Development, implementation, and evaluation of a 3D-printed high-fidelity pediatric mannequin with expected hard-to-intubate airway
Abstract
Clinical simulation is fundamental for the healthcare staff to learn and enhance their procedural skills without causing harm to the patients. Despite its importance, in literature appears a deficiency of pediatric pathological mannequins, especially those simulating difficult airway management due to the obstruction of the passage of tubes, fiberscopes, or catheters. Given the importance of simulating complex scenarios in the medical staff's training, the authors decided to realize a modular high-fidelity pathological mannequin with nasal access using reverse engineering and additive manufacturing techniques within T3Ddy, a joint laboratory between Meyer Children's Hospital of Florence and the Department of Industrial Engineering of the University of Florence. The mannequin is developed from diagnostic images of a significant 30-month-old polymalformative patient also affected by Pierre-Robin syndrome modifying the tracheobronchial tree to reproduce an abnormal status. Rigid parts and silicone cast molds are manufactured using 3D-printed ABS/ASA while platinum-cure-silicones are used for the soft ones. Meyer's anesthesiologists collaborated to the realization providing feedback during design and production. The device is evaluated with a 5-point Likert scale questionnaire and results in a useful tool for the training of procedural skills related to difficult intubation as its realism, anatomical geometry, and tactic feedback are positively evaluated.
1 INTRODUCTION
Clinical simulation is a precious tool for the medical staff to learn new procedures or enhance their knowledge about them without directly involving the patient and therefore compromising its safety. By mimicking a given aspect of clinical care, physical and virtual simulators are used to reproduce different scenarios aiming to teach diagnostic and therapeutic procedures as well as repeat processes and medical concepts.1
During simulations, operators can learn the necessary skills to manage the many variables that typically characterize real clinical cases. This educational technique can accelerate the learning curve in a safe environment, in which trainees can learn from their mistakes without causing any harm.2
Based on their characteristics, simulators are usually classified as HF (high-fidelity) or LF (low-fidelity). The formers are usually characterized by low anatomical accuracy and are easier to use and maintain than the latter. Quite the reverse, HF simulators, that is, the most advanced mannequins, allow the reproduction of complex and extremely realistic scenarios since they present highly detailed anatomical parts.
Generally, educational mannequins and simulators for the training on the management of both adult and pediatric patients are increasingly realistic, but still present limits in terms of anatomical accuracy, appropriate dimensions of airway, and tissue's mechanical properties.3
Technological limits associated with pediatric mannequins typically stem from challenges in accurately replicating numerous pathophysiological occurrences. As observed in the market and emphasized by medical professionals at the Meyer Children's Hospital of Florence (Italy), commercially accessible devices predominantly depict healthy scenarios. Medical conditions complicating simulated scenarios (e.g., obstructing the passage of tubes, fiberscopes, or catheters) are rarely considered.
To corroborate this assertion, bibliographic research was carried out with a focus on pediatric solutions. According to the state-of-the-art, physical mannequins commonly used in clinical practice for educational, training, and preoperative planning purposes can be classified into three categories. Besides commercially available devices, there were found completely custom solutions and what the authors of the present article decided to classify as hybrid ones, in which commercial mannequins have been integrated or modified with custom-made anatomical parts, realized using Additive Manufacturing (AM) techniques and usually derived from real patients' diagnostic images' segmentation.
