Mutagenicity of the agriculture pesticide chlorothalonil assessed by somatic mutation and recombination test in Drosophila melanogaster
Accepted by: B. Gollapudi
Abstract
Chlorothalonil (CTL) is a pesticide widely used in Brazil, yet its mutagenic potential is not fully determined. Thus, we assessed the mutagenicity of CTL and its bioactivation metabolites using the somatic mutation and recombination test (SMART) in Drosophila melanogaster, by exposing individuals, with basal and high bioactivation capacities (standard and high bioactivation cross offspring, respectively), from third instar larval to early adult fly stages, to CTL-contaminated substrate (0.25, 1, 10 or 20 μM). This substrate served as food and as physical medium. Increased frequency of large single spots in standard cross flies' wings exposed to 0.25 μM indicates that, if CTL is genotoxic, it may affect Drosophila at early life stages. Since the total spot frequency did not change, CTL cannot be considered mutagenic in SMART. The same long-term exposure design was performed to test whether CTL induces oxidative imbalance in flies with basal (wild-type, WT) or high bioactivation (ORR strain) levels. CTL did not alter reactive oxygen species and antioxidant capacity against peroxyl radicals levels in adult flies. However, lipid peroxidation (LPO) levels were increased in WT male flies exposed to 1 μM CTL. SMART and LPO alterations were observed only in flies with basal bioactivation levels, pointing to direct CTL toxicity to DNA and lipids. Survival, emergence and locomotor behavior were not affected, indicating no bias due to lethality, developmental and behavioral impairment. We suggest that, if related to CTL exposure, DNA and lipid damages may be residual damage of earlier life stages of D. melanogaster.
1 INTRODUCTION
Chlorothalonil (CTL) or 2,4,5,6-tetrachloroisophthalonitrile, is a broad-spectrum pesticide mainly used to control fungal foliar diseases (USEPA, 1999). Brazil's economy is primarily based on the agroindustry, which performs application of CTL-based formulations to soybean plantations (Gottems, 2018). Soybean is the main grain produced in Brazil considering the cultivated area, which was estimated at approximately 35.15 million hectares (or 57% of total grain cultivated area) in 2018 (CONAB, 2018). Whereas soybean culture and, consequently, potential CTL usage occurs in all Brazilian regions (CONAB, 2018), the approval of CTL for agriculture in the European Union was suspended due to concerns on groundwater contamination by, and risk of genotoxicity when exposed to, CTL metabolites (European Commission, 2019). Chlorothalonil, however, is also a booster biocide added to antifouling paints used in structures in contact with water aiming to prevent settlement and degradation by organisms and, for this reason, can harm the aquatic environment as well (Guardiola et al., 2012; Readman, 2006).
Chlorothalonil antifungal effect is attributed to the inhibition of glycolysis and cellular respiration by reacting with functional thiol groups of enzymes of these pathways (Tillman et al., 1973), such as the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Long & Siegel, 1975). The ability to react with low molecular weight thiols (Vincent & Sisler, 1968) implicates in glutathione (GSH) depletion as a toxic effect of CTL exposure. Decreases in GSH levels can impair cellular detoxification capacity by interfering with the phase II of xenobiotic metabolism, in which GSH is conjugated to xenobiotics by the enzyme glutathione S-transferase (GST) (Gallagher et al., 1992). Observations of GSH decrease accompanied by increase of glutamate cysteine ligase activity in CTL exposures may indicate GSH is being used to detoxify CTL. Additionally, GST and GSH are important components of the cellular antioxidant defense, and lower GSH availability can lead to cellular oxidative stress (Gallagher et al., 1992; Vincent & Sisler, 1968).
The interference on GSH-dependent pathways and in energy metabolism, and the ubiquitous presence of these pathways in living organisms, raise concerns on CTL use. Indeed, sublethal and lethal toxic effects for CTL have been reported in non-target biota representative of different ecosystem compartments. Reduced survival rate was observed in bee Apis mellifera larvae fed with food containing 113 μM (30 mg/L) CTL (Dai et al., 2018). Impairment on the capacity to resist air exposure was noticed in Perna perna mussels exposed to water containing 0.038 μM (10 μg/L) CTL for 96 h (Guerreiro et al., 2017). In the estuarine polychaete Laeonereis acuta, exposure to 0.38 μM (100 μg/L) CTL for 96 h induced oxidative stress (Barreto et al., 2018).
The ability to generate oxidative stress indicates CTL could potentially harm the genetic material and cause cancer. In mammalian models, CTL shows genotoxic activity in cellular culture, whereas in vivo exposure to CTL induces no genotoxicity. Chlorothalonil and its metabolic products increased mean comet tail moment and CTL metabolites enhanced sister chromatid exchange in Chinese hamster ovary (CHO) cells (Galloway et al., 1987). DNA strand breaks were also higher in human peripheral blood lymphocytes collected from healthy donors and afterwards exposed to CTL in vitro (Lebailly et al., 1997). Differently, no increase in DNA damage as measured by alkaline comet assay was induced in whole blood, bone marrow, thymus, liver, kidney and intestine cells of rats treated with CTL via oral route (Godard et al., 1999); or detected in blood cells collected from mice exposed to CTL via inhalation of air flowing from areas where a CTL-based pesticide were applied (Garron et al., 2012). Whether CTL and its metabolic products could be genotoxic to in vivo animal models in chronic exposure was poorly addressed. Regarding carcinogenicity, CTL is considered a possible carcinogen classified as group 2B by the International Agency for Research on Cancers (IARC, 1999). Although there is no data on CTL carcinogenicity in humans, carcinogenic effects of CTL were observed in rats, mice (Wilkinson & Killeen, 1996) and Drosophila melanogaster (Yoon et al., 1985). When mice of both genders were exposed to CTL via oral, renal tumors were observed in male mice only (Wilson et al., 1983), suggesting that gender may play a role in the CTL toxicity.
