodegradable or non-biodegradable. Biodegradable waste decomposes with microorganisms, while non-biodegradable waste, like plastic, does not easily break down [8]. Tires have a short lifespan and are made of rubber, steel, and other additives. India produces 190 million tons of waste tires annually due to high vehicle numbers and poor roads [9].
The disposal of waste tires through landfilling constitutes 53% of the total volume generated. The various methods of waste tire recycling are mentioned in Figure 1. This method impacts land allocation fertility and poses a fire hazard, so many nations prohibit it [10]. Incineration and Tire Derived Fuel (TDF) are two methods that convert waste tires into energy. TDF turns tires into liquid fuel that can be burned in combustion systems [11]. Passenger vehicle tires are primarily composed of carbon (85%), fabric materials (10%–15%), and sulfur (0.9%–1.25%). Pyrolysis is a promising method to convert organic matter in tires into valuable energy sources. This information provides a basis for further research on tire composition and its impact on performance and durability [12].

Efficient waste tire collection and sorting are vital for cost reduction and raw material supply. Streamlined logistics and marketing strategies build customer loyalty. Converting waste tires to tire oil is profitable and eco-friendly. Efficient pyrolysis processes, market demand for sustainable energy, and revenue from by-products can boost profits. Government incentives, optimized logistics, and strong customer relationships can also help. Distilling TPO is crucial in making it safe and marketable while reducing harmful emissions and promoting clean-burning fuel [16]. Table 1 mentions the different content of the waste tire oil by weight percentage in different researchers' observations.
The AVL 5402, 6.25 kW engine operated with tire pyrolyzed oil blends with diesel in 20%–40%. The exhaust gas recirculation is also used for emission reduction. Without the EGR condition, the 40% blend has 22 J/CAD of HRR, 12 CAD of ignition delay, and 38%, 30%, and 11% increased CO, HC, and NOx emissions with 7% reduced particulate matter than diesel. But with the EGR condition 25 J/CAD of HRR, 13 CAD of ignition delay, and 84%, 26% increased CO, HC, and 40%, 29% reduced NOx and particulate matters than the diesel [17].
The 3000 rpm 5.4 kW Antor 6LD400 CI engine is operated with a 10% blend of waste tire oil with diesel. The engine runs at a constant speed of 2200 rpm. At maximum load, the 10% waste tire oil blend has 54 bar in-cylinder pressure, 22 J/CAD of HRR, 1500 K of in-cylinder temperature, 110 CAD of combustion duration, 0.27 kg/kW-h of SFC, 9% of CO2, and 600 ppm of NOx these all are higher than diesel. The same blend has 1 ms of ignition delay, 6.5 MW/m2 ringing intensity, 23% thermal efficiency, 300 ppm of CO, and 2.9 m−1 of soot emission. These are less than diesel [18].
The impact of nanoparticles on internal combustion engines using various fuel types, including diesel, biodiesel, and synthetic oils. The study focused on the influence of individual nonmetals (MWCNT, SWCNT, CNT GNP, and GO), metals (Zr, Ni, Mg, Cu, and Si), oxides of metal (CeO2, AgO2, CuO, MnO, TiO2, Al2O3, and Fe2O3) and, with varying outcomes due to factors such as nanoparticle size, concentration, and blending. This research provides valuable insight into the potential effects of nanoparticles on engine performance and highlights the importance of continued exploration in this field [19].
Grapefruit waste seed biodiesel 30% blend with diesel runs the 5.5 kW CI engine. Adding 10% BHA and BHT antioxidants to the blend improves fuel atomization. Diesel results are compared between the 30% biodiesel blend, the 10% BHA added blend, and the BHT added blend. The BHA used blend has 29.4% of BTE, 0.298 kg/kW-h of BSFC, 65.12 bar of ICP, 68.08 J/CAD of HRR, 12 g/kW-h of NOx, 1.2 BSU of smoke, 1.8 g/kW-h of CO, and 0.259 g/kW-h of HC. The BHT used blend has 28.2% BTE, 0.33 kg/kW-h of BSFC, 64.35 bar of ICP, 69.75 J/CAD of HRR, 13 g/kW-h of NOx, 1.3 BSU of smoke, 2.1 g/kW-h of CO, and 0.325 g/kW-h of CO. Both results of antioxidants used blends have better than the blend results and nearly equal to the diesel results [20].
In a 5.2 kW diesel engine, run by 20% jamun biodiesel. The assessment was carried out by adding antioxidants such as l-ascorbic acid, A-tocopherol acetate, and p-phenylenediamine separately to the biodiesel at 250 mg per kg of oil. The mixture of additives, including antioxidants and p-phenylenediamine, effectively reduces NOx emissions (24.7%) while decreasing HC (62.5%), CO (52%), and smoke (12.2%) compared to the JA20 blend without additives. CP and HRR remain unchanged [21].