Regarding pediatric commercially available mannequins, there is a wide age range to choose from, from premature newborns to 6-year-old children. Unfortunately, the same cannot be said regarding the available pathologies affecting the airways and therefore complicating the patient's management for example obstructing the passage of tubes, fiberscopes, and catheters. The availability of pediatric pathological mannequins is severely restricted, even though the scientific community acknowledges the critical necessity of training medical personnel in challenging intubation scenarios.4-15
The only commercially available pediatric mannequin representing a medical condition complicating the intubation procedure is the AirSim Pierre Robin X (Trucorp®),16 representative of a 6-month-old patient affected by Pierre Robin Syndrome which unfortunately has not been successful among the medical community, who evaluated the syndrome sequence as not sufficiently pronounced.7, 8
Recent progress in reverse engineering (RE) and AM techniques allowed the development of new preoperative planning approaches, enabling the reproduction of biological tissues and the direct production of implantable medical devices.17 Therefore, using custom-made mannequins to fulfill specific training needs is now widespread, as well as their use as educational tools or as support to explain to the patient and family members surgical procedures.18
Interesting studies in which custom-made devices have been realized and that corroborate those remarks were those of Podolsky et al., who realized a pediatric high-fidelity cleft palate simulator,19 Park et al. who realized a low-cost mannequin for the training on rigid bronchoscopy using AM techniques20 and Weatherall et al. who realized a healthy 21-month-old mannequin using RE, AM and silicone casting.6
Hybrid solutions reported in the literature are the ones by Al-Ramahi et al. and Kovatch et al. who developed a high-fidelity pediatric tracheal model based on CT scans to be inserted into a mannequin for front-of-neck access training purposes.3, 9
Due to the substantial lack of commercially available pathological simulation devices that emerged from the aforementioned literature analysis and the importance that training tasks on difficult scenarios have in the education of the medical staff,4-15, 21, 22 within the T3Ddy laboratory,23 born from a collaboration between the Department of Industrial Engineering of Florence (DIEF) of the University of Florence (Italy) and the Meyer Children's Hospital, it was decided to develop a pathological mannequin with nasal access to reproduce a scenario in which fiberoptic-guided intubation (FGI) was the only option to manage the expected difficult airway.
FGI is a high-risk procedure and requires specific formation and constant training, especially with pediatric patients, whose airway dimensions are significantly reduced.24 This is also confirmed by the feedback given by a panel of doctors from different regions of Italy, who are not new to the procedure. They reported encountering a higher difficulty when performing the FGI attempt on pediatric patients with airway diseases and agreed on the importance of introducing difficult scenarios in the medical staff's learning and updating programs.
Given the substantial lack of a satisfactory fleet of pathological mannequins, the main aim of the present work is to develop a new advanced version of a modular mannequin featuring difficult airways with nasal access faithfully reproducing the anatomy, except for the tracheobronchial tree, of a 30-month-old polymalformative syndromic patient affected by hypoplasia and mandibular dysmorphism with micrognathia and lingual agenesis, whose facial features derived from the CT scans have been anonymized for privacy reasons. The patient underwent fiberscope-guided nasotracheal intubation soon after the birth followed by tracheostomy and gastrostomy placement. The manikin reproduces the patient's airway prior to mandibular advancement and mouth commissurotomy procedures.
The tracheobronchial tree has been reproduced starting from the CT scan of another patient who was 34 months old and affected by tracheomalacia (TM), a rare disease characterized by soft and flexible trachea, leading to dynamic or static collapse of the tracheal wall.25
The modularity is a key feature of the mannequin's design to allow the predisposition of additional modules reproducing different pathologies to be designed over time according to the medical staff's demand as well as an easy substitution of all those parts that are prone to wear and tear during simulation sessions.
All mannequin's anatomical regions have been accurately 3D modeled based on real patients' CT images through 3D voxel segmentation of regions of interest (ROIs) and by means of RE and computer-aided design (CAD) techniques to ensure component geometrical compatibility. The layout is fully modular, with the possibility of substituting components with ease to compose a new clinical scenario or to replace a worn part.
Finally, AM techniques were used to manufacture the parts. Rigid ones were directly 3D-printed in acrylonitrile butadiene styrene (ABS) or acrylonitrile styrene acrylate (ASA), because of easy availability, low cost, and adequate mechanical properties, while soft ones were realized by casting platinum silicones of various Shore hardness into 3D-printed molds. The choice of materials also took into account the excellent stability of ABS/ASA and silicone over time. Moreover, these materials are compatible with steel (used for threaded inserts and screws) and, when not subjected to severe environmental conditions (high temperatures and aggressive chemicals) or prolonged exposure to the sun, are stable over time.