The somatic mutation and recombination test (SMART) in D. melanogaster is a versatile tool to evaluate the mutagenic potential of substances and environmental samples as well as to identify the mechanisms of DNA damage induction. The assay detects several mutational events, such as point mutations, deletions, chromosome loss, and somatic recombination (Frei et al., 1992). Another advantage is the possibility to evaluate the indirect genotoxicity of a given substance by testing its effects on the offspring (obtained by high bioactivation (HB) cross) of ORR strain, which is a fly lineage expressing high CYP enzyme levels and, thus, having higher bioactivation capacity (Graf & van Schaik, 1992). The genotoxic potential of a wide range of compounds relevant to health, such as chemotherapeutic medicine (Allgayer et al., 2019; Danesi et al., 2010, 2012) and personal hygiene products (Rodrigues et al., 2007), as well as to assess the environmental quality of water (Jacociunas et al., 2010; Porta et al., 2017; Soares Neto et al., 2016) and air samples (Dihl et al., 2008), was successfully assessed by the SMART wing test.
Due to the disagreement on the CTL genotoxic potential in vivo and in vitro tests and to the lack of chronic studies on CTL genotoxicity, this study aimed primarily to evaluate the CTL mutagenicity on D. melanogaster. Because CTL interferes with cellular antioxidant defenses, we also evaluated whether CTL exposure affects cellular redox balance. Our study also allows insights into the gender role on the CTL toxicity, as well as addresses the CTL indirect toxicity mediated by products of CTL metabolism, since our study included experiments on flies with HB capacity.
2 MATERIALS AND METHODS
2.1 D. melanogaster strains
Four D. melanogaster strains were used in this study: multiple wing hair (mwh/mwh, or simply mwh), flare3 (flr3 /In(3LR)TM3, ri pp sep l(3)89Aa bx34e and BdS, or simply flr3), ORR-flr3 (ORR-flr3/In(3LR)TM3, ri pp sep l(3)89Aa bx34e and BdS, or simply ORR) and wild-type (WT). Multiple wing hair, flr3, and ORR strains are lineages genetically constructed for the SMART wing spot test. WT and ORR strains were used in experiments conducted to evaluate survival and emergence rates, locomotor activity, and oxidative stress parameters. ORR strain individuals present multiple copies of CYP450 gene and consequently higher CYP450 expression, therefore the ORR strain and the offspring of ORR and mwh cross (HB cross, see Section 2.3) were used in order to determine the toxicity of CTL bioactivation metabolites. WT strain was maintained at 25°C under a light/dark cycle of 12/12 h in 300 mL vials containing a banana-based medium. The medium was composed of banana (200 g), corn flour (28.8 g), wheat germ (3.24 g), common table sugar (3.24 g), NaCl (0.72 g), Nipagin (1.04 mg, Synth, N1002.02.AG, USP-NF/FCC) as a mold inhibitor, and was mixed and cooked with boiling water (400 mL). Multiple wing hair (mwh/mwh, or simply mwh), flare3 (flr3/In(3LR)TM3, ri pp sep l(3)89Aa bx34e e BdS, or simply flr3) and ORR-flr3 (ORR-flr3 /In(3LR)TM3, ri pp sep l(3)89Aa bx34e e BdS, or simply ORR) were provided by Universidade Luterana do Brasil and maintained on the same conditions of the WT strain, except for the medium (37.5 g baker's yeast, 4.25 g agarose, 70.7 g table sugar, 138.6 g corn flour, 3.8 mg Nipagin, 3.5 mL acid solution (propionic acid (Sigma, 100% purity): phosphoric acid (Quimex, 95% purity), 10:1), cooked in 750 mL boiling water). Bananas, wheat germ baker's yeast, common table sugar, and corn flour suitable for human consumption were obtained at the local supermarket.
2.2 Preparation of chlorothalonil solution and exposure substrate
Because CTL possesses low solubility in water (<0.1 mg/mL), we prepared a stock solution of 22.056 mM analytical standard CTL (PESTANAL®, Sigma-Aldrich, product number 36791, ≥ 98% purity) in dimethyl sulfoxide (DMSO, Dinâmica Química Contemporânea LTDA, analytical standard, 99.9% purity), approaching the solubility limit in this solvent (5–10 mg/mL or 18.8–37.6 mM). The stock solution was diluted in DMSO to working concentrations of 20, 10, 1, and 0.25 mM. These solutions were then diluted in water to prepare the test solutions at 20, 10, 1, and 0.25 μM, maintaining the final DMSO concentration constant at 0.1% v/v. A vehicle control group treated with 0.1% DMSO and a control group treated with water were also evaluated. Since there is no information available for CTL toxicity in flies, this concentration range was chosen based on acute and chronic toxicity data from other invertebrates (Caux et al., 1996), on the knowledge that DMSO concentrations higher than 0.1% are known to interfere in the analyzed parameters, and on the fact that no mortality was observed in our pilot experiments. The higher CTL concentration in the experimental substrates was estimated at approximately 4.6 mg/kg. For comparison, the maximum residue level tolerated by the Brazilian Agency for Health Surveillance (Agência Nacional de Vigilância Sanitária—ANVISA) for apple and cucumber are 1 and 11 mg/kg, respectively (ANVISA, 2023).