From this piece of literature, tire waste can be used to run the engine with lower blending with diesel, but this has higher emissions and lesser performance than diesel. Also, antioxidants are used to improve performance and emissions. Waste tire oil with antioxidants used work in CI engine is very less. In this investigation, the waste tire oil is derived by distillation of pyrolyzed waste tire oil. This is supported by diesel blending. The 20% waste tire oil blend with 80% diesel (D80WTO20). This blend performance and emission were tried to improve the Butylated hydroxy anisole antioxidants in different amounts, such as 250, 500, 750, and 100 ppm. These BHA-added blends and D80WTO20 were used to run the CI engine at different loads, and the results were compared with the diesel. The following pretest observations show this study's preferred antioxidant concentrations. For oxidation stability, the D80WTO20 blend used the well-known antioxidant BHA. It initially improved oxidation resistance at 250 ppm. The study measured performance and emission improvements while maintaining fuel stability during storage and combustion by gradually increasing the concentration to 1000 ppm. By improving oxidation and catalytic reactions, BHA affected fuel blend calorific value and combustion efficiency. The chosen range allowed systematic examination of these effects at higher concentrations. Choice of concentration range struck a realistic balance between economic viability and performance enhancement. Higher concentrations (750–1000 ppm) improved combustion efficiency and emission reduction, while lower concentrations (250–500 ppm) were cheaper but less effective. This justified the higher cost at higher concentrations. Concentration determined antioxidants' ability to reduce smoke, NOx, CO, and HC. The 250–1000 ppm range was chosen to reduce emissions without over-adding additives or raising costs. The range was also influenced by preliminary research and industry standards that recommended similar antioxidant concentrations for fuel blends. This allowed the study to accurately assess BHA's effects in this range while adhering to best practices. Concentrations above 1000 ppm were avoided to avoid excessive fuel property changes (like viscosity) or engine component incompatibility. Capping the concentration at 1000 ppm improved performance and emissions while ensuring safety and compatibility. The following pretest observations show this study's preferred antioxidant concentrations. For oxidation stability, the D80WTO20 blend used the well-known antioxidant BHA. It initially improved oxidation resistance at 250 ppm. The study measured performance and emission improvements while maintaining fuel stability during storage and combustion by gradually increasing the concentration to 1000 ppm. By improving oxidation and catalytic reactions, BHA affected the fuel blend's calorific value and combustion efficiency. The chosen range allowed systematic examination of these effects at higher concentrations. The choice of concentration range struck a realistic balance between economic viability and performance enhancement. Higher concentrations (750–1000 ppm) improved combustion efficiency and emission reduction, while lower concentrations (250–500 ppm) were cheaper but less effective. This justified the higher cost at higher concentrations. Concentration affects antioxidants' ability to reduce smoke, NOx, CO, and HC. The 250–1000 ppm range was chosen to reduce emissions without over-adding additives or raising costs. The range was also influenced by preliminary research and industry standards that recommended similar antioxidant concentrations for fuel blends. This allowed the study to accurately assess BHA's effects in this range while adhering to best practices. Concentrations above 1000 ppm were avoided to avoid excessive fuel property changes (like viscosity) or engine component incompatibility. Capping the concentration at 1000 ppm improved performance and emissions while ensuring safety and compatibility.
2 Experimentation
The waste tire pyrolysis oil is purchased from Srinivasa Industries Lit. in Chennai. This is made by the pyrolysis process at 400°C. This oil is highly viscous and has a heavy odor. This could not be used directly by the engine. It will spoil the engine parts. So, to reduce the convenient fuel of this oil by the distillation process. The distillation arrangement is shown in Figure 2. The electric heater provided a constant and uniform heat source to the distillation flask. A manipulable control knob was used to adjust the heating rate, while a thermometer with a range of 0°C–360°C accurately measured the temperature.
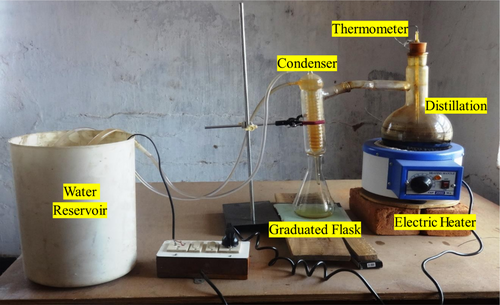
An electric heater heated the waste tire pyrolysis oil. The study focused on temperatures ranging from 230°C to 250°C. The oil is converted into vapor, condensed into a gaseous state through a condenser facilitated by water, and then collected in a receptacle. A pump was used to introduce water into the condenser in this experimental setup continuously. The condensed liquid is collected in a graduated flask. This fuel is called waste tire oil (WTO). To ensure that distilled waste tire oil (WTO) characteristics remained consistent across batches, quality control procedures were implemented during distillation. First, temperature and pressure were monitored to ensure distillation consistency. The temperature range of 230°C–250°C was maintained to avoid overheating or underheating the distilled oil's chemical composition. Second, periodic sampling during distillation allowed real-time analysis of fuel properties like density, calorific value, viscosity, and sulfur content. This kept these parameters reasonable for the intended use.
Oxidation stability tests assessed the distilled oil's chemical stability to ensure fuel quality. To improve consistency, the finished product was tested batch-to-batch in a controlled engine environment. This ensured the oil's combustion properties. Meet engine and blending requirements.
The blending is created with diesel on a 20:80 percentage of volume basis. This fuel is called D80WTO20.
- D—Diesel.
- D80WTO20—Diesel 20% and Waste tire oil 80% by volume.
- D80WTO20 + 250 BHA—250 ppm of BHA mixed per liter of D80WTO20.
- D80WTO20 + 500 BHA—550 ppm of BHA mixed per liter of D80WTO20.
- D80WTO20 + 750 BHA—750 ppm of BHA mixed per liter of D80WTO20.
- D80WTO20 + 1000 BHA—1000 ppm of BHA mixed per liter of D80WTO20.
