LPG Dispersion in Confined Spaces: A Comprehensive Review
Funding: This research was supported by the Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Ministry of Trade, Industry and Energy (MOTIE) (RS-2023-00285272, RS-2024-00420993, and RS-2024-00434535).
ABSTRACT
The growing environmental concerns and increasingly stringent air pollution regulations for maritime emissions are driving the search for sustainable marine fuels. Among the various alternatives, LPG has emerged as a promising, environmentally friendly option, offering a viable solution during the transition to zero-emission vessels. However, concerns remain regarding the potential hazards of LPG leaks, especially in confined spaces such as storage rooms, fuel preparation areas, and engine rooms, where they can pose significant risks to seafarers and vessel safety systems. This study provides a comprehensive analysis of LPG leakage and risk management in confined spaces aboard vessels. It reviews recent experimental and theoretical research on LPG leakage behavior in such environments, offering insights into the dispersion process and associated risks. Key factors influencing LPG dispersion, including the initial temperature and pressure of the gas, atmospheric stability, leak characteristics, and ventilation conditions, are discussed in detail. The study emphasizes the importance of proper ventilation specifically in ceiling zones, side spaces, and inactive exhaust chimneys based on the arrangement of LPG equipment, to prevent gas accumulation and reduce the associated risks. Finally, the paper highlights future research directions aimed at mitigating the risks of using LPG as a marine fuel.
Abbreviations
-
- C3H8
-
- propane
-
- C4H10
-
- butane
-
- CFD
-
- computational fluid dynamics
-
- CO
-
- carbon monoxide
-
- GHG
-
- greenhouse gas emissions
-
- H2
-
- hydrogen
-
- HAC
-
- hazardous area classification
-
- HC
-
- hydrocarbon
-
- HFO
-
- heavy fuel oil
-
- IGF Code
-
- The International Code of Safety for Ships using Gases or other Low-flashpoint Fuels
-
- IMO
-
- International Maritime Organization
-
- KG
-
- deflagration index
-
- LNG
-
- liquefied natural gas
-
- LPG
-
- liquefied petroleum gas
-
- NOx
-
- nitrogen oxide
-
- PPO
-
- plasma partial oxidation
-
- RH
-
- relative humidity
-
- SOx
-
- sulfur oxide
-
- VCE
-
- vapor cloud explosion
-
- VOC
-
- volatile organic compound
1 Introduction
The global marine propulsion engine market is projected to generate $12 billion in revenue by 2022 [1], with an annual growth rate of approximately 4.1% from 2016 to 2022 [2]. This growth is driven by continued government investments in the shipbuilding sector and inland waterways during the forecast period [3, 4]. However, the emissions from ships, such as sulfur oxides and nitrogen oxides, significantly harm air quality, posing serious health risks to nearby communities and negatively impacting marine ecosystems [5]. Recent studies have revealed that shipping plays a significant role in human-generated SOx and NOx emissions, accounting for 13% of the world's sulfur oxide emissions and 15% of nitrogen oxide emissions [6]. In response, the International Maritime Organization (IMO) has established a bold goal to eliminate greenhouse gas emissions (GHGs) [7, 8] from global shipping by 2050 [9, 10]. Achieving this objective requires a shift toward zero-carbon propulsion systems, such as hydrogen and ammonia, over the long term. To bring this low-carbon maritime vision to life, reducing the current heavy dependence on fossil fuels, which are the predominant marine energy source, is crucial. Technological innovations, especially in alternative fuel-powered engines [11, 12], are expected to fuel market expansion. Among the various alternative options, LPG is particularly notable for its ability to significantly reduce air pollutants, offering environmental benefits comparable to other alternatives [13, 14]. Moreover, the annual fuel consumption and costs associated with LPG are comparable to those of conventional heavy fuel oil (HFO) [15]. LPG is already a viable marine fuel, with the capability to retrofit diesel engines for its use, positioning it as a key player in the transition to zero-emission shipping [16]. Currently, LPG is the preferred fuel choice for LPG carriers, but its potential goes beyond this niche market [17]. To fully realize its benefits, LPG propulsion must expand its adoption across the broader shipping industry, where it deserves wider recognition and utilization [2].
LPG [18], primarily composed of propane (C3H8) and butane (C4H10), is predominantly sourced from natural gas fields [19], which contribute over 60% [20] of its production. One of LPG's key advantages over other fuels is its ability to be easily liquefied at pressures of 10–20 bar under normal conditions [21], facilitating simple transportation [22]. Additionally, LPG can be stored in its liquid form for long periods without significant degradation [23]. Unlike LNG [24], LPG [25] does not require cryogenic storage, simplifying temperature management. However, LPG has a lower auto-ignition temperature than LNG [26], which demands careful control of surface temperatures on electrical equipment. Despite these benefits, LPG presents certain hazards [27], including its higher gas density, narrower ignition range, and a lower explosion limit of approximately 2% [21]. From an economic standpoint [28], LPG is just as attractive as LNG [29], offering shorter payback periods, lower upfront investment costs, and greater stability against fuel price fluctuations. Unlike traditional marine fuels such as HFO, LPG does not cause marine pollution in the event of leaks [30]. It also provides emissions reductions similar to LNG, without restrictions on vessel size. Increasingly, maritime industry research supports using LPG in smaller vessels [31], like narrowboats, speedboats, and fishing boats, as a cleaner alternative to gasoline and diesel [32]. These smaller vessels often operate in rivers, lakes, and inland waterways, where pollution poses significant risks to wildlife, aquatic life, and the surrounding ecosystem. Furthermore, the materials used in LPG storage systems are typically compatible with ammonia [12], facilitating a smoother transition to ammonia-fueled [33] vessels in the future [34]. LPG emerges as a promising solution for sustainable transportation [15], providing a clean energy option [34] for today and an even cleaner alternative for the future with the advancement of near-zero-emission renewable LPG [35]. Its compatibility with existing fuel systems makes it a practical and future-ready choice for shipowners, regardless of vessel size or purpose.
However, the LPG-fueled vessels face significant risks [36] due to their susceptibility to a range of factors, including environmental conditions, storage practices, and human error [37]. These factors can contribute to equipment malfunctions [38], fuel leaks [39], and potentially catastrophic explosions [39, 40]. Therefore, a comprehensive understanding of LPG storage and handling is critical for effectively investigating the impact of LPG leakage and preparing for disaster scenarios [41].