Individuals were exposed from third instar larval stage to early adult fly stage (approximately 8 days) in vials containing solid substrate hydrated with water, 0.1% DMSO, or CTL solutions. For the SMART assay, solid substrate consisted of 1.5 g of dry Drosophila instant medium (Carolina Biological Supply, Burlington, NC) hydrated with 5 mL of the control or test solutions. For the evaluation of survival, locomotor assay, and oxidative stress parameters, the substrate consisted of potato flake (4.5 g, suitable for human consumption, obtained at the local supermarket) rehydrated with 31 mL of control or test solutions, and Nipagin mold inhibitor 0.83% (v/v). Four vials were prepared for each experimental group in each experiment, except in the experiment to evaluate the locomotor activity, in which each experimental group was tested in triplicates. The solid substrates work as food source, of which the larvae vividly feed for approximately 48 h until pupation, as well as physical substrate for larvae and adult flies. Adult flies were collected after emergence from pupae and properly stored or processed for analysis.
2.3 Experimental design for SMART
For the SMART assay (Figure 1), two strain crosses were prepared: (1) the standard (ST) cross, in which females from flr3 were mated with mwh males (Graf et al., 1989), and (2) the HB cross, in which females from ORR were mated with mwh males (Graf & van Schaik, 1992). To that, 100 virgin female flies were collected within 8 h from pupae emergence and were put to mate with 50 male flies and lay eggs in bottles containing solid agar (Dinâmica, product number P.10.0036.000.00) base (3% w/v) and an oviposition medium, consisting of 500 g baker's yeast with table sugar (4 tbs) and 40 mL distilled water. After 8 h, adult flies were removed and bottles kept in incubators for 3 days. On the third day, third instar larvae were cautiously collected and transferred to experimental vials, prepared according to Section 2.2. Individuals were exposed to CTL in the experimental vials for approximately 8 days, while they completed larval stage, pupate and emerged from pupae as flies. To check the effect of the chlorothalonil on survival under the conditions of this experimental design, in the first experiment, 30 larvae were put in an experimental vial per group and individuals were counted at the adult fly stage. Adult flies were collected and stored in 70% ethanol until used for slide preparation. Slides were prepared by fixing wings removed from flies with Faure's solution and left to dry. Dry slides were examined under a microscope (Olympus CX 41, 400 × magnification) first for quality and then for frequency and size of two spot categories: single and twin spots. Single spots present only mwh or flr3 hair phenotype, and twin spots present both mwh and flr3 hair phenotypes. Each spot category indicates different mutagenic events. Single spots can result from deletions, point mutations, specific chromosome aberrations, or from somatic recombination occurring between the two marker genes. Twin spots are produced by somatic recombination between the proximal marker flr3 and the centromere of chromosome 3. The frequency of each spot category per fly in each experimental group was obtained and compared to the frequency of the control 0.1% DMSO, using the Kastenbaum–Bowman statistical test for proportions followed by the multiple-decision procedure described by Frei and Würgler (1988). Urethane (Sigma-Aldrich) 20 mM is a mutagenicity inducer and was applied as positive control. Two independent experiments were conducted.
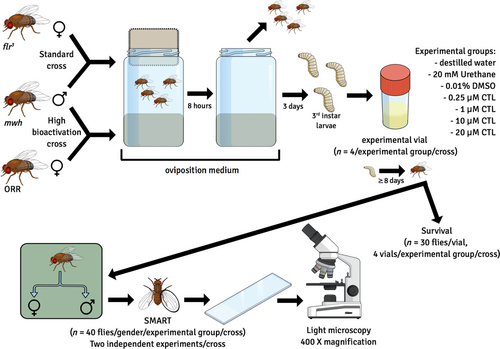
2.4 Experimental design for survival and emergence rates, locomotor behavior, and oxidative stress parameters
To assess survival and emergence rates, oxidative stress parameters, and locomotor behavior, either WT or ORR adult flies were put in bottles containing oviposition medium, prepared as described in Section 2.3, left for 8 h to mate and lay eggs, and then removed (Figure 2). The bottles were kept in incubators for 3 days when third instar larvae were cautiously collected, counted, and transferred to experimental vials (n = 30/vial). The experimental vials were prepared as mentioned in section 2.2. Exposure to CTL in the experimental vials lasted from third larval stage to adult flies stage, which lasted approximately 8 days. Adult flies were collected, sexed, and tested for their locomotor activity or processed for the determination of the levels of reactive oxygen species, antioxidant capacity against peroxyl radicals (ACAP), or lipid peroxidation. These three oxidative stress parameters were evaluated in the same experiment, and two independent experiments were conducted to collect these data. Survival and emergence rates and locomotor behavior were evaluated in separate experiments. Two independent experiments were performed to evaluate survival and emergence rates, and two independent experiments, to assess the locomotor activity.