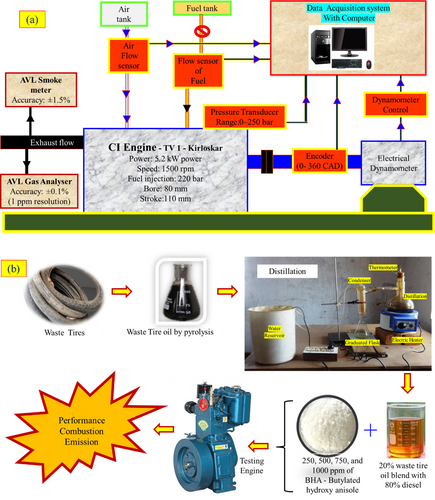
These fuels were evaluated, and corresponding properties were mentioned in Table 2. These fuels were used to run the CI engine. The arrangement of the engine testing is shown in Figure 3a. The engine details and the individual parameters of the experimental arrangements are mentioned in Table 3. The emissions and smoke of the engine exhaust were measured with the AVL five gas analyzer and smoke meter. Their details are also mentioned in Table 3. The test fuels were individually tested with the fuels at zero to full load conditions, and the investigation flow is mentioned in Figure 3b. The equations mentioned below help identify the results [22, 23].
| Characteristics | Density (kg/m3) | Kinematic viscosity @40°C (cST) | Calorific value (MJ/kg) | Cetane number | Flash point (°C) |
|---|---|---|---|---|---|
| ASTM—Standard | D 4052 | D 445 | D 240 | D 4737 | D 92 |
| Diesel | 830 | 3.05 | 42.92 | 51 | 65 |
| WTO | 885 | 3.58 | 37.02 | 44 | 96 |
| D80WTO20 | 841 | 3.20 | 40.94 | 49 | 71 |
| D80WTO20 + 250BHA | 838 | 3.17 | 41.41 | 50 | 74 |
| D80WTO20 + 500BHA | 835 | 3.15 | 41.88 | 52 | 78 |
| D80WTO20 + 750BHA | 832 | 3.13 | 42.35 | 53 | 81 |
| D80WTO20 + 1000BHA | 830 | 3.10 | 42.83 | 55 | 85 |
| Uncertainty and accuracy details | Engine details | ||||
|---|---|---|---|---|---|
| Content | Name | Accuracy | U-uncertainty (%) | Model | TV1 |
| BP | UBP | 0.001 kW | ±1.5 | Compression ratio | 17.5 |
| In-cylinder pressure | UICP | 0.001 bar | ±1.0 | Speed | 1500 rpm |
| BTE | UBTE | 0.01% | ±0.02 | Power | 5.2 kW |
| Temperature—T | UT | 0.05°C | ±1.0 | Max load | 18.24 kg |
| Smoke—S (AVL smoke meter) | US | 0.02% | ±0.05 | Injection pressure | 200 bar |
| CO (AVL gas analyzer) | UCO | 0.00% | ±1.0 | Timing of injection | 23° TDC |
| HC (AVL gas analyzer) | UHC | 1 ppm | ±0.05 | Cooling | Water |
| NOx (AVL gas analyzer) | UNOx | 2 ppm | ±0.01 | Bore | 0.0875 m |
| Total uncertainty | ±2.29 | Stroke | 0.11 m | ||
3 Results and Discussion
Waste to useful product production is the evergreen idea for sustainable development [24]. The performance, combustion, and emission results were taken from this computerized experimental arrangement at different loads. The performance was calculated with Equations (1) and (2). Combustion-based heat release rates were calculated [22, 23] with Equation (3). The cumulative uncertainty is computed by Equation (4). The experimentally measured result of the BHA oxygenates added blends was compared with the base blend and the diesel. These all are discussed one by one in the following section. The statistical analysis was carried out and marked the error bars in the graph at a 95% confidence interval with a ±1 standard deviation.
In the performance analysis brake, specific fuel consumption is the major parameter to realize, which helps identify the brake thermal efficiency. BHA antioxidant impact on the BSFC of the D80WTO20 blend was mentioned in Figure 4. Here, Diesel and D80WTO20 have 0.257 and 0.270 kg/kW-h of BSFC at full load. This increase in the BSFC blend is due to the reduction in the calorific value and the increase in the viscosity of the blend compared to diesel [25]. This will require more fuel to produce equal power at the same operating conditions [26].
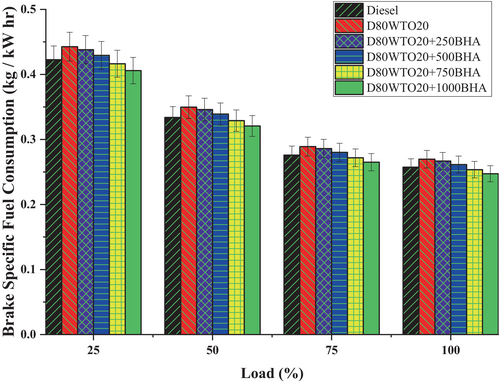
Similarly, D80WTO20 + 250BHA has 0.267 kg/kW-h, D80WTO20 + 500BHA has 0.261 kg/kW-h, D80WTO20 + 750BHA has 0.254 kg/kW-h, and D80WTO20 + 1000BHA has 0.247 kg/kW-h of BSFC at maximum load. The increase in the BHA amount increases the BSFC than the diesel. This increase in the BSFC is mainly due to the need for more fuel to create the energy to produce similar power [13-15]. The less calorific value increases the fuel supplied to the engine [21, 25].
BHA antioxidant impact on BTE of the D80WTO20 blend was shown in Figure 5. Diesel and D80WTO20 have 31.62% and 30.19% of BTE at full load. This decrease in BTE of the blend due to the participation of the 20% WTO involvement decreased the calorific value, increased the blend's viscosity, and delayed the vaporization during combustion [15, 25]. Similarly, D80WTO20 + 250BHA has 30.51%, D80WTO20 + 500BHA has 31.13%, D80WTO20 + 750BHA has 32.10%, and D80WTO20 + 1000BHA has 32.92% at maximum load.