This project pioneers a cutting-edge pediatric mannequin, addressing the critical gap in pathological simulation devices by incorporating high-fidelity anatomical accuracy and modular design. Utilizing advanced reverse engineering and additive manufacturing techniques, this innovative solution replicates complex pediatric airway scenarios, enhancing the training of medical professionals. The fully customizable and realistic mannequin significantly elevates clinical training, enabling precise simulation of difficult airways to ensure safer and more effective medical procedures.
2 MATERIALS AND METHODS
2.1 Mannequin's layout
The structure of the mannequin is designed to allow the combination of multiple components made of different materials while ensuring ease of component assembly and interchangeability. Referring to Figure 1, the upper airway component is designed to be the core of the mannequin around which to place the cranial shells securing them by threaded connections. The epiglottis, vocal cords, and tracheobronchial tree are meant to be separate components to ensure easy replacement when necessary. The lower airway components, corresponding to epiglottis, vocal cords, and tracheobronchial tree are connected to the head via the lower airway joint.
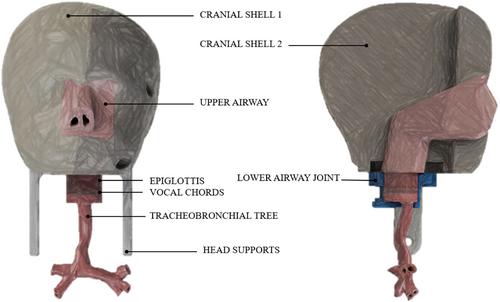
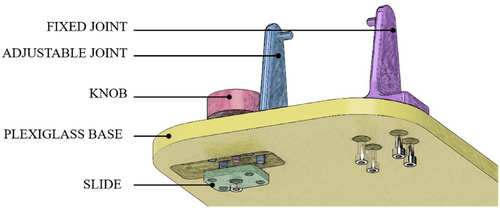
To ensure stability during simulation sessions, the head of the mannequin is ensured to a plexiglass base by means of a support system composed of two joints, one of which is fixed and the other can be moved in a transverse direction by means of a slide and secured in position using a knob. A sketch of the support system is shown in Figure 2.
2.2 Modeling and material definition
CT and MR images of a 30-month-old patient affected by a polymalformative syndrome were obtained thanks to the collaboration of Meyer's Children's Hospital medical staff. The syndrome is a polymalformative congenital condition characterized by severe mandibular hypoplasia, lingual agenesis, and micrognathia. Children affected by this anatomical condition develop an acute respiratory failure requiring prompt intubation or an emergency tracheostomy at birth.
Depending on the nature of the part, namely mechanic or anatomical, the workflow to obtain it has followed different paths.
Purely mechanical parts (lower airway joint, head supports, and base support system) have been entirely developed in a 3D CAD and Computer Aided Engineering (CAE) environment, using SolidWorks (Dassault Systèmes, France).
Anatomical-based components have been modeled following the widely known procedure of geometrical reconstruction based on real patients' CT and MR images (see Figure 3).
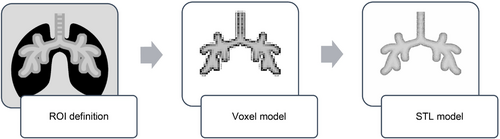
The first step aims to define the region of interest (ROI) of the target anatomical region directly on the patient's CT and MR images by using common selection tools based on the Hounsfield Unit (HU). The result of this operation consists of a draft voxel reconstruction that is further processed through the Marching Cubes algorithm26 to obtain a proper polygonal geometry that can be exported using the Standard Triangulation Language (STL) format. All these operations have been carried out using Mimics Medical 21.0 software (Materialize, Belgium) and repeated to reconstruct each of the following anatomical regions to be used as templates for designing the parts of the mannequin ensuring adherence to the anatomical structures of the real patient: skin, cranial bone structure, airway lumen, epiglottis, vocal cords, and tracheobronchial tree. Editing and parts modeling have been carried out using Geomagic Design X (3D Systems Inc., California), a RE software for CAD reconstruction starting from raw scan data or polygonal models.