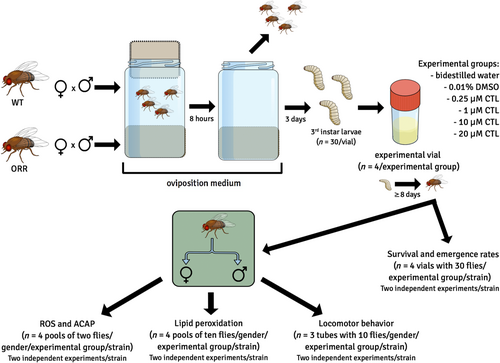
2.5 Survival and emergence rates
Survival was always checked in all experiments. Survival rates consist in the percent of number of adult flies in relation to the number of larvae (n = 30) in a given test tube. For the emergence rate, the rate at which flies emerge from the pupae was monitored daily and was expressed as percent of cumulative emergence in days since the first emergence in any of the experimental groups was observed.
2.6 Locomotor assay
2.7 Determination of reactive oxygen species (ROS) levels and ACAP
ROS levels and antioxidant capacity measured against peroxyl radicals (ACAP) were determined based on the protocol by Myhre and Fonnum (2001) and Amado et al. (2009), respectively. The D. melanogaster samples were prepared according to de Aguiar et al. (2016) and Figueira et al. (2017). For each experimental group, four pools (n = 4) of two flies per sex were homogenized in cold buffer (200 μL, Tris (Sigma, 99% purity)-HCl (Dinâmica, 99.9% purity) 10 mM, pH 7.2) and centrifuged (2000 × g, 20 min at 4°C). Pellets were discarded and supernatant was used for further analyses and for protein content determination using Bradford (1976) method, with a bovine serum albumin curve as standard. ROS levels were determined using a fresh 10 μL aliquot incubated at 25°C with 2,7-dichlorofluorescein diacetate (H2DCF-DA 2.4 mM, Sigma, ≥95% purity). H2DCF-DA oxidation was determined every 5 min over a 120 min period using a fluorimeter (488 nm excitation, 525 nm emission). The values for area under curve (AUC, fluorescence × time) for each sample were obtained, and normalized for μg of protein, the average levels of ROS for each experimental group were calculated and then ROS levels were expressed as percentage of the water control group.
Determination of ACAP was performed in a 10 μL aliquot incubated with and without 4 mM 2,2-azobis (2-methylpropionamidine) dihydrochloride (ABAP, Sigma, ≥95% purity) by monitoring the fluorescence (488 nm excitation, 525 nm emission) for 40 min in 5 min intervals, at 37°C. This temperature is necessary for ABAP thermolysis and the generation of peroxyl radicals. The AUC (fluorescence × time) for each sample was determined in the presence and in the absence of ABAP and normalized by protein content, following the equation: 1/(AUC with ABAP − AUC without ABAP)/(AUC without ABAP)/μg protein. The mean for each experimental group was calculated and the values were transformed in percentage of the water control group.
2.8 Lipid peroxidation
For lipid peroxidation analysis, four pools (n = 4) of 10 female or male flies were collected after emergence and frozen at −80°C. The animals were homogenized in 100 μL of cold methanol following centrifugation at 1000 × g for 5 min at 4°C. A 15 μL aliquot was added to 175 μL of ultra-pure water, 35 μL of xylenol orange (1 mM, Sigma-Aldrich, product number 398187), 35 μL of sulfuric acid (0.25 mM, Dinâmica, 99.9% purity) and 90 μL of ferrous sulfate (1 mM, Synth, >99% purity). Absorbance of samples was determined spectrophotometrically at 550 nm, and lipid peroxidation levels were calculated based on 0.175 mM cumene hydroperoxide (CHP, Sigma, ≥95% purity) standard (Jiang et al., 1992).
2.9 Statistical analyses
SMART assay results were analyzed using Kastenbaum–Bowman statistical test for proportions followed by the multiple-decision procedure described by Frei and Würgler (1988). Data from survival, emergence rates, locomotor assay, ROS, ACAP, and lipid peroxidation fitted normal distribution, as verified with Shapiro–Wilk test, and outliers were identified using Grubbs' test. After excluding outliers, data of emergence rates was analyzed by two-way ANOVA followed by Tukey test (assuming experimental groups and time as factors), and data of survival, locomotor assay, ROS, ACAP, and lipid peroxidation were checked for statistical differences using one-way ANOVA followed by Tukey's multiple comparison test. We also tested whether there was an effect of gender on LPO levels in WT flies using two-way ANOVA followed by Tukey's multiple comparison test. All significance levels were set at p < .05.
3 RESULTS
3.1 SMART assay
No significant differences in frequency of spots for standard and HB crosses were observed between water and DMSO 0.01%, indicating no mutagenic effect of the solvent at the concentration used for CTL solubilization (Table 1). In standard cross, urethane 20 mM increased the frequencies of small single spots, large single spots and total spots, whereas, in HB cross, 20 mM urethane additionally increased the frequency of twin spots (Table 1). In general, the treatment with CTL ranging from 0.25 to 20 μM did not alter the frequencies of single, twin and total number of spots. Nevertheless, chlorothalonil at 0.25 μM increased the frequencies of large single spots in standard cross flies (Table 1).