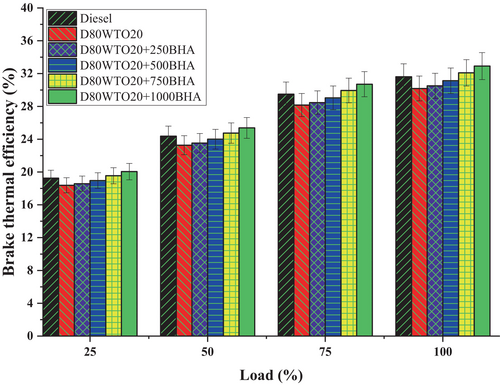
Up to 500 ppm of BHA-used blends have lesser BTE; beyond that, up to 1000 ppm of BHA blends have higher BTE than diesel. This BTE difference is because the amount of the BHA used in the blend increases the calorific value and improves the vaporization. These improved properties and will help with atomization in combustion, so the thermal efficiency increases with the higher involvement of the BHA antioxidants [19, 27]. The increase of BHA in the blend increases the fuel's oxygen content, which improves the combustion than the D80WTO20 [21, 28].
BHA antioxidant impact on in-cylinder pressure of D80WTO20 blend expressed in Figure 6. Here, diesel and D80WTO20 have 71.99 bar and 73.07 in-cylinder pressure at full load. This increase in the blend is due to the higher viscosity and lesser cetane number of the D80WTO20, creating rapid combustion with higher fuel consumption. So, the maximum pressure increases than diesel [15, 25, 29].
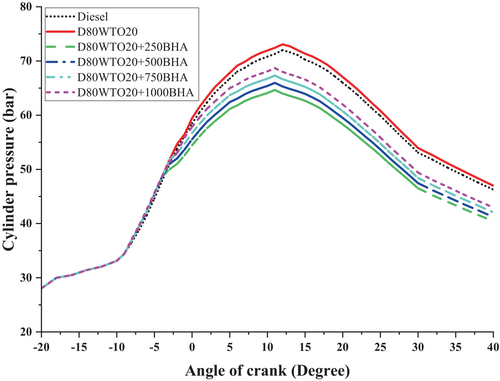
Similarly, D80WTO20 + 250BHA has 65.55 bar, D80WTO20 + 500BHA has 66.89 bar, D80WTO20 + 750BHA has 68.25 bar, and D80WTO20 + 1000BHA has 69.64 bar at maximum load. These maximum pressures were slightly shifted toward the positive crank angle side. These differences were created by the BHA involved in the D80WTO20 blend. D80WTO20 + 1000BHA produced better combustion. An increase in BHA increases the pressure due to the higher oxygen content available on the BHA utilized in the combustion [21, 26]. It increases the premixed combustion and pressure but less than the diesel fuel [27, 28].
BHA antioxidant impact on the HRR of D80WTO20 blends is plotted in Figure 7. Diesel and D80WTO20 have 38.10 and 43.03 J/CAD heat release rates at full load. This increase in the blend is due to the reduced calorific value and lesser cetane number by the participation of the WTO [12, 19]. More fuel participation increases the ignition delay period and the heat release rate [25].
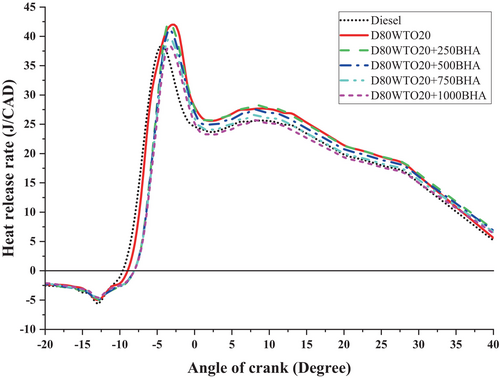
Similarly, D80WTO20 + 250BHA has 41.91 J/CAD, D80WTO20 + 500BHA has 40.69 J/CAD, D80WTO20 + 750BHA has 39.31 J/CAD, and D80WTO20 + 1000BHA has 37.98 J/CAD at maximum load. BHA reduces the ignition delay and improves the combustion [23]. The high concentration of the BHA antioxidant improves the combustion through an improved cetane number and increased calorific value than the D80WTO20 [26-28]—the least HRR obtained by the D80WTO20 + 1000BHA, which is nearly equal to the diesel.
Insufficient oxygen during combustion leads to incomplete combustion, which is responsible for the emission of carbon monoxide. BHA antioxidant impact on the CO emission of the D80WTO20 blend is shown in Figure 8. Diesel and D80WTO20 have 0.254% and 0.320% CO emissions at full load. This increase in CO emissions by the blend is created due to the incomplete combustion formed by the lesser calorific value and higher density of D80WTO20 [17, 25, 29].