Research into LPG dispersion in confined spaces involves a multifaceted approach, combining experimental investigations with advanced computational techniques such as computational fluid dynamics (CFD) simulations [24, 42]. These studies aim to replicate and analyze various scenarios of LPG leakage to assess their potential impact [43] on both safety and operational efficiency [44]. By using mathematical models and simulation software [45], researchers can predict the behavior of LPG in different environmental and operational conditions, allowing for the development of more effective risk management strategies [46]. In the process industry, where LPG leakage incidents are a concern, this research is particularly valuable [47]. It helps in understanding the dynamics of LPG dispersion in enclosed areas, such as engine rooms or cargo holds, where leaks can have severe consequences [48]. By integrating experimental data with theoretical models, researchers can provide a detailed analysis of LPG dispersion patterns, identify critical risk factors, and propose mitigation measures to enhance safety and prevent accidents [49]. Demichela et al. [50] presented an analysis of an industrial LPG storage accident involving the release of propane from a tanker, which ultimately led to the collapse of a storage tank. A model from consequence event analysis is introduced that accounts for both the released gas flows and their contribution to radiant thermal energy in the presence of flames. The progression of the accident is further evaluated through the digitization and analysis of footage capturing the fireball, which resulted from the ignition of approximately 500 L of LPG, with an estimated fireball diameter of 50 m.
Schoor et al. [51] conducted a risk analysis for a 30 × 30 m enclosed car park where LPG vehicles are permitted to park. The analysis employed an event tree approach to identify and evaluate 26 different incident scenarios along with their probabilities. Simulation results indicated that a release from a 70-L LPG fuel tank could produce vapor clouds up to 200 cubic meters in volume, potentially filling the entire height of the car park. The explosion simulations demonstrated that these vapor clouds could create overpressures exceeding 30 kPa throughout the car park. Ventilation simulations revealed that high airflow rates of approximately 0.060 cubic meters per second per square meter of car park floor area are necessary to effectively dilute these substantial vapor clouds. Yang et al. [52] presented a plasma partial oxidation (PPO) method for mitigating hazardous gas leaks in confined spaces. The experiments were conducted with an initial butane concentration (simulating LPG) of 5%, evaluating the impact of discharge power, gas flow rate, and their combined effects on reaction performance. By establishing a relationship between butane and CO concentrations and energy density (discharge power/gas flow rate), it was found that a 5% initial butane concentration could be reduced to below 1.9% when the energy density reached 2.17 kJ/L. This reduction effectively mitigates the explosion risk associated with leaking butane. Simultaneously, the CO concentration was reduced to 0.8%, indicating a low level of CO toxicity. Jia et al. [53] demonstrated the interconnected relationship between accident causes that involve multiple organizations and factors of LPG tanker in the petrochemical industry. The results indicated that five key areas, including maintenance and design, loading and unloading valves, emergency training, management of subsidiaries and contractors, multilevel supervision, and task organization, must be thoroughly reviewed and assessed from top to bottom by the relevant organizations.
In maritime applications, LPG is commonly used in the engine room, where the fuel supply system is typically housed within confined spaces. While emergency responders must be prepared to handle incidents as they occur, maritime officers are responsible for proactively managing the risks associated with potential accidents before they happen. This can be achieved through various strategies, such as implementing mitigation measures, reducing fuel inventory, or altering the transportation patterns of hazardous materials. However, there is a notable lack of research on comprehensive methods for analyzing and responding to LPG leaks in confined spaces aboard vessels.
This study aims to provide a thorough and holistic overview of LPG leakage analysis and risk management in confined maritime environments. It reviews and evaluates the leakage process, influence factors affecting the dispersion of LPG, and other factors related to LPG safety. Additionally, recent experimental and theoretical studies on LPG leakage are discussed in detail, offering a comprehensive perspective. The study also examines trends in LPG dispersion behavior within enclosed spaces, contributing to a deeper understanding of the risks and providing guidance on effective safety measures.
Overall, this research aims to improve safety protocols and disaster response plans by offering a deeper insight into LPG leakage dynamics. It also supports the development of advanced safety measures and guidelines to manage the risks associated with LPG storage and handling in confined maritime environments.
2 LPG as Marine Fuel
LPG, primarily composed of propane and butane, is a widely adopted alternative fuel [54] for ships. These components are by-products of oil and natural gas production and refining [55]. LPG's lower hydrocarbon content leads to reduced CO2 emissions [56], with potential carbon reduction rates of up to 17% over its entire life cycle, making it a promising option for achieving low-carbon shipping [57]. The fuel can be readily liquefied at pressures between 10 and 20 bar and remains stable in its liquid state for extended periods [58], which simplifies transportation. However, LPG is prone to leakage [59] and is highly corrosive, requiring careful handling [60]. It must be stored in well-ventilated areas and managed with suitable materials to ensure safe storage and refueling [61].
2.1 LPG Properties
LPG is primarily composed of light hydrocarbons, specifically propane and butane [62]. Although it exists as a gas under normal atmospheric conditions, it can easily be converted into a liquid at ambient temperatures with the application of moderate pressure [22]. It is largely derived from the removal of heavier hydrocarbons [63] from natural gas and through petroleum refining processes [64]. LPG can be distributed using similar infrastructure as LNG, such as pipelines, pumps, and storage tanks, making it a viable alternative to LNG [23]. However, it is more commonly used for heating rather than propulsion purposes. For a comprehensive summary of LPG properties, see Table 1 [28, 65-69].
| Properties | LNG | LPG | Unit | |
|---|---|---|---|---|
| Propane | Butane | |||
| Chemical formula | CH4 | C3H8 | C4H10 | |
| Specific gravity | 0.55 | 1.55 | 2.07 | At 15°C and 1 atm |
| Octane number [70] | 120 | 105 | 92 | |
| Cetane number | — | −2 | −2 | |
| Density | 420 | 503 | 500 | kg/m3 |
| Critical temperature | −82.95 | 96 | 152 | |
| Auto ignition temperature | 595 | 459 | 405 | |
| Lower flammable limits | 5 | 2.1 | 1.86 | Volume in air (%) |
| Boiling point at 1 bar absolute | −161 | −42 | −1 | |
| Cp/Cv (static pressure/static heat ratio k) | 1.31 | 1.13 | 1.096 | |
| Lower heating value [71] | 45 | 46.34 | 45.55 | MJ/kg |
| Vapor pressure at 45°C | — | 15 | 4 | Bar |
| Upper flammable limits | 15 | 10.1 | 8.41 | Volume in air (%) |
| Critical pressure | 45.99 | 42.6 | 38 | Bar |
LPG boasts a higher energy density and a smaller environmental footprint compared to other petroleum-based fuels. With a calorific value between 46 and 50.3 MJ/kg, it exceeds the energy content of most gaseous fuels. LPG's high energy density and rapid combustion characteristics make it particularly suitable for use in marine diesel engines, especially in dual-fuel configurations. Its high hydrogen-to-carbon ratio promotes cleaner combustion, leading to reduced particulate matter and lower CO2 emissions compared to conventional marine diesel. Additionally, the high-octane rating of LPG enhances knocking resistance, allowing for optimized ignition timing and improved thermal efficiency [72]. When utilized in dual-fuel marine engines operating on the Trinkler cycle, LPG effectively enhances combustion stability and increases overall engine efficiency, contributing to more sustainable and efficient maritime operations. Moreover, its extensive global storage infrastructure makes it a more accessible alternative to LNG. In dual-fuel engines, increasing the LPG ratio in the fuel mix has been shown to enhance torque output [57].