| Experimental groups | Number of flies (N) | Frequency of spots per fly (total number of spots) | Number of spots with mwhc | |||
|---|---|---|---|---|---|---|
| Small single spots (1–2 cells)b m = 2 | Large single spots (>2 cells)b m = 5 | Twin spots m = 5 | Total spotsb m = 2 | |||
| Standard cross | ||||||
| Water | 40 | 0.65 (26) | 0.15 (06) | 0.00 (00) | 0.80 (32) | 30 |
| DMSO 0.01% | 40 | 0.75 (30) | 0.05 (02) | 0.05 (02) | 0.85 (34) | 32 |
| Urethane 20 mM | 20 | 4.55 (91) + | 0.45 (09) + | 0.15 (03) i | 5.15 (103) + | 103 |
| CTL 0.25 μM | 40 | 0.63 (25) − | 0.23 (09) + | 0.00 (00) i | 0.85 (34) − | 34 |
| CTL 1 μM | 40 | 0.50 (20) − | 0.00 (00) i | 0.05 (02) i | 0.55 (22) − | 22 |
| CTL 10 μM | 40 | 0.93 (37) − | 0.18 (07) i | 0.03 (01) i | 1.13 (45) − | 44 |
| CTL 20 μM | 40 | 0.68 (27) − | 0.08 (03) i | 0.03 (01) i | 0.78 (31) − | 31 |
| High bioactivation cross | ||||||
| Water | 40 | 1.13 (45) | 0.18 (07) | 0.05 (02) | 1.35 (54) | 54 |
| DMSO 0.01% | 40 | 1.23 (49) | 0.13 (05) | 0.00 (00) | 1.35 (54) | 54 |
| Urethane 20 mM | 20 | 25.20 (504)+ | 9.80 (196) + | 3.35 (67) + | 38.35 (767) + | 752 |
| CTL 0.25 μM | 40 | 0.75 (30) − | 0.23 (09) i | 0.03 (01) i | 1.00 (40) − | 40 |
| CTL 1 μM | 40 | 0.83 (33) − | 0.08 (03) i | 0.10 (04) i | 1.00 (40) − | 39 |
| CTL 10 μM | 40 | 1.35 (54) − | 0.13 (05) i | 0.05 (02) i | 1.53 (61) − | 60 |
| CTL 20 μM | 40 | 0.88 (35) − | 0.10 (04) i | 0.10 (04) i | 1.08 (43) − | 42 |
- Note: According to Frei and Würgler (1988), possible statistical diagnoses (p ≤.05) are (+) positive; (−) negative or (i) inconclusive in relation to the respective control. Two independent experiments were conducted.
3.2 Survival and emergence rates
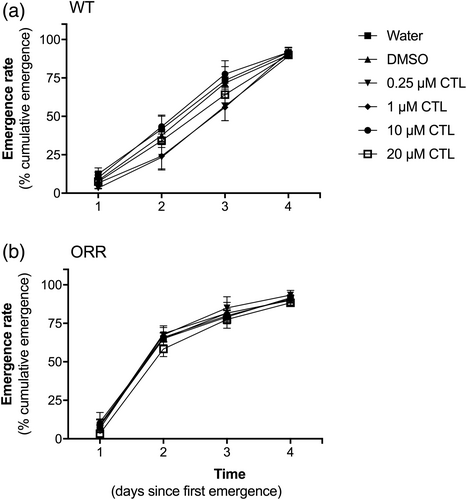
Survival rate of WT and HB (ORR) strains was measured as the percentage (%) of adult flies that emerged within 4 days, setting day one as the day we observed the first fly emergence in any experimental group in a given experiment. For both strains, survival was above 80% in all experimental groups and did not differ among groups (Supplemental Figure 1A). Emergence rates of WT and ORR flies were monitored daily until day 4, which was the fourth day since the first fly emerged in a given experiment, regardless of the experimental group. For both strains, the emergence rates did not differ among the experimental groups (Figure 3a, b). An effect of time on emergence rates, however, was pointed out by two-way ANOVA (Supplemental Figure 1B).
3.3 Locomotor behavior
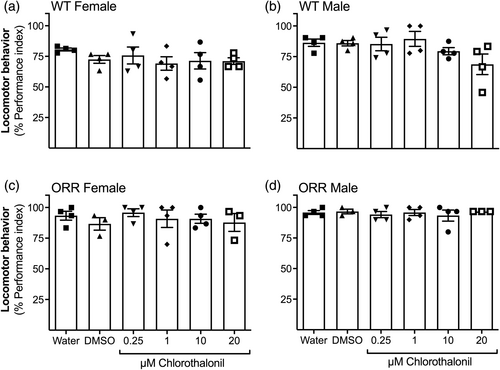
Locomotor behavior was assessed separately for female and male flies of both WT and ORR strains. Chlorothalonil treatment between 0.25 and 20 μM did not alter the capacity to move to the top of the vial, as expressed as % of PI, of neither female nor male flies of any strain (Figure 4).