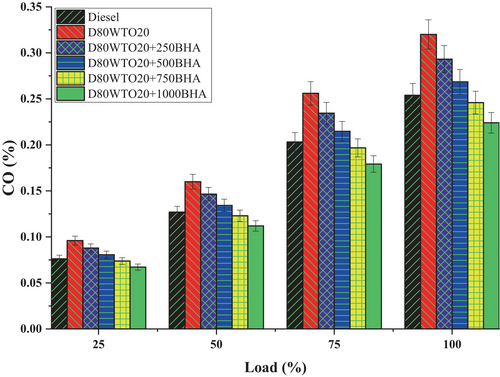
Similarly, D80WTO20 + 250BHA has 0.293%, D80WTO20 + 500BHA has 0.268%, D80WTO20 + 750BHA has 0.246%, and D80WTO20 + 1000BHA has 0.224% at maximum load. BHA antioxidants improve combustion and reduce CO emissions by increasing their concentration in the D80WTO20 blend [20, 26]. The improved cetane number and calorific value are involved in better combustion. The higher BHA antioxidant availability improves continuous oxidation during combustion [21, 30]. This will convert the possible CO emissions into carbon dioxide through oxidation [27, 31].
The leading cause of hydrocarbon emissions is unburnt and partially burnt fuels. The BHA antioxidant impact on unburnt hydrocarbon emissions of the D80WTO20 blend is mentioned in Figure 9. Here, diesel and D80WTO20 have 77 and 85 ppm of unburnt hydrocarbon emissions at full load. This increase in HC emissions of the blend is due to a higher hydrocarbon fuel content available by fuel consumption during combustion. Also, rapid combustion creates improper combustion, producing unburnt hydrocarbons [14, 32-34].
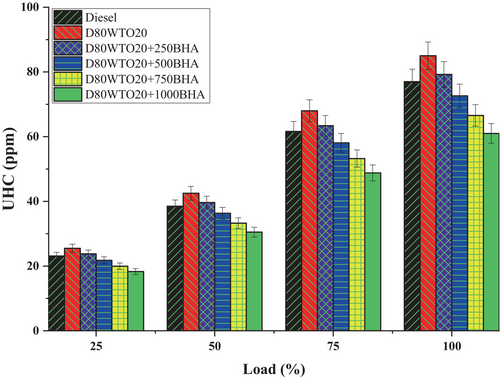
Similarly, D80WTO20 + 250BHA has 79 ppm, D80WTO20 + 500BHA has 73 ppm, 80WTO20 + 750BHA has 67 ppm, and D80WTO20 + 1000BHA has 61 ppm at maximum load. Compared to D80WTO20, HC emission gets reduced with increasing BHA antioxidants [21]. D80WTO20 + 1000BHA has the least HC emission, even less than diesel. This reduction is because of the improved oxidation of the unburnt hydrocarbons into further combustion products like carbon monoxide and carbon dioxide [35, 36]. However, this oxidation is limited by the premixed combustion and the lesser available duration for oxidation [28, 31].
NOx emission formation depends on temperature, oxygen, and gas residence time. BHA antioxidant impact on NOx of D80WTO20 blends is plotted in Figure 10. Here, Diesel and D80WTO20 have 1719 and 1886 ppm of NOx emission at full load. This increase in the blend is due to the maximum cylinder temperature because of the heat release rate produced by the D80WTO20 during the combustion. The increase in premixed combustion increases the NOx emission formation by converting the nitrogen content in the air-fuel mixture [15, 17, 18, 37].
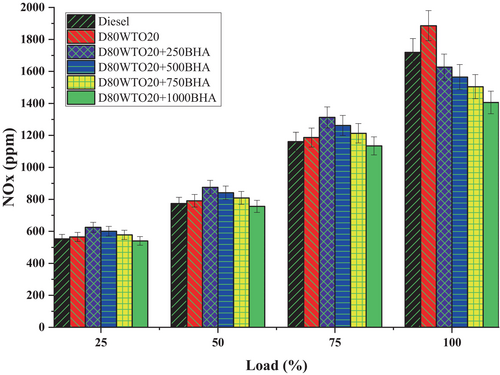
Similarly, D80WTO20 + 250BHA has 1627 ppm, D80WTO20 + 500BHA has 1565 ppm, D80WTO20 + 750BHA has 1504 ppm, and D80WTO20 + 1000BHA has 1406 ppm at maximum load. D80WTO20 + 1000BHA has the least NOx emission than diesel and D80WTO20. This reduction in the NOx emission is caused by the lesser heat release rate produced during combustion, which reduces the in-cylinder temperature that will produce the lesser NOx emission [31, 38]. The BHA is involved in the combustion catalytic reaction, which also reduces the NOx emission based on the availability of the BHA concentration in the blend [35, 38-40].
Increased BHA concentration reduces NOx emissions due to its phenolic antioxidant properties. BHA breaks NOx chain reactions during combustion as a radical scavenger. High-temperature NOx production usually occurs through the Zeldovich mechanism, in which oxygen radicals react with nitrogen molecules in the air. BHA inhibits oxygen radicals by stabilizing them with phenolics. Thus, fewer NOx-forming active oxygen species are available [41-43]. BHA also improves fuel blend oxidation stability, resulting in more complete combustion at lower peak temperatures. Lower combustion temperatures naturally produce less NOx because thermal NOx formation is temperature-dependent. BHA improves combustion and prevents precursor production to reduce NOx emissions without affecting engine performance. Its radical inhibitor and stabilizer properties show how BHA reduces alternative fuel blend emissions.
Incomplete combustion, oxygen deficiency, fuel atomization, and auto-ignition temperature of the fuel are the key factors contributing to smoke emission in diesel engines. BHA antioxidant impact on the Smoke of D80WTO20 blends is mentioned in Figure 11. Here, Diesel and D80WTO20 have 66.40% and 70.62% of smoke emissions at full load. This increase in the blend is due to the maximum fuel consumption of the diesel [14, 17]. The higher viscous and density of D80WTO20 fuel produced incomplete combustion, increasing the emission of smoke [25].