LPG is a non-toxic fuel that plays a crucial role in enhancing air quality [57], as it does not produce particulate matter (PM) emissions. Its lower carbon-to-hydrogen ratio helps reduce CO2 emissions, though methane leaks can still contribute to GHGs [73]. Compared to HFO, LPG generates 17% fewer GHGs throughout its life cycle. It also completely eliminates sulfur oxide (SOx) emissions and cuts nitrogen oxide (NOx) emissions by 10%–20%, depending on the equipment and combustion conditions. Volatile organic compound (VOC) emissions are also significantly lower with LPG. Furthermore, LPG [57] can remove PM emissions entirely and reduce CO2 emissions by 10%–14%. When used in gasoline and dual-fuel diesel engines, it also helps lower carbon monoxide (CO) and hydrocarbon (HC) emissions [74].
LPG faces considerable challenges, including strong competition from other alternative fuels, slow technological advancements, market constraints, and a limited supply infrastructure [75-78]. One critical issue is that LPG [79], being denser than air, can be difficult to detect in case of leaks, and its low flash point poses significant safety risks. As a result, storage and distribution systems must be well-ventilated to prevent accidents. The operational consideration when utilizing LPG as a marine fuel is the mandatory degassing and inertization of cargo spaces to ensure safety and prevent flammable gas accumulation. These procedures are essential for compliance with safety regulations but can lead to increased cargo operation times and higher energy consumption. Specifically, the need for inert gas production and extended ventilation periods contributes to additional fuel usage and operational complexity. To mitigate these impacts, advanced ventilation systems and optimized inertization strategies can be employed. Despite these challenges, the environmental and efficiency benefits of LPG can outweigh the operational costs when managed effectively. Proper crew training is also essential for the safe handling of LPG, as inhaling high concentrations can lead to suffocation. Other key obstacles include a lack of operational experience and an underdeveloped supply network.
Although LPG is one of the most widely accepted alternative fuels worldwide, its adoption in the maritime sector remains limited. It is compatible with all types of vessels, and, unlike natural gas, it benefits from an extensive and well-established global infrastructure [80]. However, challenges persist due to restricted production capacity and supply facilities, meaning ships powered by LPG need to refuel, or bunker, every few weeks. While LPG can be used effectively by ships in both domestic and international operations, it is currently a lower priority compared to other alternative fuels in the maritime industry.
2.2 LPG Storage and Supply System
In marine transport, LPG is typically carried by specialized vessels designed for pressurized, semi-pressurized, or refrigerated conditions [81]. Pressurized ships, operating at 18-bar pressure with ambient temperatures, transport volumes between 3000 and 10,000 m3 [82]. Semi-pressurized vessels, working at 5–8 bar and temperatures between −10°C and −20°C, can handle 10,000–30,000 m3 [67]. For larger volumes, refrigerated transport is used, keeping ambient pressure while cooling the LPG to as low as −43°C for pure propane [83]. These fully refrigerated ships usually carry between 35,000 and 100,000 m3. The global LPG shipping market is increasingly shifting toward large-scale refrigerated transport [84]. Offshore terminals support all three LPG transfer methods, with the entire loading and unloading process, including mooring and departure, typically completed within a day [85].
Dual-fuel engines can combust alternative fuels like natural gas and other accessible options without requiring modifications or retrofitting. These engines offer several potential benefits, including fuel flexibility, increased compression ratios, reduced emissions, and enhanced efficiency. While these engines require a small amount of diesel fuel for operation even in gas mode, it can also run entirely on liquid fuels like diesel or HFO, highlighting their dual-fuel capability. In this setup, LPG is injected at high pressure into the cylinder after the ignition of the diesel pilot fuel, a process known as the “Diesel DF principle” or “GD principle.” These engines can also operate solely on liquid fuels (diesel or HFO), preserving their versatility as dual-fuel systems.
LPG storage can be seamlessly integrated into a ship, along with the necessary bunkering systems and pipelines, allowing for a continuous fuel supply to generators while docked. Onboard LPG storage is typically handled in one of two ways: using LPG cylinder containers or an LPG storage tank.
Figure 1 illustrates the fundamental process of the LPG supply system onboard a vessel. During this process, several confined spaces pose a potential risk of LPG leakage. This study focuses on three critical confined spaces: the fuel storage area (Target 1), the fuel preparation room (Target 2), and the engine room (Target 3).
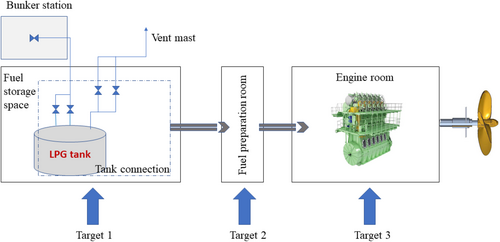
For vessels powered by LPG, the IGF Code [86] establishes safety standards for using LPG as a gas or low-flashpoint fuel. These regulations provide key guidelines for the arrangement and installation of propulsion and auxiliary systems to minimize risks to the ship, crew, and environment, considering the specific properties of LPG. While these standards are mandatory for all ships using gaseous or low-flashpoint fuels, the detailed provisions currently focus primarily on LNG. As a result, the IMO is working on developing international regulations for other gaseous and low-flashpoint fuels, including LPG [27, 87].
2.3 LPG Leakage Characteristics
The hazards associated with LPG are diverse and can pose significant risks in case of leakage [88]. Potential dangers include cryogenic burns, pool fires, deflagrations, detonations, and vapor cloud fires. Due to its extremely low temperature, LPG can cause material embrittlement upon direct contact. When LPG leaks onto a surface, it rapidly boils due to the substantial temperature difference, as its boiling point is −42°C. As LPG vapor disperses into the atmosphere, it creates thermal hazards from various combustion scenarios, such as vapor cloud fires or explosions. Therefore, thorough safety assessments and hazard analyses are crucial in managing LPG leakage risks. Understanding the entire leakage process is a key step in conducting these evaluations.
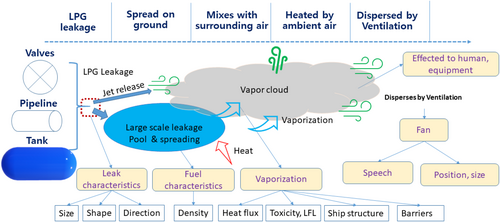
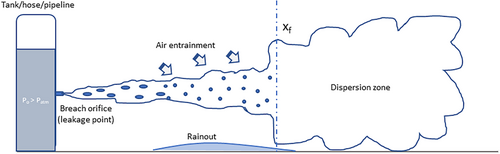
The release and spread of LPG follow the behavior typical of heavy gas dispersion [89]. The leakage process can be divided into five key stages. Initially, LPG escapes from storage tanks, pipes, hoses, or similar systems, coming into contact with the surrounding air. Due to its low temperature and higher density than air, LPG forms a cold pool on the ground or water surface. In the second stage, this cold pool begins to disperse over a wider area. Next, because the ambient temperature is usually higher than LPG's boiling point, the liquid begins to evaporate, creating a large vapor cloud with a lower temperature. Finally, the vapor cloud spreads further, driven by wind forces.