3.4 Oxidative stress parameters
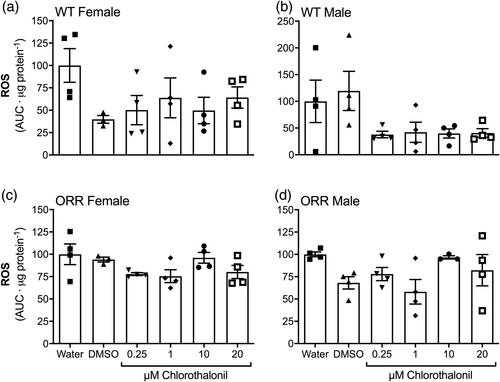
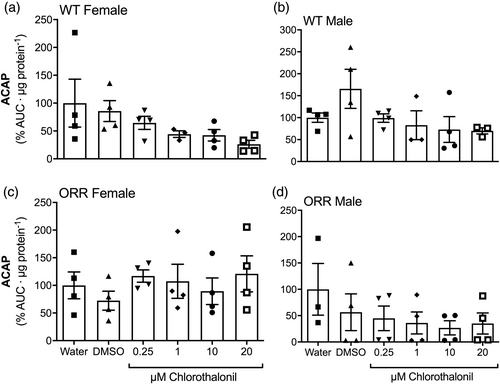
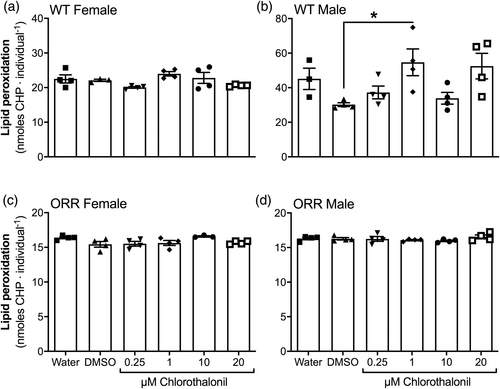
The generation of ROS, the ACAP, and the levels of lipid peroxidation (LPO) were assessed in WT female and male, and ORR female and male adult flies exposed, since third instar larval phase, to CTL or to control conditions. For any gender and strain, no difference was observed among the experimental groups regarding ROS generation (Figure 5a–d) or ACAP levels (Figure 6a–d). Regarding LPO, WT male flies exposed to 1 μM CTL showed higher levels than those males exposed to 0.01% DMSO (Figure 7b). An effect of gender on LPO levels in WT flies was also observed (p < .0001, two-way ANOVA).
4 DISCUSSION
Our study focused primarily on evaluating the mutagenic potential of CTL using the SMART for wing spots in D. melanogaster. A substance is considered mutagenic in the SMART wing spot assay only when the number of total spots is altered, thus, CTL did not present mutagenic potential under the experimental conditions of our study. Moreover, this absence of mutagenicity was observed in standard and HB crosses, indicating, respectively, no direct genotoxic activity for CTL as well as no direct and indirect genotoxicity caused by intermediates of CTL metabolism via CYP450 bioactivation enzymes (Table 1). The lack of mutagenic effects here observed is in agreement with the findings of previous in vivo studies (EFSA, 2016; Garron et al., 2012; Godard et al., 1999; USEPA, 1999), which opposes the results obtained from in vitro studies (Galloway et al., 1987; Lebailly et al., 1997).
The apparent disagreement between in vitro and in vivo evaluations of CTL genotoxicity may reflect the way the genotoxic potential is assessed. The genotoxic effects observed in vitro experiments may be a result of the direct CTL exposure to cells, whereas the absence of genotoxicity in vivo experiments may be related to the protection conferred by the systemic metabolization and thus to higher tolerance capacity presented by whole organisms. DNA damage in SMART assay is only detected if genotoxic events take place in the wings' precursors' cells, are not repaired, do not cause cell death, and lead to mutations in the wings of adult flies. Exposure between 50 and 500 μM CTL for 1 h increased DNA damage in human blood lymphocytes, as measured as tail moment and as image length using the comet assay (Lebailly et al., 1997). In the latter study, after 24 h of CTL exposure, the DNA strand breaks remained and the cell viability decreased, indicating that the lymphocyte repair system could not cope with the DNA damage, which probably led to cell death (Lebailly et al., 1997). Under our experimental conditions, if CTL were able to damage D. melanogaster larvae DNA, that damage would either be repaired during pupation or lead to cell death and could not be evidenced as spots in the wings of adult flies.
We evidenced that CTL exposure increased the number of large single spots in flies of the standard cross exposed to 0.25 μM CTL (Table 1). In SMART, D. melanogaster is exposed at third larval stage. At this stage, larvae have specialized clusters of cells, called the imaginal discs, which undergo cellular divisions in order to form different body structures, such as the eyes, legs, and wings, in adult flies (Beira & Paro, 2016; Nöthiger, 1972). Considering the wings' imaginal discs, the earlier a mutagenic event arises, the larger the wing spot in the adult fly is. Therefore, considering that the increased frequency of large single spots is related to CTL exposure, our results may suggest a direct genotoxic potential of CTL exposure to an earlier larval phase. Such life stage effect has been already described for CTL-associated toxicity in another insect. Topical application of 2.1 mg/mL (7.9 μM) of a chlorothalonil-based pesticide in eggs or as first-instar larvae of Japanese beetle (Popillia japonica) reduced the survival, but no lethal effect was observed when the application was performed in second or third-instar larvae (Obear et al., 2016). Although, under our experimental conditions, the CTL exposure was not able to cause relevant DNA damage, there is a possibility that CTL be genotoxic to D. melanogaster at earlier developmental stages than third instar.