Parts were modeled following the layout previously defined to allow the modularity of the system while focusing also on other important aspects that the medical staff underlined. Namely, the dimensions of the airway lumen must guarantee the passage of a 2.40 mm fiberscope, and the wall thickness of the tracheobronchial tree had to measure between 1.50 and 2.00 mm to be anatomically realistic. The tracheobronchial tree has to precisely replicate the haptic sensation provided by the instruments during the procedure on actual patients in addition to adhering to the wall thickness constraint. In order to determine the ideal material and wall thickness combination, a thorough preparatory investigation was conducted. This led to the creation of a 1.75 mm wall-thickness tracheobronchial tree, which was made from Shore 40A platinum cure silicone.21
Continuous feedback by the medical staff with whom the T3Ddy laboratory collaborates was crucial in this phase to evaluate the many aspects to be combined to reach a high level of realism of the airways (upper airways, epiglottis, vocal cords, and tracheobronchial tree) of the mannequin such as haptic feedback, anatomical accuracy, usability of the device in terms of fiberscope passage.
A first validation of the overall internal geometry of the mannequin has been performed directly in a 3D virtual environment reproducing the airway inspection using SolidWorks walk-through functionality which gives the endoscopic view obtained through the fiberscope.
Then, further experimental tests were carried out using physical prototypes of the airways to evaluate the correct passage of the medical equipment during navigation. From those tests, issues related to the passage of the fiberscope in the pharyngeal area emerged. Since the patient had swollen tonsils due to ongoing inflammation, the lumen in the pharyngeal area resulted in being partially obstructed, so a manual correction of the polygonal model has been necessary to resolve the issue. More than one attempt was required to find an optimal solution.
The upper airways' external geometry was designed to allow an easy coupling with the cranial shells in which the part is embedded, while to reproduce the internal geometry of the choanae and pharynx the STL resulting from the segmentation was used. The first CAD prototype was modeled enlarging the internal geometry extracted from the CT scan of the reference patient and validated by the medical staff by means of a walk-through simulation to show them the internal geometry of the part.
Another issue that emerged during experimental tests was due to the absence of tracheal rings, which represent the standard anatomical reference for the medical staff during navigation. This was because they were barely visible from the reference CT images. This was fixed by manually modeling the rings under the medical staff's supervision.
Due to the limited CT image resolution, which lacked sufficient detail for certain areas such as the epiglottis and vocal cords, manual adjustments were necessary to ensure accurate representation.
All the other head parts, both anatomical and mechanical, were modeled in consequence of the airway. Among those, the lower airway joint deserves particular emphasis because of its key role in allowing easy assembly and interchangeability of epiglottis, vocal cords, and tracheobronchial tree. It is composed of two halves held together by a pin and two magnets (see Figure 4). The pin serves also as a reference for the correct positioning of the parts.
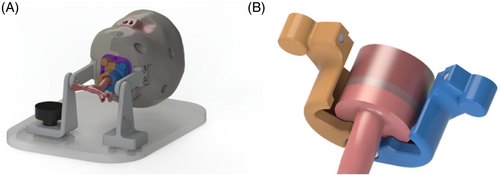
Virtual geometry of the parts corresponding to the upper airway, epiglottis, vocal cords, and the tracheobronchial tree is shown in Figure 5, where the geometry to ensure the correct fitting of the parts into the lower airway joint can be also appreciated.
