Establishment of a nonradioactive DNA ligation assay and its applications in murine tissues
Accepted by: S. Yan
Abstract
As final process of every DNA repair pathway, DNA ligation is crucial for maintaining genomic stability and preventing DNA strand breaks to accumulate. Therefore, a method reliably assessing DNA ligation capacity in protein extracts from murine tissues was aimed to establish. To optimize applicability, the use of radioactively labeled substrates was avoided and replaced by fluorescently labeled oligonucleotides. Briefly, tissue extracts were incubated with those complementary oligonucleotides so that in an ensuing gel electrophoresis ligated strands could be separated from unconnected molecules. Originally, the method was intended for use in cerebellum tissue to further elucidate possible mechanisms of neurodegenerative diseases. However, due to its inhomogeneous anatomy, DNA ligation efficiency varied strongly between different cerebellar areas, illuminating the established assay to be suitable only for homogenous organs. Thus, for murine liver tissue sufficient intra- and interday repeatability was shown during validation. In further experiments, the established assay was applied to an animal study comprising young and old (24 and 110 weeks) mice which showed that DNA ligation efficiency was affected by neither sex nor age. Finally, the impact of in vitro addition of the trace elements copper, iron, and zinc on DNA ligation in tissue extracts was investigated. While all three metals inhibited DNA ligation, variations in their potency became evident. In conclusion, the established method can be reliably used for investigation of DNA ligation efficiency in homogenous murine tissues.
Abbreviations
-
- APC
-
- aphidicholin
-
- ATP
-
- adenosine 5′-triphosphate
-
- BCA
-
- bicinchoninic acid
-
- BER
-
- base excision repair
-
- BSA
-
- bovine serum albumin
-
- Cu
-
- copper
-
- DTT
-
- dithiothreitol
-
- EDTA
-
- ethylenediaminetetraacetate
-
- Fe
-
- iron
-
- ICP-MS/MS
-
- inductively coupled plasma-tandem mass spectrometry
-
- Mg
-
- magnesium
-
- Mn
-
- manganese
-
- PMSF
-
- phenylmethanesulfonyl fluoride
-
- ROS
-
- reactive oxygen species
-
- RSD
-
- relative standard deviation
-
- Se
-
- selenium
-
- SEM
-
- standard error of mean
-
- XRCC
-
- x-ray cross-complementing protein
-
- Zn
-
- zinc
1 INTRODUCTION
Located in an aqueous and oxygen-rich environment, due to its chemical structure the DNA is exposed to a variety of threats to its integrity. Under physiological conditions, these include damage by hydrolytic cleavage, hydrolytic deamination, alkylation or oxidation catalyzed by reactive oxygen species (ROS; Lindahl, 1993). Fortunately, cells are equipped with a well-functioning machinery of DNA damage response and adequate DNA repair pathways to handle these damages and manage genomic stability (Carusillo & Mussolino, 2020). All DNA repair pathways comprise a complex network of cooperating proteins but each one is finalized by DNA ligation. This process of repairing nicks in the phosphodiester backbone is catalyzed by the DNA ligase family, which consists of three functional isoforms. While DNA ligase I and IV are found exclusively in the nucleus, ligase III can also be expressed as polypeptide able to translocate into mitochondria (Sallmyr et al., 2020). Besides, the three ligases predominantly participate in different repair pathways, even though significant functional overlap allows for compensation (Le Chalony et al., 2012). Ligase I is predominantly responsible for joining Okazaki fragments during DNA replication but also plays an important role for DNA repair. While ligase III, in complex with x-ray cross-complementing protein 1 (XRCC1) completes the short-patch base excision repair (BER), ligase I performs the final DNA ligation step in long-patch BER. Also in nucleotide excision repair, both ligases I and III are involved, depending on cell cycle stage (Kemp, 2019; Spivak, 2015). For double-strand break repair, especially nonhomologous end-joining, ligase IV in complex with XRCC4 enables repair (Clarke & Mostoslavsky, 2022). These specializations are determined by variations of binding motifs in C- and N-terminal regions which also cause the ligases to partner with different proteins. However, the catalytic core and therefore ligation mechanism is shared by all eukaryotic DNA ligases (Tomkinson & Della-Maria, 2013). Condensed, in the first step a covalent enzyme-AMP intermediate is formed upon reaction with adenosine 5′-triphosphate (ATP) which allows recognition of nicked DNA. AMP is then transferred to the 5′-phosphate terminus of the DNA strand which can finally be esterized to the 3′-hydroxyl group by nucleophilic attack (Tomkinson & Della-Maria, 2013). This reaction is not only dependent on ATP, but also on magnesium ions (Mg2+) which are essential for both, the adenylyl transfer as well as nick-sealing reaction (Taylor et al., 2011).