In order to check whether mortality in our experimental design could interfere with the evaluation of CTL mutagenicity, we determined the survival rates of adult flies in the SMART assay. No lethality related to CTL were observed in adult individuals of standard or HB crosses exposed since third stage larvae (Supplementary Figure 1). Therefore, we assumed no bias in the evaluation of CTL mutagenic potential due to interference on D. melanogaster survival under our experimental conditions. We also monitored the survival of adult flies of WT and ORR strains and observed no differences in the number of flies emerging from pupae (Figure 3a, b). Our findings corroborate with the report for another invertebrate indicating that five times higher CTL concentrations (30 mg/L or 113 μM) than our highest concentration tested (20 μM) are necessary to reduce the survival of bee (Apis mellifera) larvae, which was also exposed by feeding (Dai et al., 2018). We acknowledge the possibility that CTL at the concentration range we tested might cause mortality in D. melanogaster if exposure were performed in earlier life stages, that is, eggs, first or second instar larvae, similarly to previously reported (Obear et al., 2016). Nevertheless, CTL exposure did not affect survival of D. melanogaster under our experimental conditions, which then was considered suitable for the evaluation of sublethal effects.
Differently from strict aquatic animals submitted to water-borne exposure, exposure by feeding or contaminated substrate can be at least partially counteracted by behavioral strategies, provided that individuals can avoid food or contact with the contamination source. If organisms did avoid feeding, the caloric restriction could negatively impact the development from larvae to adults, which could be perceived as alteration in larval pupation capacity and fly emergence delay. By counting the number of pupae in each experimental vial, we inferred no effect on pupation capacity of larvae exposed to CTL at concentrations ranging from 0.25 to 20 μM (data not shown). By monitoring the emergence of flies from the pupae in the experimental vials, no delay in the fly development related to CTL exposure was observed (Figure 3a, b). Interestingly, we observed a difference in emergence profiles among strains (Figure 3), which had no relation to CTL exposure. Nevertheless, our data indicated CTL exposure did not impair the development of D. melanogaster under the experimental conditions of our study.
The negative geotaxis assay assesses impairment in the neuromotor function of D. melanogaster by evaluating the locomotor behavior based on the reflex movement of flies to the top of a tube. No alterations in the locomotor behavior performance were observed in the flies exposed to CTL under our experimental conditions (Figure 4). On one hand, we expected CTL exposure to affect the behavior of flies, since previous studies on aquatic invertebrates demonstrated that exposure to CTL altered acetylcholinesterase (AChE) enzyme activity. AChE modulates the signaling of acetylcholine, the most abundant excitatory neurotransmitter in the central nervous system of insects, involved in learning and memory processing (Gauthier, 2010). Decreased AChE activity levels were reported to the polychaete Laeonereis acuta exposed to 0.38 μM CTL for 96 h (Barreto et al., 2018). Similarly, 96 h-exposure to 0.038 μM of chlorothalonil resulted in significant inhibition of AChE enzymatic activity in gills of pacific oyster Crassostrea gigas and blue mussel Mytilus edulis (Haque et al., 2019). This inhibitory effect was probably due to a reaction of CTL with sulfhydryl groups present in AChE, inactivating the enzyme (Barreto et al., 2018). On the other hand, AChE is not considered a specific target of CTL and sublethal concentrations (until 0.047–0.075 μM) of CTL for 96 h did not changed AChE activity in muscle of tadpoles of Agalychnis callidryas, Isthmohyla pseudo puma, and Smilisca baudinii (Méndez et al., 2016). Taking into account that our exposure lasted 8–9 days and used CTL concentrations of up to 20 μM, and that we observed no behavior impairment, it is possible that CTL did not alter AChE activity in D. melanogaster under the experimental conditions of our study.
Similarly to other pesticides, CTL was identified as an inductor of oxidative stress on several tissues and animals (Baustian et al., 1997; Bessi et al., 1999; Barreto et al., 2018; Méndez et al., 2016; Wang et al., 2010). The mechanism for this pro-oxidant action was argued to be the depletion of GSH, potentiated by high GST activity in phase II biotransformation (Gallagher et al., 1991). As a result of oxidative stress, oxidative damage is inflicted to biomolecules, such as DNA and lipids. Since we observed no genotoxic potential for CTL, we questioned whether CTL could promote oxidative stress and oxidative damage to lipids in our experimental design. We observed a two-fold increase in lipid peroxidation levels in WT males exposed to 1 μM CTL (Figure 7b), but no other significant result was observed at any other CTL exposed groups. Gender-specific (Wilson et al., 1983), as well as no clear dose–response relationship (McMahon et al., 2012; Shelley et al., 2009; Wilson et al., 1983) were observed in previous CTL exposure studies. The nonmonotonic response in the lipid peroxidation levels of males WT exposed to CTL may reflect that (1) CTL concentrations equal or higher than 1 μM are able to cause oxidative stress, and (2) antioxidant defense mechanisms may effectively prevent lipid peroxidation caused by CTL exposure at higher concentrations than 1 μM. Noteworthily, female WT flies presented lower levels of lipid peroxidation than male WT flies (Figure 7a, b, p < .0001), which likely reflects the higher antioxidant enzyme activity in female flies (Niveditha et al., 2017) and may be the reason why the lipid oxidative damage levels are not increased in females. We, however, did not detect oxidative imbalance in adult flies by CTL exposure of WT and ORR D. melanogaster strains (Figures 5 and 6), suggesting the increased LPO levels are possibly reminiscent oxidative damage in lipids caused by CTL induced-oxidative stress in earlier life stages of D. melanogaster. Similar to our results, no oxidative stress was observed in hemocytes of mussels Perna perna exposed to 0.38 nM or 0.038 μM (0.1 or 10 μg/L) CTL for up to 96 h via contaminated water (Guerreiro et al., 2017).