Figure 2 illustrates the various stages involved in LPG leakage and dispersion. It is important to note that the condition of the released LPG, the amount leaked, and the extent of its spread differ at each phase of the process. Additionally, since LPG requires time for release, heat absorption, evaporation, and eventual dispersion, the length of the leakage eventis affected.
2.4 Risk Assessment of LPG Leakage
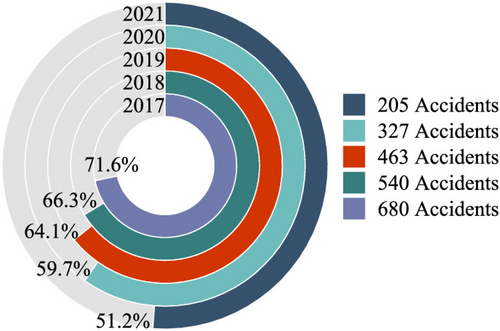
Between 2017 and 2021, gas explosion incidents occurred frequently, with over half resulting from indoor explosions, primarily linked to gas-burning appliances such as closed areas (Figure 3). These indoor explosions are the leading causes of injuries and fatalities. Accident investigations consistently point to gas microleakage, often caused by aging or corroded gas pipelines, as a major contributing factor. This microleakage leads to the uncontrolled diffusion and accumulation of gas within household appliances, ultimately increasing the risk of explosion.
The diffusion and accumulation of leaking combustible gases in confined spaces are the primary causes of indoor combustion and explosion accidents. To better understand these risks, researchers have conducted extensive studies analyzing the behavior of leaking gases in enclosed environments. While some researchers have utilized theoretical models to explore how gases diffuse and accumulate in confined spaces, these models often fall short in capturing the short-term fluctuations in gas concentrations near the leak source.
In risk assessment for LPG leakage, two primary methods are used to define decision making: the deterministic approach and the risk-based approach (as in Figure 4). The choice between these depends on the specific bunkering scenario. Typically, for standard operations on board, such as fuel preparation room, leakage in the engine room, and small spaces of confined space, a qualitative (deterministic) method is used. For more complex scenarios, a quantitative (risk-based) method is preferred. The scenario is modeled under worst-case or specific leak conditions, and the safety distance around the facility is estimated based on the flammable limit and dispersion behavior of LPG.
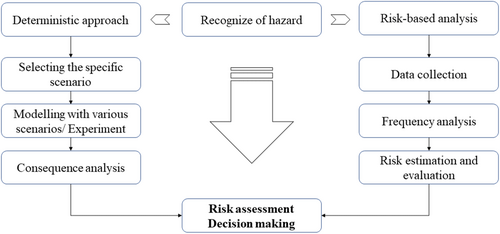
The traditional approach to risk assessment of LPG leakage involves several key steps: collecting relevant data, conducting scenario analyses, assessing event frequency, evaluating potential outcomes, and performing a comprehensive risk assessment. For the LPG bunkering process, a thorough risk assessment includes identifying hazards, analyzing consequences, evaluating risks, pinpointing control measures, implementing those controls, and ensuring ongoing monitoring and review. Taking these factors into account is crucial for maintaining safety during bunkering operations. This systematic process helps to mitigate accident risks and ensures the safe execution of LPG transfers.
3 Risk Assessment Method of LPG Leakage in Confined Space
When LPG, whether cooled through refrigeration or liquefied under high pressure, leaks into the atmosphere, it begins to disperse, forming a gas cloud (see Figure 2). The gas concentration at the release point defines the initial concentration of the cloud. The movement of this cloud in an enclosed space follows a predictable pattern influenced by ventilation (position, fan speed) and the gas's density. Lighter gases, which are less dense than air, rise, while colder, denser gases sink. Over time and distance, the uniformity of the cloud changes regardless of its density or buoyancy, meaning it does not remain consistent. Heavier gases can disperse both as a gas cloud and as liquid puddles on the surface.
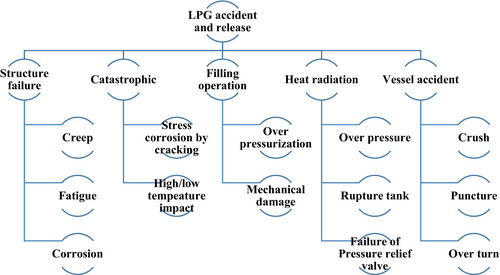
A significant number of dispersion and explosion incidents involving LPG as marine fuel have been reported recently during its storage, transport, and usage in the engine room [91]. The failure of LPG containers typically occurs due to mechanical damage, overfilling of storage tanks, which can lead to cracks or weld failures, or mechanical impacts [92]. Loading and unloading operations are particularly hazardous when handling LPG. Most accidents involving LPG occur at storage depots, processing plants, during transportation in pipelines, and in the engine room (as shown in Figure 5).
Zhao et al. [93] studied the impact of hydrogen (H2) volume fraction on the explosive behavior of LPG, focusing on the combustion dynamics of the gas mixture. The analysis examined how varying levels of hydrogen influence the explosion characteristics and underlying combustion mechanisms of the LPG-H2 blend.
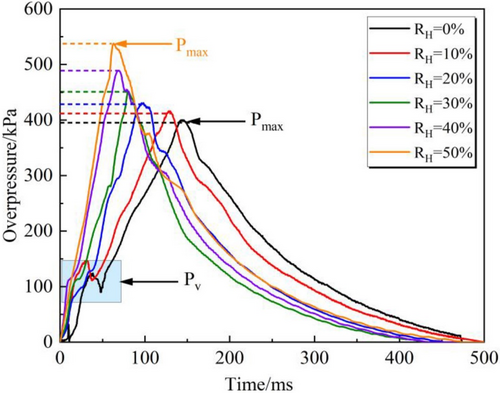
The results show that the flame development of LPG/H2 mixtures under varying relative humidity (RH) conditions follows four distinct growth patterns. As RH increases, the maximum pressure (Pmax) rises, while the explosion relief pressure decreases.
The equivalence ratio has a significant impact on the Thermo-Diffusive and Darrieus-Landau instabilities in the LPG/H2 mixture (as Figure 6). The initial pressure peak in the explosion pressure curve of the LPG/H2 mixture is identified as the peak value (Pv). As the hydrogen volume fraction increases, the Pv gradually decreases. When the hydrogen content is 0% or 10%, the explosion pressure curve exhibits relatively higher peak values. However, as the hydrogen concentration reaches approximately 20%–40%, a significant drop in blowout pressure is observed. Beyond a hydrogen ratio of 50%, the pressure peak in the explosion pressure curve becomes nearly undetectable. Under the same conditions, noticeable differences are observed in both flame duration and explosion pressure duration. The primary mechanism through which hydrogen affects the LPG/H2 reaction is a chain reaction (H2 + O → OH + H and H2 + OH → H2 + OH), producing critical radicals like H and OH that enhance reaction activity (as in Figure 7).