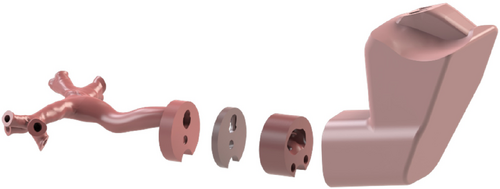
Different magnet types and placements to find the optimal solution in terms of stability were tested to avoid joint failure during mannequin navigation. An additional component that functions as a bridge between the cranial shells and magnet “housing” was added. This allowed testing different settings without requiring the cranium shells to be remodeled and remanufactured at any change (see Figure 6).
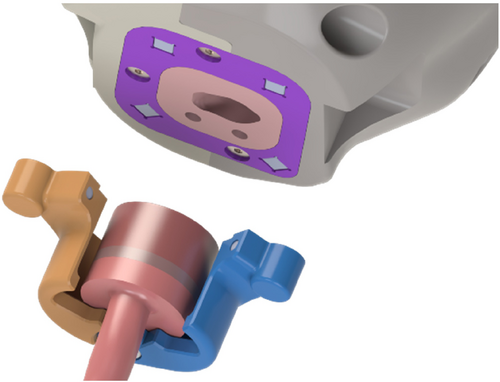
Then, the focus was made on the holding system. As planned during the layout definition, two hooks on either side of the cranial shells were designed to be secured with two couples of screws and threaded inserts. To guarantee the movement of the head while the mannequin is attached to the base, which was designed as a 12-millimeter-thick plexiglass panel, and to guarantee an easy disassembly if needed, two more hooks with different geometry were designed. The one on the left side of the mannequin is fixed by means of four screws to the base, while the one on the right was designed to allow its sliding once loosened a fastening knob that couples with a sliding plate positioned on the back of the base to allow tool-free detachment of the head.
Once the internal structure of the mannequin was designed and validated, the skin was designed as a 5 mm layer under medical staff indication.
The final structure of the mannequin is reported in Figure 7.

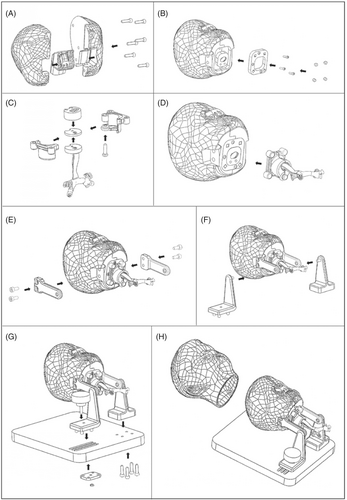
All the other parts are independent and can be assembled starting from the airways which is the core of the mannequin. After framing it in the shaped area of the shells representing the cranium by means of five screws (see Figure 8A), a mounting plate can be secured to the base with three more screws (see Figure 8B). The lower airway attachment system can be easily assembled by connecting the two parts of it with a pin and positioning the epiglottis, the vocal cords, and the tracheobronchial tree into it (see Figure 8C). The system is connected to the upper part of the mannequin thanks to the presence of four couples of cubic magnets in the mounting plate at the base of the cranium and in the airway attachment system itself (see Figure 8D). The presence of magnetic connections further facilitates the possible substitution of the parts also speeding the procedure up. The support system has a detachable structure too in which the easy removal of the mannequin head is ensured by the presence of a slide kept in position by a fastening knob. The other hook is kept in position with four screws (see Figure 8E–G). Finally, the silicone cover simulating the skin can be easily positioned thanks to the presence of proper reference points in the oronasal area (see Figure 8H).
Once the final layout had been defined, molds were designed to fabricate the soft components corresponding to upper airways, epiglottis, vocal cords, trachea, and skin by silicone casting. While for the molds of epiglottis, vocal cords, and skin, no particular issues other than to avoid undercuts emerged, the design of the molds of upper airways and trachea components required attention.
Theoretically, the presence of the numerous undercuts could have been overcome by subdividing cores into multiple parts, but practically this was difficult due to their limited dimensions. In the case of the tracheobronchial tree, for example, the lumen's cross-section average anteroposterior and transverse diameters in anteroposterior and transverse directions are 5.3 mm and 6.4 mm along the tracheal trait in a 20-months healthy pediatric patient,27 but in the case of tracheomalacia, the diameter of the minimum internal cross-section can easily reach a value of 1 mm.