Interestingly, the toxic effects were evidenced in flies with basal bioactivation capacity (standard cross, Table 1, and WT flies, Figure 7b), whereas they were absent in flies with HB capacity (HB cross and ORR flies). These results point to direct toxic effects of the CTL molecule as well as raise the question of whether high expression levels of cytochrome P450 (CYP450), and thus high biotransformation capacity, is protective against CTL. CYP450 enzymes are monooxygenases involved in biotransformation of xenobiotics and in steroid genesis (Hannemann et al., 2007), and are induced mainly by several endogenous and exogenous agents (Kizawa et al., 1991). In insects, CYP450 is a known catalyst for detoxification reactions of exogenous compounds (Feyereisen, 1999). Flies of the ORR HB strain carry chromosomes 1 and 2 from a DDT-resistant Oregon R(R) strain and, therefore, present multiple copies of CYP450 gene and consequently higher CYP450 expression (Saner et al., 1996). Recently, it was reported that an isoform of CYP450 (CYP561) found in a species of fungus was capable of converting CTL into its metabolite 4-hydroxy-2,5,6-trichloroisophthalonitrile (Green et al., 2018). To the best of our knowledge, CTL metabolism by CYP450 has not yet been demonstrated in animals. When comparing the results of WT and ORR strains, we cannot attribute the differences observed only to CYP metabolism because we cannot rule out differences in genetic constitution or antioxidant defense levels among these strains. However, it is possible that CTL be a substrate for CYP isoforms present in the HB cross' offspring and in the ORR flies and, thus, that the high CYP levels be protective against DNA and lipid damages induced by CTL.
As well as life stage, the route of exposure, and the time lapse between exposure and effect assessment are factors known to affect the experimental outcome. In this study, genotoxicity and other toxic effects were evaluated using the experimental design of the SMART assay. In the SMART assay, the main route of exposure is oral contamination while feeding. Studies on mammals demonstrated that when administered orally, CTL is in great part excreted by the gastrointestinal system as feces or biles (Parsons, 2001). We did not assess the contribution of these pathways, however it is likely that most CTL be eliminated as excretory products by D. melanogaster. By choosing to perform the SMART experimental design to evaluate all parameters, we acknowledge the possibility of missing CTL non-lethal acute effects in larvae that might have been coped by the organism defenses during pupation and early adult fly periods. On one hand, exposing D. melanogaster as larvae and evaluating CTL effects on adult flies decrease the possibility of detecting short-term deleterious effects. On the other hand, the long-term evaluation could be an indicator of the physiological capacity one organism has to respond to a deleterious event and activate defense mechanisms, such as antioxidant responses and xenobiotic metabolism, and also of its behavioral responses. Besides the limitations, long-term experimental designs, such as the SMART assay, allow investigations on the toxicology of a substance and the resilience of a given species into an ecologically relevant picture.
5 CONCLUSION
This study reported the effects of CTL exposure, initiated at third instar larval stage, on adult flies of D. melanogaster strains with different CYP450 activity levels. Under our experimental conditions, CTL cannot be considered mutagenic. The lack of mutagenicity may be linked to the absence of an oxidative imbalance observed in CTL exposure. However, an increase in the frequency of large wing spots in WT flies and not in ORR flies, suggests that, if CTL is mutagenic, it presents direct mutagenicity in earlier developmental phases of D. melanogaster. Moreover, it is possible that DNA damage induced by CTL may be repaired before it leads to a mutation. An increase of LPO in male flies of WT strain was observed in an intermediary CTL concentration (1 μM), whereas no alteration was observed in the ORR strain flies of the respective experimental condition. These results reinforce CTL direct toxic effects and also suggest a protective role of high CYP450 bioactivation in D. melanogaster. In addition, the higher LPO levels found in WT males may reflect gender-specific differences in the antioxidant enzyme defense in D. melanogaster. No CTL effects were detected on survival, emergence rates and locomotor skills, suggesting our experimental conditions posed no impairment on development and behavior. We highlight that these data may be analyzed with caution, since CTL toxicity to D. melanogaster may be related to the life stage in which exposure occurred and in which the effects were evaluated, as well as to the route of CTL exposure.
AUTHOR CONTRIBUTIONS
This work was conceived and designed by BV, CER, and MLMH. BV, MAF, ML, and MLMH worked on data acquisition and analysis. BV, ML, CER, and MLMH interpreted the data and drafted the article. All authors revised the article and approved the final version to be published. BV, CER, and MLMH had complete access to the study data.
ACKNOWLEDGMENTS
The authors thank Dr. Fiamma Eugenia Lemos Abreu (IO-FURG) for the preparation of chlorothalonil stock solutions, and Dr. Isadora D'Ávila Tassinari and Mr. Leandro Amaro da Silva Felix for helping with Figures 1 and 2. This study was partly granted by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Brasil [Finance Code 001] and FINEP [CT-Hidro 1111/13–AIBRA-SIL2]. This work was also supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the CAPES—Brasil as ML and BV scholarships. MLMH was supported by a CAPES Post-Doctoral fellowship in PPG-CF/ FURG during the data collection and analyses, and in PPG-CB: Fisiologia/UFRGS during manuscript preparation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest regarding the conduction of the research, results, conclusions, and opinions here reported.
ETHICS STATEMENT
The funding agencies had no involvement in the study design, in the data collection, analysis, and interpretation, in the article writing, and decision to submit for publication.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