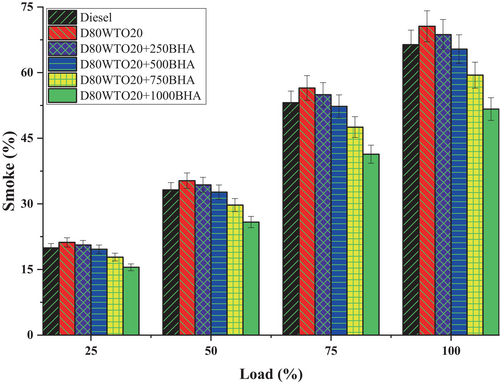
Similarly, D80WTO20 + 250BHA has 68.71, D80WTO20 + 500BHA has 65.37, D80WTO20 + 750BHA has 59.43, and D80WTO20 + 1000BHA has 51.68 at maximum load. The smoke emissions get reduced with increased BHA antioxidant involvement in the D80WTO20 blend. This difference is due to the improved combustion through the better oxidation created by the BHA [25, 31, 38]. This antioxidant is involved in the improved oxidation of the fuel in the combustion process, so the smoke reduces with a higher concentration of the BHA [39, 44].
Incorporating waste-derived tire oil into energy policies can help create a circular economy by addressing environmental issues and decreasing dependence on traditional diesel. The following are the main implications [45-47]: By promoting alternative fuels, such as waste tire oil blends, we can reduce our reliance on fossil fuels and improve energy security. (Diversifying Sources of Fuel.) Using waste tire oil reduces the amount of tire waste that cannot be broken down by nature and lowers greenhouse gas emissions, which is in line with global sustainability goals. With the right investment in waste management and refining technologies, it is possible to increase the production of tire oil through distillation. Adding antioxidants such as BHA can help ensure that performance and emissions meet industrial standards. Subsidies, tax benefits, and regulations that encourage cleaner alternatives are ways that policymakers can motivate the use of fuels that come from waste. Using fuels made from waste is a cost-effective solution because it creates value from waste materials, generates revenue, and lowers fuel costs [30, 48]. Table 4 is a comparative analysis to highlight the uniqueness of the current study.
| Study/reference | Key focus | Key findings | Uniqueness of current study | Improvements in current study |
|---|---|---|---|---|
| Sharma and Murugan [34] | Effect of tire-derived fuel on biodiesel oxidation stability and engine performance and emissions. | Tire pyrolysis oil (TPO) had natural antioxidant properties. Emissions were reduced, but engine performance was not as efficient as diesel. | Investigates antioxidant additives instead of relying solely on natural antioxidants in TPO. | Used Butylated Hydroxyanisole (BHA) in precise concentrations to enhance performance and further reduce emissions. |
| Balaji et al. [36] | Effects of antioxidant addition and catalytic converter on diesel-tire oil blends. | Tire oil blends reduced dependency on diesel but required a catalytic converter and antioxidants for improved emissions. | Focused specifically on antioxidant concentration and performance without dependency on catalytic converters. | Demonstrated improved combustion and emissions with optimized antioxidant concentration (1000 ppm). |
| Gnanasikamani et al. [37] | Fuel modification and after-treatment of tire oil-diesel blends. | Added antioxidants (up to 300 ppm) to reduce emissions, but performance gains were marginal. | Explored higher antioxidant concentrations (250–1000 ppm) systematically for performance and emission optimization. | Achieved substantial performance (32.92% BTE) and emission reductions compared to lower antioxidant concentrations. |
| Kumaravel et al. [40] | Use of nano-additives in tire oil-diesel blends to improve fuel properties. | Nano-additives enhanced the calorific value and reduced emissions, but they increased costs significantly. | Used cost-effective antioxidants (BHA) instead of nano-additives. | Balanced performance improvements and cost-effectiveness with an optimal antioxidant concentration. |
| Yaqoob et al. [41] | Review of tire pyrolysis oil as an alternate fuel. | Tire oil was viable as an alternative fuel but required significant fuel property modifications. | Combined waste tire oil blending with antioxidant optimization to enhance both fuel properties and engine performance. | Improved cetane number, calorific value, and viscosity for better engine performance. |
| Saravanan et al. [43] | Emission evaluation of tire oil blends using nano-pyrogallol as antioxidants. | Nano-pyrogallol (500 and 1000 ppm) reduced emissions and enhanced fuel properties, but engine performance gains were limited. | Achieved both higher performance (BTE of 32.92%) and lower emissions without relying on expensive nano-antioxidants. | Used cost-effective BHA antioxidants with comparable results in emissions and better performance. |
| Raj et al. [45] | Comparison of waste plastic oil and tire oil as alternative fuels. | Waste tire oil blends showed potential as diesel alternatives but required further optimization of fuel properties and additives. | Focused on systematic optimization of tire oil blends with precise antioxidant concentrations. | Demonstrated practical engine performance improvements with optimized fuel properties using 1000 ppm of BHA. |
| Hosseinzadeh-Bandbafha et al. [46] | Comprehensive review of biodiesel antioxidants and their impact on engine performance. | Phenolic antioxidants were found to improve biodiesel stability and reduce emissions. | Applied phenolic antioxidants (BHA) to waste tire oil blends for CI engines, an area less explored. | Proved the effectiveness of BHA in enhancing fuel properties and reducing emissions in tire oil-diesel blends. |
| Kumaravel et al. [47] | Review on tire pyrolysis oil as an alternative fuel. | Tire pyrolysis oil was recognized as a viable alternative fuel but required property enhancements for effective engine application. | Optimized fuel properties through antioxidant addition to address performance and emission challenges. | Achieved significant improvements in engine efficiency and emissions with an optimized D80WTO20 blend. |
| Subramani et al. [33] | Evaluated methyl esters from novel feedstocks and their combustion/emission characteristics. | Insights into fuel blending strategies for alternative biofuels. | Focused on waste-derived fuels (tire oil) instead of novel biofuel feedstocks. | Extended blending strategies by integrating antioxidants for performance and emission optimization. |