To overcome this issue, soluble cores had to be forecasted to realize the complex geometries of the tracheobronchial lumen, and the same strategy was used for nasal choanae and pharyngeal traits of the upper airway component.
In the perspective of realizing a set of different pathological tracheobronchial trees, a parallel study was also carried out to develop an interactive semi-automatic procedure to speed up the design of the relative molds.28
2.3 Fabrication
All the parts of the mannequin but the plexiglass base were fabricated using 3D printing and molding and casting techniques. The rigid parts corresponding to cranial shells and lower airways attachment system, as well as the molds for the soft parts, were realized in ABS/ASA using the FDM (Fused Deposition Modeling) 3D printer F370 by Stratasys Inc. (Minnesota, USA), which was also used to produce the soluble cores in SR-30 (Stratasys Inc. Minnesota, USA) to be inserted in the molds of the upper airways and the trachea. SR-30 is soluble in a 60°C soda bath with a 10% concentration, which does not damage the platinum silicone chosen to realize the soft parts.
The coupling plate and the parts of the support systems (hooks and sliding plate) were realized in photopolymer White Resin (Formlabs Inc., Massachusetts, USA) using the SLA (Stereolithography) 3D printer Form3B by Formlabs Inc. (Massachusetts, USA).
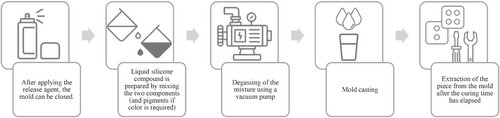
The parts of the airway system (upper airways, epiglottis, vocal cords, tracheobronchial tree) were realized using Smooth Sil 940 (Smooth-On Inc., Pennsylvania), a pink platinum-cure silicone with a 40 Shore-A hardness.21 For the skin Dragon Skin 30 (Smooth-On Inc., Pennsylvania), a transparent platinum-cure with a 30 Shore-A hardness silicone that can be colored using specific concentrated color pigments, was used. Regardless of the specific type of silicone used, the molding and casting process features the same simple steps. After applying a silicone-free release agent, having positioned the core if any and closed the molds, the liquid silicone compound was prepared by mixing the two components respecting the ratio specified by the manufacturer, namely 1A:1B for Dragon Skin 30 and 100A:10B for Smooth-Sil 940,29 while adding specific-colored pigments if needed. Once mixed, the compound is degassed using a vacuum pump and then gently poured into the mold to avoid new bubble formation. Once cured, the piece is ready to be removed from the mold. The workflow for the production using molding and casting technique is presented in Figure 9.
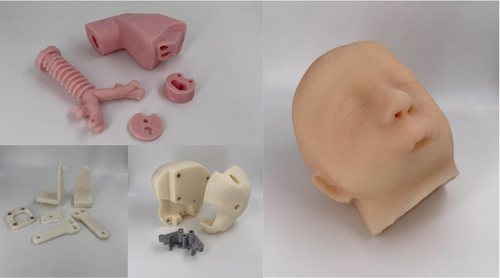
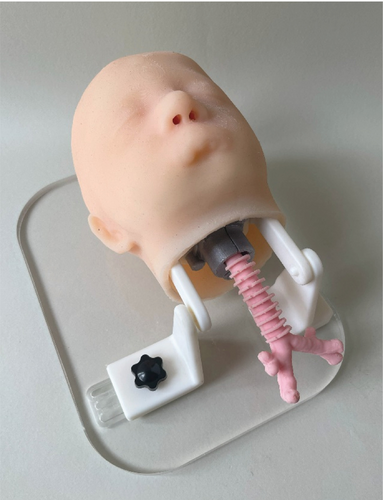
After inserting the magnets and the thread inserts where required, the mannequin is ready to be easily commissioned. Single parts before assembly are shown in Figure 10, while the assembled mannequin is reported in Figure 11.