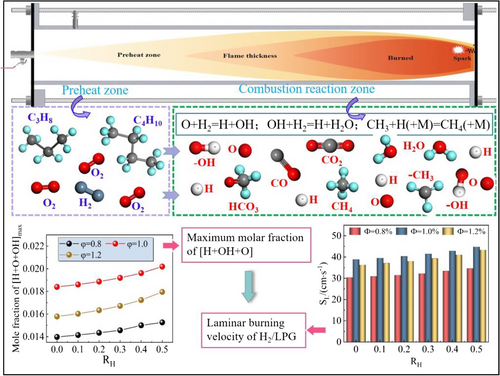
The production rate of OH radicals during the combustion of the LPG/H2 mixture mirrors the trend observed in the peak molar fraction of [H + OH + O]. This is due to the hydrogen-enhanced O + H2 → H + OH chain reaction, which generates a high concentration of reactive radicals, accelerating the laminar flame speed of the LPG/H2 blend. As a result, it becomes evident that the laminar flame speed is highly sensitive to the rate at which OH radicals are produced, highlighting the critical role of these radicals in influencing the combustion dynamics of the mixture. The role of hydrogen in the reaction mechanism is further confirmed by its ability to increase the sensitivity coefficient of the fundamental reactions driving LPG/H2 explosions.
These dispersion characteristics align with the findings of Duong et al. [94], who conducted a detailed study comparing the dispersion patterns of LNG and NH3 during the bunkering process to find our common trend of dispersion for low-flash point fuels, including LPG. Their research highlighted the similarities in the behavior of vapor clouds formed by both fuels, particularly in terms of how they spread under various environmental conditions (Figure 8). The study's comparison of the dispersion profiles revealed that both LNG and NH3 exhibit similar tendencies in vapor cloud formation, movement, and dissipation, influenced by factors such as temperature, wind speed, and atmospheric stability. This correlation supports the understanding that low-flash point fuels, like LPG, LNG, and NH3, follow analogous dispersion trends in real-world bunkering scenarios, making the findings applicable across various operational environments.
Simulating the entire process of LNG/NH3 leakage, evaporation, and gas dispersion in a single model presents significant technical challenges. In this scenario, the use of the “flash utility” offers a practical and efficient alternative. This tool allows for the calculation of key parameters such as the leak area, flow rate, and equivalent concentration (fuel concentration) at point xf, which represents the location where the initial LNG/NH3 leak has completely vaporized. Once this point is reached, simulations can then focus on analyzing the gas dispersion behavior within the gas–air mixture, starting from point xf. This approach may yield similar dispersion trends for low flash point fuels such as LPG and LNG, NH3.
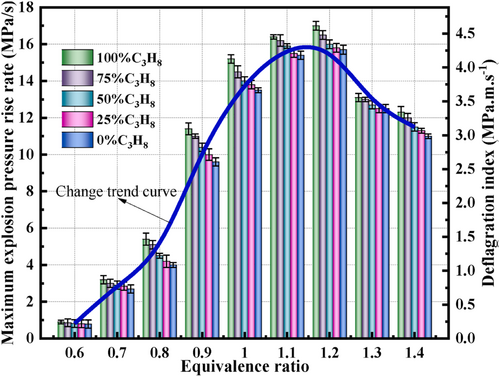
Wei et al. [95] evaluated the hazardous effects of LPG by examining its macro- and micro-level deflagration behavior under different fuel compositions and equivalence ratios. A 20-L spherical experimental setup was used alongside CHEMKIN-Pro kinetic software to analyze the combustion dynamics and assess the associated risks.
The study's results indicate that when the equivalence ratio was 1.2, LPG reached its highest values for maximum explosion pressure (pmax), maximum rate of pressure rise (dp/dt)max, deflagration index (KG), and average flame propagation speed (v) (Figure 9). Additionally, (dp/dt)max, KG, and v increased as the proportion of C3H8 in the LPG mixture rose. In the LPG combustion chain reaction, the most influential steps for enhancing and inhibiting reactions were H + O2 → O + OH and 2CH3(+M) → C2H6(+M), respectively. C2H4 accumulated gradually during the chain initiation phase but was rapidly consumed as the temperature spiked. To effectively suppress LPG explosions, the study recommends developing inhibitors that neutralize reactive radicals like H*, O*, and OH*, as well as inhibitors specifically targeting reactions involving C2H4. Moreover, increasing the proportion of C3H8 in LPG can reduce the emission of harmful gases such as CO and CO2. The composition of LPG significantly influences the combustion byproducts. Increasing the proportion of C3H8 in the LPG mixture can help reduce the release of toxic pollutants like CO and lower the emission of greenhouse gases such as CO2.
Jin et al. [90] studied integrated stoves to investigate how microleakage gas behaves in domestic appliances. It examined how varying leakage rates and locations influence the diffusion and accumulation patterns of microleakage LPG inside the stove. The analysis was conducted using CFD simulations to model these characteristics (Figure 10).
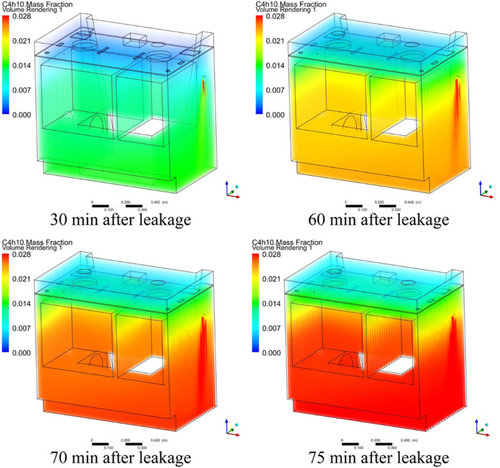
The findings reveal that microleakage LPG tends to diffuse downward due to gravity, and its concentration within the stove cavity increases as leakage time progresses, though the distribution remains highly uneven.
The volume of the hazardous zone expands rapidly with the ongoing accumulation of microleakage LPG, eventually occupying more than 50% of the cavity. While the diffusion and accumulation patterns are similar across various leakage rates, the location of the leak plays a significant role in these characteristics. Diffusion and accumulation patterns vary considerably depending on where the leak originates. For instance, when the leak occurs above the stove baffle, there is a concentration difference of 0.1%–0.2% between the top and bottom. However, when the leak occurs below the baffle, this difference increases to 1.0%–1.3%. Additionally, the location of the leak has a marked effect on how quickly LPG concentrations reach critical levels, underscoring the relationship between leak location, combustion, and explosion risks.
In contrast to LNG [96, 97], which is lighter than air, the dispersion behavior of LPG exhibits distinct characteristics due to its density and molecular properties [98, 99]. LPG, primarily composed of propane and butane, is heavier than air at ambient temperatures, leading to a unique dispersion pattern upon leakage.