| Venu et al. [30] | Investigated nano-additives' role in emission reduction and combustion improvement. | Demonstrated significant reductions in emissions and enhanced combustion efficiency using nano-additives. | Focused on cost-effective antioxidant solutions (BHA) instead of expensive nano-additives. | Balanced economic feasibility with comparable improvements in emissions and performance. |
3.1 Practical Implications for Large-Scale Adoption of D80WTO20 + 1000 ppm BHA
Reusing tires for fuel reduces landfill waste and non-biodegradable waste. Scaling this process reduces waste tire pollution. The optimized blend (D80WTO20 + 1000 ppm BHA) reduces NOx, smoke, HC, and CO emissions compared to diesel, meeting international climate goals. Widespread adoption may reduce greenhouse gas emissions and air pollution. The blend seems ready for Bharat Stage VI and Euro VI emission standards. The study found that D80WTO20 + 1000 ppm BHA reduces NOx emissions by 25.45% (1406 vs. 1886 ppm for the base blend). Lower NOx emissions result from better oxidation, combustion stability, and BHA antioxidant catalysis. These cuts meet strict Bharat Stage VI and Euro VI NOx limits. The study does not provide PM data, but the drop in smoke emissions (from 70.6% to 51.68%) suggests lower PM levels. Emission regulations require lower particulate emissions for the environment and human health. From 89 ppm in the base blend to 61 ppm in D80WTO20 + 1000 ppm BHA, HC emissions dropped 31.96%. BHA's oxidation stability and the blend's cetane number reduce unburned hydrocarbons, improving combustion efficiency. From 0.32% to 0.224%, CO emissions dropped 30%. This improvement meets international standards because CO is a key target for reducing air pollution and health risks. Future widespread adoption (scalability) of D80WTO20 + 1000 ppm BHA depends on the following factors. A reliable and affordable supply of waste tire oil (WTO) and antioxidants like BHA is needed to scale up BHA-enhanced D80WTO20 blends. Expanded waste tire pyrolysis facilities with efficient logistics are needed to maintain oil quality and quantity. Optimize antioxidant synthesis to save money. The fuel blending infrastructure can accommodate BHA doping because it only requires basic mixing. However, industrial antioxidant dispersion may require equipment upgrades. BHA is cheaper than nano-additives, but buying and adding it to WTO blends costs more than diesel. An economic feasibility analysis of this blend with other alternative fuels would be easier with a lifecycle cost analysis. BHA is cheaper than nano-additives, but buying and adding it to WTO blends costs more than diesel. An economic feasibility analysis of this blend with other alternative fuels would be easier with a lifecycle cost analysis. Engine testing is needed to determine WTO blends' long-term effects on injectors, fuel pumps, and combustion chambers. Long-term storage stability is essential, especially in different climates. BHA's antioxidant properties should improve fuel stability, but large-scale testing is needed.
The effectiveness of butylated hydroxy anisole (BHA) and nanoparticles as additives in alternative fuel blends is contrasted in the table below. Based on published research and experimental results, parameters like cost-effectiveness, scalability, emission reduction, and combustion efficiency are assessed. Table 5 illustrates the importance of BHA over the addition of nanoparticles in the fuel blends by highlighting the effectiveness in different aspects.
| Parameter | BHA antioxidant | Nanoparticles |
|---|---|---|
| Mechanism of action | Improves fuel stability by scavenging free radicals, reducing oxidative degradation. | Acts as a catalyst to enhance combustion, oxidation, and reduce emissions. |
| Effect on combustion | Enhances combustion efficiency by improving cetane number and calorific value. | Further enhances combustion due to catalytic properties, leading to better heat release rates. |
| Reduction in NOx | 25%–30% reduction in NOx (from 1886 to 1406 ppm in D80WTO20 blend). | Up to 40% reduction in NOx emissions due to superior thermal and catalytic effects. |
| Reduction in smoke/PM | Reduces smoke by 27% (from 70.6% to 51.68% in D80WTO20 blend). | Greater reduction in PM (up to 45%) due to effective combustion and particle agglomeration. |
| Reduction in HC | HC emissions reduced by ˜31% (89–61 ppm). | Higher reduction in HC (˜35%–40%) due to complete oxidation. |
| Reduction in CO | CO emissions reduced by 30% (from 0.32% to 0.224%). | Comparable reduction in CO (˜30%–35%), with higher variability depending on particle type. |
| Fuel stability | Excellent oxidative stability over extended periods. | Limited impact on oxidative stability; primary role is combustion enhancement. |
| Environmental concerns | Minimal, as BHA is biodegradable and non-toxic. | Potential environmental toxicity and disposal challenges for nanoparticles. |
| Cost | Low cost; readily available and scalable for blending. | Higher cost due to synthesis, processing, and dispersion challenges. |
| Scalability | Easily scalable for industrial applications. | Requires advanced dispersion techniques, limiting large-scale use. |
| Engine compatibility | No significant wear on engine components. | May cause abrasive wear if dispersion is not uniform. |
| Ease of implementation | Simple blending with fuels, no special modifications required. | Requires advanced equipment for nano-dispersion to prevent agglomeration. |
The pretest results determined the range, which was rounded off for safe experiments. Thus, this study tested BHA concentrations up to 1000 ppm, which showed significant emissions and performance improvements. Despite improved fuel qualities (cetane number and calorific value), decreased emissions (NOx, HC, CO, and smoke), and combustion efficiency at 1000 ppm, higher concentrations may yield more information. Higher concentrations, like 1250 or 1500 ppm, may reveal whether increased viscosity, antioxidant saturation, or engine combustion dynamics reduce or plateau the benefits. At high concentrations, other antioxidants and nano-additives have shown declining benefits or even negative effects, like soot formation. Higher BHA concentrations may be needed to find the best balance between performance, emissions, and cost-effectiveness without compromising engine compatibility or long-term operational stability.