3 EVALUATION QUESTIONNAIRE
On a flexible and rigid pediatric bronchoscopy course held by Meyer's Children's Hospital intended for anesthesiologists and intensivists, otolaryngologists, neonatologists, in-hospital and out-of-hospital emergency physicians, pneumologists, and pediatricians.
To validate the pediatric mannequin presented in this manuscript, a questionnaire was developed and submitted to 8 medical experts who filled it out anonymously after having attended a course on bronchoscopy organized by Meyer's Children's Hospital (see Figure 12).
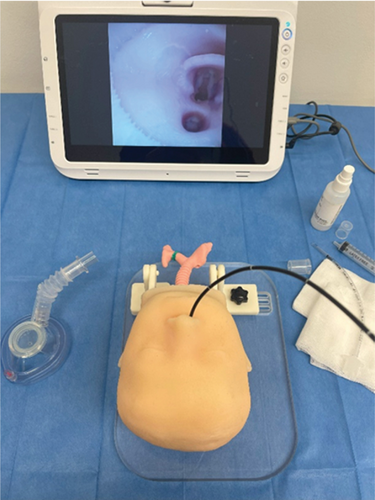
The course was a comprehensive training program focused on bronchoscopy techniques, including both theoretical knowledge and practical skills. It covered various aspects such as anatomy, instrumentation, diagnostic procedures, and therapeutic interventions related to bronchoscopy. The course aimed to make the participant gain expertise in performing bronchoscopy examinations and managing related clinical scenarios.
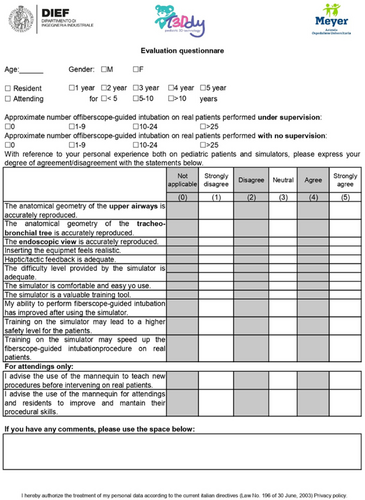
The questionnaire featured 13 statements to which the trainees had to express their agreement using a modified 5-point Likert scale (0 = not evaluable, 1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, and 5 = strongly disagree). The questionnaire layout was based on a previously validated format utilized by Barsness et al. in assessing a novel thoracoscopic neonatal simulator.30 Besides declaring their age, gender, educational level, and previous experience in performing the procedure, the trainees had to declare their level of agreement with the 13 (11 addressed to all trainees and 2 addressed to attendings only) statements that have been selected from the literature.3, 8, 10, 11, 14, 15, 19, 30-47 The statements aimed to evaluate the realism of the mannequin, the realism of the simulation, and its usefulness by evaluating the overall satisfaction. A comment section was also provided. Metrics regarding the physician's performance (e.g., time taken to tracheal intubation, number of attempts, or failed intubation percentage) were not included since the focus of the study was to evaluate the mannequin and not the trainees' skills. The questionnaire is reported in Figure 13.
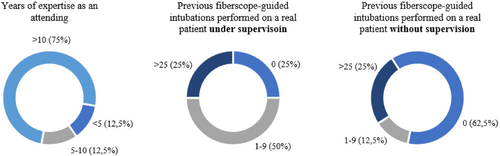
The average age of the eight trainees that filled the questionnaire was 47.5 ± 9.4 years and, as mentioned before, they were all attending the course. Two of them were male (25%), while the remaining six were female (75%). Six trainees had previous experience in the fiberscope-guided intubation on real patients. The fact that two participants have not experienced the procedure on a real patient before, led to the exclusion of their feedback regarding the comparison with the scenarios involving real patients (namely statements n. 4, n. 5, and n. 6). Other statements have been still considered valid since referring to visual aspects of the mannequin and the simulation experience.