As essential components of genomic stability maintenance, DNA ligases and how they are affected by various parameters still hold much research potential. So far, there are already methods in place to determine their activity. Most of them involve oligonucleotides containing radioactively labeled phosphorus (Audebert et al., 2004; Chen & Yu, 2019; Parsons & Dianov, 2012; Zhao et al., 2020). However, as will be introduced in this article for the first time, also fluorescently labeled oligonucleotides may be suitable as a substrate for DNA ligases in murine tissue extracts which obviously improves handling safety as well as reduces need for specialized detection. The present assay is based on the previously published method adopted to assess incision activity towards certain DNA lesions repaired by base excision repair in mouse liver extracts (Winkelbeiner et al., 2020). After adoption, the method was validated in isolated tissue extracts. Further, the impact of selected trace elements on DNA ligation activity has been investigated.
2 MATERIALS AND METHODS
2.1 Material
Acrylamide solution, ammonium persulfate, boric acid, disodium ethylenediaminetetraacetate (EDTA), glycerol, N,N,N′,N′-tetramethylethylenediamine, sodium chloride (NaCl), Tris-(hydroxymethyl)-amino methane (Tris), Tris hydrochloric acid (Tris–HCl) and urea were obtained from Roth (Karlsruhe, Germany). Acetylated bovine serum albumin (BSA), ATP, Amicon ultra-0.5 centrifugal filter units (10 kDa), aphidicholin (APC), aprotinin, bicinchoninic acid (BCA) solution, blue dextran, dithiothreitol (DTT), formamide, hydrogen peroxide (H2O2), iron(II) chloride tetrahydrate (FeCl2), leupeptin, magnesium chloride hexahydrate (MgCl2), nitric acid (HNO3, 65% Suprapur®), pepstatin, phenylmethanesulfonyl fluoride (PMSF), sodium fluoride (NaF), sodium orthovanadate (Na3VO4), and zinc chloride (ZnCl2) were obtained from Sigma/Merck (Darmstadt, Germany). For inductively coupled plasma-tandem mass spectrometry (ICP-MS/MS) analysis certified reference material from the Joint Research Centre, European Commission was used. Fluorescently labeled oligonucleotides were obtained from Eurogentech (Seraing, Belgium).
2.2 Preparation of protein extracts
Aliquotation was performed on dry ice to prevent tissue from thawing. Additionally, liver tissue was homogenized in a mortar under liquid nitrogen. 15–30 mg (cerebellum) or 25–50 mg (liver) tissue were subjected to bead beating with around 30 beads (Bead Ruptor 12, zirconium beads, both biolabproducts, Bebensee, Germany) in 20 μL cold extraction buffer per mg tissue. As previously published (Winkelbeiner et al., 2020), extraction buffer contained 20% glycerol, 250 mM NaCl, 50 mM NaF, 50 mM Tris–HCl (pH 7.1), 1 mM EDTA, 1 mM Na3VO4, 0.5 mM DTT, 0.5 mM PMSF, 1.5 μM APC, 10 μg/mL aprotinin, 5 μg/mL leupeptin and 1 μg/mL pepstatin and was prepared freshly before use. Following three cycles of bead beating, extracts were centrifuged at 15,000 ×g and 4°C for 20 min. Supernatants were collected and centrifuged again for purification with the same parameters. Afterwards, the tissue extracts were placed on 10 kDa molecuar weight cut-off filters and centrifuged at 15,000 ×g and 4°C for 60 min. Finally, remaining extracts were collected by inverting filter units and another centrifugation at 1200 ×g and 4°C for 6 min. Protein concentrations were determined by BCA protein assay and protein extracts stored at −80°C.
2.3 DNA ligation assay
The DNA ligation assay is based on two compatible Cy5-labeled oligonucleotides. Their sequences are similar to a previously published method to assess incision activity towards certain DNA lesions (Winkelbeiner et al., 2020). Additionally, to ensure function of DNA ligases, a 5′-phosphate group was added, which resulted in the following base sequences:
5′-PO43−-CGA-CCA-GTC-CTG-CTT-TTG-CAG-GAC-TGG-TCG-CGC-GTG-TTA-TTA-TTG-Cy5-3′ and 5′-CAA-TAA-TAA-CAC-GCG-3′.