The D80WTO20 fuel blend with 1000 ppm BHA may be cheaper than diesel. In areas with high diesel prices, adding 20% waste tire oil reduces diesel use and fuel costs. Although BHA antioxidants cost more, their use is low because only low ppm levels are needed. BHA's improved fuel properties and combustion efficiency reduce fuel consumption (BSFC), offsetting the cost impact. Improved engine performance and lower emissions can extend engine life and reduce maintenance costs, lowering overall costs. The blend uses waste management to turn non-biodegradable tire waste into fuel, making it a cheaper and greener diesel alternative. The market price of BHA antioxidants may slightly raise the initial production cost, but less diesel use, lower emissions-related fines, and possible government incentives for using alternative fuels could make this blend economically advantageous in the long run. More research on large-scale antioxidant manufacturing and bulk acquisition can help maximize cost-effectiveness.
4 Conclusions
- D80WTO20 has lesser BTE (30.19%), higher NOx (1886 ppm), smoke (70.6%), and CO (0.32%) emissions than diesel fuel due to the higher viscosity, density, and lesser calorific value and cetane number of the D80WTO20 blend than diesel.
- Different concentrations of Butylated hydroxyanisole in the D80WTO20 blend can run the engine on all loads.
- Increase of BHA improves the blend properties like calorific value, cetane number, and viscosity.
-
Because of the better oxidation, the catalytic reaction produced improved combustion. 1000 BHA mixed D80WTO20 produced.
- –
Higher BTE (32.92%),
- –
Lesser BSFC (0.247 kg/kW-h),
- –
Lesser NOx (1406 ppm), smoke (51.68%), HC (61 ppm), and CO (0.224%) emissions than diesel and D80WTO20 blends.
- –
- 1000 ppm BHA antioxidant mixed with D80WTO20 blend is a better alternative fuel for CI engines, with lower emissions and improved performance.
Nomenclature
-
- WTO
-
- waste tire oil
-
- TPO
-
- tire pyrolysis oil
-
- BHA
-
- butylated hydroxyanisole
-
- BHT
-
- butylated hydroxytoluene
-
- GO
-
- graphene oxide
-
- GNP
-
- graphene nano platelet
-
- CNT
-
- carbon nano tube
-
- SWCNT
-
- single-walled carbon nanotube
-
- MWCNT
-
- multi-walled carbon nanotube
-
- MnO
-
- manganese oxide
-
- ZnO
-
- zinc oxide
-
- CeO2
-
- cerium oxide
-
- TiO2
-
- titanium oxide
-
- Al2O3
-
- aluminum oxide
-
- AgO2
-
- silver oxide
-
- Ni
-
- nickel
-
- Cu
-
- copper
-
- Zr
-
- zirconium
-
- NP
-
- nano particle
-
- BTE
-
- brake thermal efficiency
-
- BSFC
-
- brake specific fuel consumption
-
- EGT
-
- exhaust gas temperature
-
- EGR
-
- exhaust gas recirculation
-
- P
-
- pressure (bar)
-
- Ø
-
- angle (°)
-
- V
-
- volume
-
- ICP
-
- inner cylinder pressure
-
- HRR
-
- heat release rate
-
- HC
-
- hydrocarbons
-
- CO
-
- carbon monoxide
-
- CO2
-
- carbon dioxide
-
- NOx
-
- nitrous oxide
-
- PM
-
- particulate matter
-
- ppm
-
- parts per million
-
- BP
-
- brake power (kW)
-
- CV
-
- calorific value
-
- dQ
-
- change in heat release
-
- dØ
-
- change in crank angle
-
- dV
-
- change in volume
-
- dP
-
- change in in-cylinder pressure
Author Contributions
R. Saravanan: conceptualization, investigation, funding acquisition, visualization, writing – review and editing, writing – original draft, methodology, software, project administration, data curation, resources. D. Chandrasekar: conceptualization, investigation, writing – original draft, writing – review and editing, formal analysis, data curation. Sivakumar Karthikeyan: conceptualization, writing – review and editing, validation, funding acquisition, software, project administration, supervision. T. Sathish: conceptualization, investigation, writing – original draft, writing – review and editing, methodology, visualization, software, formal analysis, data curation, resources. Jayant Giri: conceptualization, investigation, funding acquisition, visualization, validation, methodology, writing – review and editing, resources, project administration, supervision. J. Isaac Joshua Ramesh Lalvani: writing – original draft, writing – review and editing, conceptualization, methodology, validation, data curation, funding acquisition. Bashar Tarawneh: investigation, visualization, writing – review and editing, formal analysis, resources. A. Anderson: writing – review and editing, funding acquisition, investigation, validation, software, project administration, supervision.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that has been used are confidential.