Initially, LPG tends to disperse downward due to its higher molecular weight, resulting in the gas sinking toward ground level when released into the atmosphere [100]. This downward movement is driven by the greater density of LPG compared to air, which creates a gravity-driven flow of the gas, particularly in confined or low-lying areas. This phase of dispersion increases the potential hazard [101], as LPG can accumulate in bilge spaces created by equipment or other low-lying areas of the engine room, fuel preparation room, or fuel storage areas.
- As LPG absorbs heat from its environment, its temperature rises, causing a reduction in density. Once it approaches a critical threshold where the gas density becomes comparable to or less than that of air, buoyant forces begin to dominate, leading to upward movement.
- Atmospheric turbulence and wind contribute to the dilution of LPG, further assisting in its eventual upward dispersion. The gas spreads out vertically and horizontally, reducing its concentration as it disperses into thes atmosphere.
4 Effect of Initial Pressure and Temperature on the Dispersion Law of LPG
It has been determined that the dispersion of a heavy gas cloud is influenced by various factors [104], including the properties of the gas itself and the surrounding atmospheric conditions [105]. Comparisons between experimental observations and numerical simulations in this study indicate that heavy gas cloud dispersion is particularly affected by the following gas parameters [106]: (i) Dispersion velocity, (ii) Cloud density.
When LPG leaks [107], it is quickly released into the lower-pressure environment [108], causing the liquid to vaporize almost immediately [109]. This rapid vaporization creates a vortex at the edges of the gas cloud. The vortex ring captures a significant portion of the leaked gas due to the suction effect generated by the vortex. Along with the dense gas, some air is also drawn into the vortex [110]. In the intermediate phase, the vortex formed by the heavy gas cloud begins to disperse. As the discharge velocity decreases, the effects of temperature and density become more significant [111]. Due to its low temperature and high density, the heavy gas tends to settle and flow toward lower areas, behaving similarly to a liquid. Surface friction and heat transfer with the environment induce turbulence in the gas, which enhances the diffusion process. For heavy gases, horizontal dispersion extends much farther than vertical dispersion, primarily due to the gas's higher density. In the final stage, the heavy gas cloud reaches maturity. At this point, areas with high turbulence contain the highest concentrations of the gas. As the cloud continues to disperse and more air mixes with the heavy gas, the densities of the gas and the surrounding atmosphere gradually equalize.
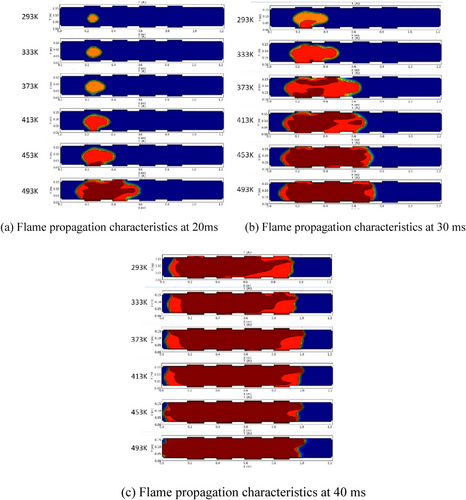
Liang et al. [112] presented a mathematical model of an LPG explosion within a confined space, examining key parameters such as explosion overpressure, induction time, maximum pressure rise rate, and flame propagation in an LPG-air mixture at different initial temperatures and pressures. Additionally, the gray correlation analysis method is applied to thoroughly assess the factors influencing these explosion characteristics. The results show that at room temperature, both the maximum explosion pressure and the rate at which it rises increase with higher LPG volume fractions but begin to decrease after reaching a peak value.
The Figure 11 illustrates that as the initial temperature rises, the reaction accelerates, causing both an increase in flame propagation distance and speed. Comparative analysis reveals that higher temperatures lead to a faster combustion rate of LPG and significantly expand the area of flame propagation. Additionally, the diagram shows that when the initial temperature increases by 40 K, the temperature in the burning zone rises by more than 40 K, indicating that the initial temperature's effect on explosion temperature is not merely additive. The overall impact on the reaction rate and end-flow dynamics plays a key role in the complex behavior of the explosion process. Furthermore, the diagram highlights the significant influence of temperature on the flame's front structure transformation. The propagation of premixed flames in pipelines has attracted much attention from researchers. As the flame travels down the pipe, its shape shifts from convex to concave, creating the characteristic “tulip” flame. The development and spread of this tulip flame can be divided into two stages: the initial outward expansion of the flame, followed by the evolution of its front into a finger-like shape as the flame structure progresses.
The velocity of heavy gas plays a crucial role in determining the area that a gas cloud will cover when released into the environment, both horizontally and vertically. Higher velocities lead to a larger dispersion area of the gas cloud, a relationship confirmed by current CFD simulation results that demonstrate direct proportionality. Additionally, the density of the heavy gas cloud is influenced by the gas's molecular mass. The lighter the gas, the greater its dispersion, as lighter gases are more easily carried by the wind. Comparisons between experimental observations and numerical simulations indicate consistent results regarding the gas's density.
5 Effect of Atmospheric Stability Class on the LPG Dispersion
The uniform dispersion of a gas cloud can be understood through the principles of gas buoyancy and the relative densities of both the gas and the surrounding atmosphere. Gas buoyancy is influenced by factors such as concentration, volume, and temperature. As the gas occupies a larger volume, its buoyant force increases, causing it to rise [113]. Upon release, the gas spreads horizontally in the x-direction while its upward movement in the z-direction is driven by buoyancy. This interaction leads [114] to the formation of a distinct cloud shape, which is largely determined by the gas's density and temperature.
- In the lower part of the tank/ pipelines (around 0.3 m from the ground), where the liquid phase of LPG is located, a leak of the liquid phase occurs.
- In the upper part of the tank/ pipelines (from 0.7 m from the ground), where the gas phase is present, a leak of gaseous LPG occurs.
In the second scenario, the leak originates from the part of the tank containing liquid LPG. This results in the formation of a boiling pool of propane-butane on the ground (boiling point −41.2°C). The vapors generated by the boiling liquid mix with the air, forming a combustible, explosive atmosphere.
Salamonowicz et al. [117] simulated LPG leakage and dispersion with 6 different wind speed includes 1, 3, 5, 7, 3 and 2 m/s, in equivalent with 06 stability classes of A, B, C, D, E, and F. The example of simulation results with stability class A is presented in Figure 12.
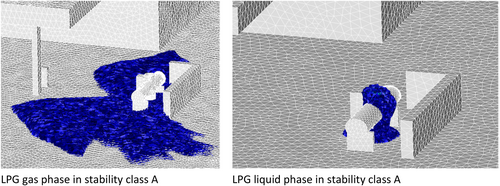
All analyzed cases showed that the explosive zone was situated several dozen centimeters above the ground. The extent of the explosion hazard zone is highly influenced by weather conditions, particularly wind speed. The results indicate that lower wind speeds correspond to a larger explosion hazard zone. This effect is most pronounced under low wind speed conditions (classified as wind speed classes A, E, and F).