Participants' details in terms of professional expertise and previous experience in performing the fiberoptic procedure on real patients are reported in Figure 14.
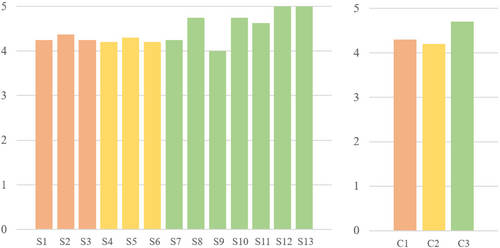
The 13 statements and the results obtained calculating the mean between the answers can be considered satisfactory (details are reported in Figure 15, on the left). Mean values obtained for each of the three categories (realism of the simulator, realism of the simulation, overall satisfaction) were calculated (see Figure 15, right) and show that the overall scores are higher than 4, meaning that there has been a general agreement on the proposed statements regarding the adequacy of the realism of the simulator, the simulation, and the overall experience.
4 DISCUSSION AND CONCLUSIONS
First feedback on the developed mannequin was obtained through an anonymized questionnaire submitted to the attention of 8 attendings of the Meyer's bronchoscopy course.
Participants were satisfied with the anatomical geometry and dimension of the components, as well as the difficulty of the simulated scenario. The mannequin has been assessed to provide an anatomically accurate reproduction of the airway of a polymalformative congenital condition characterized by severe mandibular hypoplasia, lingual agenesis, and micrognathia, configuring a difficult expected airway.
Besides the anatomical structure of the components, the medical staff positively evaluated the tactic feedback given by the materials, which means that the compliance of the real tissues is correctly reproduced, and the realism as well as the level of difficulty provided by the simulation, even if some aspects as the insertion of the equipment can be improved.
At the moment, the mannequin is a useful training tool on difficult airway conformation due to craniofacial malformations that somehow cause an obstruction in the passage of tubes, laryngoscopes, and catheters, but there is already the willingness to realize a novel version of the mannequin featuring both nasal and oral access as well as expanding the available pathologies, especially to the tracheobronchial tree.
The final goal was, in fact, to manufacture an advanced and complete mannequin using RE and AM techniques in which different pathological modules can be combined and substituted according to the medical and surgical staff's requests, to fill an existing gap regarding pathological mannequins to train on the management of the difficult airways and thus complement the commercially available devices.
From what has been assessed, it can be stated that the developed modular mannequin is an extremely useful and innovative tool, which promises to expand the world of simulation toward clinically difficult scenarios.
This model, despite being a high-fidelity reproduction of the syndromic airways of a 30-month-old real patient, does have some limitations. It features only nasal access and this characteristic, despite being desired to force the trainee to practice the procedure of nasal access using a fiberscope, represents a limitation to the realism of the model since no other approach for intubation can be performed. A further limitation is due to the lack of an internal lubrification system that makes continuous manual lubrification from an operator necessary. Finally, adding the thorax to the model would enhance its perceived realism.
Currently, work is being done on the development of tracheobronchial tree-related pathological modules, and a new high-fidelity mannequin prototype featuring oral access and movable jaw is already in the pipeline.
AUTHOR CONTRIBUTIONS
Luca Puggelli: Conceptualization; methodology; validation; writing – review and editing. Marta Mencarelli: Writing – original draft; validation. Paola Serio: Conceptualization; validation. Rocco Furferi: Supervision; writing – review and editing. Francesca Amoretti: Resources. Yary Volpe: Supervision; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
As part of the collaboration between Meyer's Children Hospital and DIEF within T3Ddy laboratory, all data necessary to carry out the activity of making models for training that reproduce non-patient-specific anatomy but are the result of aggregating and synthesizing information from multiple anonymized diagnostic images, the General Data Protection Regulation (abbreviated GDPR) does not apply. The necessary data were fully anonymized by the Meyer's Children Hospital before sharing with DIEF, respecting the confidentiality of the data of those involved.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