It is also recommended that LPG tanks be located in open areas to allow for the proper dispersion of released gases. Additionally, the location of the leak plays a significant role in determining the size of the danger zone. When the leak occurs below the liquid surface, LPG is released in its liquid form, whereas leaks above the liquid surface result in gas-phase LPG release. The most extensive hazard zone was observed in cases of liquid-phase leakage.
The observations indicate that neutral gas rapidly ascends in the vertical direction upon release from its source. In this case, the temperature difference between the neutral gas and the surrounding atmosphere is negligible, leading to no density difference between the two. As a result, buoyancy is effectively zero, resulting in reduced dispersion of the neutral gases, as demonstrated by the simulation.
Ambient atmospheric conditions, such as temperature, ventilation fan, and pressure, directly impact the dispersion of heavy gas. Among these factors, ventilation plays the most significant role in influencing the temperature, concentration, and overall dispersion of the gas.
6 Effect of Leak Characteristics on LPG Dispersion
In the context of LPG release and dispersion, the leakage point refers to the location where LPG escapes into the environment. The leak flow rate and direction significantly influence how the vapor cloud forms and disperses. This leakage point acts as the initial reference for dispersion simulations, playing a crucial role in determining the spread and concentration of the gas cloud [118]. It has been observed that neutral gases tend to rise rapidly in the vertical direction when released from their source. Unlike other gases, the cloud formed by neutral gas typically remains elevated above ground level. The dispersion of neutral gas is most pronounced in the upward (z) direction, primarily due to its low density, which results in minimal buoyancy.
The density of a heavy gas cloud is influenced by the gas's molecular mass. A lower molecular mass results in greater dispersion of the gas cloud, as lighter gases are more easily carried by the wind. Comparisons between experimental observations and current numerical simulations show consistent results regarding density. Kim and Lee [119] identified the differences between actual experimental results, CFD simulations using FLACS, and calculations based on the KGS CODE GC101, which is the classification code for explosive hazardous areas in gas facilities. To facilitate convenience and wider application, the research developed the KGS HAC (hazardous area classification) program, which is based on the KGS GC101 guidelines. The research results revealed that when using calculation methods like CFD and KGS HAC, the dispersion distance increases proportionally with the flow rate, assuming ideal conditions. In real-world gas leaks, the dispersion of gas can vary due to the influence of localized wind conditions. During actual gas leakage scenarios, maintaining a consistent dispersion pattern is challenging due to fluctuating factors such as ambient air temperature, pressure, and wind.
Zhou et al. [120] experimented on LPG released with various influencing factors, including the release velocity of LPG (as in Figure 13). The results show that the gas velocity in module 1 is significantly higher than in the other modules. Specifically, the gas velocity in module 2 is slightly greater than in modules 3 and 4, which exhibit nearly identical velocity measurements.
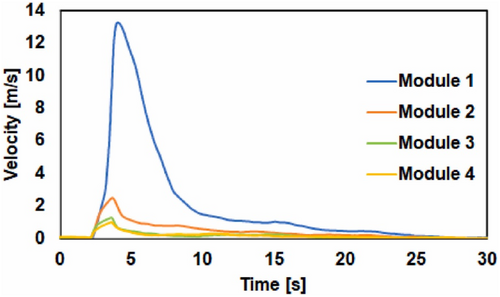
In the experiment with module 1, the gas velocity peaks at 13.32 m/s approximately 2 s after the gate opens. As the rupture size increases from modules 1 to 4, the maximum gas velocity decreases. Notably, the maximum velocity in module 1 is more than six times higher than in module 2 and over 10 times higher than in modules 3 and 4. Additionally, the time taken to reach the peak velocity decreases with increasing rupture size. These velocity measurements indicate that the gas release behavior in module 1 differs markedly from that of the other three modules, consistent with the pressure measurement results.
The experiment demonstrates characteristics of a continuous discharge, featuring high gas velocity and concentration in the flow field, which suggests a low risk of explosion initiation. In contrast, the experiments with modules 3 and 4 exhibit blowdown depressurization properties, where the released gas has a lower velocity and a wider spatial distribution, with gas concentrations within the flammability range of LPG. This indicates a higher potential for a vapor cloud explosion (VCE) under blowdown depressurization conditions in an LPG tank. Thus, rupture size is a key factor in differentiating these two gas release behaviors. The experimental results further suggest that the threshold for distinguishing these outcomes is slightly smaller than, but close to, the rupture size of module 2, which corresponds to 1.5‰ of the tank's surface area (approximately 0.124 m2).
In an actual accident, the rapid vaporization of LPG during depressurization absorbs a significant amount of heat, causing a substantial drop in the temperature of the tank's structural material. This thermal–mechanical interaction during the release process can intensify tank damage, potentially leading to a highly destructive VCE. This phenomenon, referred to as “depressurization-escalated damage,” presents a serious risk to the safe storage of flammable energy sources and requires thorough investigation. Additionally, the embrittlement of pressurized vessel materials due to this effect should be considered when assessing storage safety for substances like LPG. The findings indicate that low-alloy steel may not be the most suitable material for constructing LPG tanks.
The flashing jet exhibits a distinct characteristic during its release: the formation of small droplets suspended within the heavy gas cloud. These droplets can be carried away by rainwater and remain in the atmosphere for extended periods. The diameter of these droplets has been measured through both experimental observations and current numerical simulations, revealing that it depends on factors such as velocity, nozzle diameter, and the density of the heavy gas.
- Nozzle diameter or source size
- Droplet diameter
- Pressure within the cylinder containing the liquefied gas
7 Effect of Ventilations
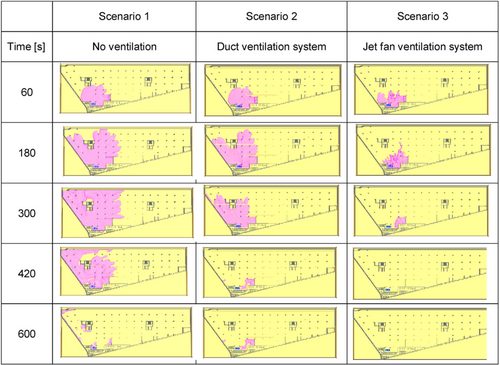
The ventilation system plays a crucial role in removing pollutants from enclosed spaces, such as the LPG fuel preparation room and engine room. Openings in the structure, combined with external atmospheric conditions like wind, greatly affect air quality within the enclosure. Jet fan systems are commonly employed to enhance the ventilation process in closed area from ship. These fans are mounted under the ceiling or wall and generate high-speed airflow, promoting the induction, mixing, and movement of polluted air. Jet fans function as a ductless ventilation system, simultaneously using multiple fans to transport air or smoke from intake points toward exhaust outlets. In normal operation, they run at a lower setting to ventilate daily emissions from vehicles. When a fire occurs or if LPG is detected, the system switches to a higher setting to efficiently remove smoke or hazardous gases. Jet fans are particularly effective when large volumes of air need to be moved at high speed.
Experiments of Brzezinska et al. [121] have demonstrated that in the event of an LPG release, traditional duct-based ventilation systems are significantly less effective compared to jet fan systems. The tests were carried out in an industrial car park measuring 2900 m2 with a ceiling height of 2.7 m, where both types of ventilation systems were installed. In the duct system, 50% of the exhaust air was drawn from near the floor and 50% from near the ceiling. In contrast, the jet fan system maintained the same overall ventilation capacity, but instead of ducts, jet fans were used, with air being exhausted from a single ventilation point. For the experiment, dry ice was used to create CO2 clouds, mimicking the behavior of LPG in terms of gravity and dispersion. The experiment focused on a qualitative comparison of the two systems' effectiveness.
As in Figure 14, the spread of the flammable gas cloud (where the concentration exceeds the lower explosive limit) in the seconds after LPG is released. It is evident that the jet fan ventilation system disperses the gas concentration much more rapidly compared to the duct ventilation system, which proves to be less effective in this scenario. Notably, the jet fan system requires only 5 min to eliminate the risk of fire or explosion, whereas the gas cloud remains significantly large after this time with duct ventilation.
8 Future Prospect
The safety of LPG as marine fuel during the process of installations, storage facilities, and LPG fuel oil supply systems is critical, especially as many LPG-fueled vessels are now operated in densely populated areas. Although these installations are equipped with safety valves, alarms, and robust multilayer protection systems, maintaining constant vigilance is essential. Strict compliance with housekeeping and maintenance protocols, along with conducting regular safety drills, is necessary to reduce the risk of incidents and ensure operational safety. This research provides an in-depth review of key safety considerations and engineering techniques for LPG as marine fuel, as well as their transportation and storage processes.
As discussed, extensive research has been conducted on LPG leakage and dispersion, particularly using CFD simulations to model the evolution of leakage, vapor cloud formation, affected areas, and explosion risk under the combined influence of various factors. For large LPG storage tank leaks, numerical simulation offers high precision and operational flexibility. This approach not only expands on theoretical and experimental findings but also allows for the study of LPG leakage and dispersion under complex conditions. Despite progress in understanding the factors influencing LPG leakage, research on the specific mechanisms behind jet formation, pool expansion, and vapor cloud dispersion following continuous leakage from large storage tanks remains in its early stages. This is due to the large size of LPG storage tanks and the unique wind field dynamics around them, including recirculation, backflow, and stagnant flow. Further investigation is needed to understand the impact of factors like jet length, pool area, and hazard range on the leakage and dispersion process.
- Expand experimental studies on pool spreading and heavy gas dispersion, especially in conditions where substantial variations at the leakage source occur, such as the presence of two-phase jets, flash atomization, or when the source is in motion.
- Adopt a more holistic approach to factors affecting the spread of pool liquids and heavy gas dispersion, considering elements such as air humidity, solar radiation, heat radiation, ventilation speed, ventilation hole position, and surface roughness.
- Examine the impact of obstacles, including those of varying heights, on heavy gas dispersion. Focus on the morphological changes and diffusion dynamics that arise when heavy gases interact with these barriers.
- There is a lack of experimental data on failures involving LPG tankers and LPG pipelines during transportation. Well-designed experiments are essential in these areas to collect relevant data. Existing theoretical models need to be rigorously tested against experimental results and real-world incidents, with carefully conducted case studies to validate these models.
- Significant variability exists in key input parameters, such as release size, weather and humidity conditions, and ignition scenarios. Therefore, models should be refined to improve accuracy and confidence in determining these input parameters.
- While setting up large-scale experiments related to gas ignition, fire, and explosions is expensive, there is no alternative to well-structured experimental work combined with theoretical analysis. Companies with similar interests should collaborate and pool resources to create a state-of-the-art, well-instrumented experimental facility. This facility would serve as a hub for data collection, which can then be used to validate existing models.
- There is a notable absence of experimental data on large-scale LPG dispersion, and current mathematical models lack validation for key factors such as release amounts, gas cloud formation, dispersion patterns, ignition timing, and fire spread, particularly in unconfined spaces. Addressing these gaps is crucial for improving safety.
To enhance the accuracy of predictions, further development of advanced turbulence models, such as large eddy simulations and associated numerical methods, is necessary. This represents a key area for future research.
Another critical consideration regarding the hazards posed by heavy combustible gas clouds involves the chemical reactions that can occur during gas dispersion. Due to time constraints, this aspect was not addressed in the current study, making it a recommended area for further investigation.
9 Conclusions
Field experiments on LPG leakage and dispersion are resource-intensive, requiring substantial manpower, materials, and financial investment. Additionally, the complexity of environmental conditions and challenges in monitoring certain variables makes these studies difficult to execute. While engine room experiments can provide valuable insights, they are constrained by difficulties in simulating low wind speeds and low Reynolds numbers. Consequently, theoretical analysis has become the primary approach for studying LPG leakage and dispersion. With advancements in computational power, the use of CFD simulations has become increasingly common. This paper provides an overview of how different ground conditions influence LPG leakage and dispersion, compares the behavior of leakage over land versus water, and compiles findings from both field and engine room studies. It further evaluates the methods and mathematical models used to study pool spreading and vapor dispersion, aiming to offer practical guidance for preventing accidents.
- Initially, LPG tends to disperse downward due to its higher molecular weight, resulting in the gas sinking toward ground level when released into the atmosphere. This downward movement is driven by the greater density of LPG compared to air, which creates a gravity-driven flow of the gas, particularly in confined or low-lying areas.
- It is crucial to develop a detailed understanding of LPG dispersion within engine rooms, fuel preparation rooms, and other closed spaces of facilities that utilize LPG. This knowledge is vital for ensuring the safety of plant personnel and aiding chemical and environmental rescue teams from the marine engineering department in managing emergencies involving ammonia releases in confined spaces.
- The proper ventilation of ceiling zones and any inactive exhaust chimneys is strongly advised to prevent the accumulation of LPG, which poses significant risks.
- To effectively control the dispersion of LPG released from top and bottom stub pipes, it is important to activate the side ventilation ducts.
- Effected from heat radiation and floor material on dispersion characteristics of LPG are recommended for further experiments and studies.
- Utilizing numerical methods in engineering enables rapid and efficient resolution of ventilation issues, whether in ammonia engine rooms, smoke exhaust systems for buildings, or ventilation systems in residential complexes. This approach offers a cost-effective alternative to conducting physical investigations, which would otherwise incur additional research expenses.
The results of this study offer fresh insights into how adjustments to key operational parameters impact LPG dispersion over the surface of an enclosed area. The findings also highlight the potential reductions achievable by modifying these parameters. This knowledge is valuable for developing effective operational strategies and mitigation measures for LPG as a marine fuel.
Author Contributions
Phan Anh Duong: conceptualization, investigation, funding acquisition, writing – original draft, methodology, validation, data curation, resources, project administration. Jinuk Lee: formal analysis, supervision, visualization. Hokeun Kang: conceptualization, investigation, writing – review and editing, project administration, resources, writing – original draft.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