The oligonucleotides were combined in hybridization buffer containing 100 mM Tris–HCl (pH 8), 50 mM NaCl, and 10 mM EDTA and hybridized so that a stable double-strand hairpin structure was formed. This was achieved by heating to 90°C for 15 min and afterwards allowing the solution to slowly cool down to 30°C before storage at −20°C. This process allows annealing of the smaller oligonucleotide so that a hairpin structure is formed. That is enabled by four thymidine nucleosides in the oligonucleotide sequence which do not pair with each other. Compared to duplex oligonucleotides, hairpin configurations were shown to be more stable in face of non-specific decomposition (Hamann et al., 2009).
The 50 fmol of those two hybridized oligonucleotides were combined with 50 mM Tris, 50 mM NaCl, 10 mM MgCl2, 1 mM ATP, 0.1 μg/μL acetylated BSA and tissue extracts to desired protein concentrations in a total volume of 10 μL and incubated at 37°C and 250 rpm for 60 min. ATP and Mg concentrations were derived from literature research (Chen & Yu, 2019; Parsons & Dianov, 2012). In case of metal inhibition experiments, extracts were incubated at 25°C and 250 rpm for 30 min prior to addition of oligonucleotides. Subsequently, the ligation reaction was stopped by addition of 10 μL denaturation buffer (90% formamide, 1 mM EDTA, 0.5% blue dextran) and heating to 95°C at 250 rpm for 5 min. Finally, samples were subjected to polyacrylamide gel electrophoresis (20% polyacrylamide gel [7 M urea, 89 mM Tris-borate, 2 mM EDTA]) for 3 h at 15 W per gel in order to separate repaired from nicked oligonucleotide structures. For detection and quantification, a Chemidoc MP imaging system and appropriate software (Image Lab [both Biorad, München, Germany]) was used. Sample preparation and measurements were carried out in technical duplicates (establishment, metal inhibition) or triplicates (animal experiment). A schematic workflow illustration can be found in Figure 1.
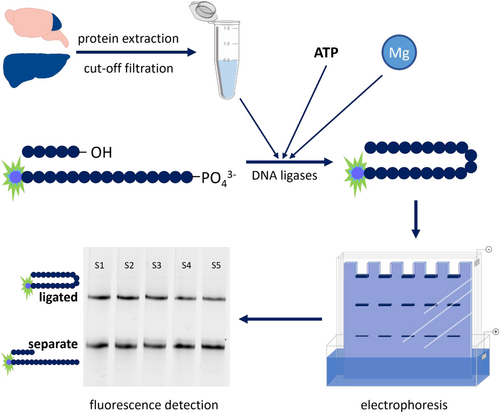
2.4 Animal experiments
Assay establishment was conducted in cerebella of healthy untreated wild-type C57BL/6Jrj mice of both sexes aged between 9 and 76 weeks. For application of the DNA ligation assay, homogenized liver tissue of male and female adults (24 weeks) and old wild-type C57BL/6Jrj mice (109–114 weeks) from a previously published experiment were used (Lossow et al., 2020). Animals were housed on a quotidian 12:12-h light/dark cycle and supplied with a commercially available chow diet (V1534, Ssniff, Soest, Germany) and water ad libitum. Anesthetization was performed with isofluorane (Cp-pharma, Burgdorf, Germany) and tissues were immediately snap-frozen in liquid nitrogen after extraction and stored at −80°C until use. All animal procedures were approved and conducted following national guidelines of the Ministry of Environment, Health and Consumer Protection of the federal state of Brandenburg (Germany, 2347-44-2017) and institutional guidelines of the German Institute of Human Nutrition Potsdam-Rehbruecke.
2.5 Quantification of trace elements in tissue extracts
For analysis of trace element concentrations by ICP-MS/MS, 50 μL of cerebellar tissue extract were subjected to microwave-assisted acid digestion in polytetrafluoroethylene (PTFE) microwave vessels using 100 μL of HNO3 and H2O2, each, as well as 20 μL of a solution containing 500 μg/L Ge and 50 μg/L Rh as internal standards in a total volume of 1 mL. After digestion, extracts were directly analyzed via ICP-MS/MS (8800 ICP-QQQ-MS, Agilent Technologies, Waldbronn, Germany) with the following working parameters: 1550 W RF power, plasma gas flow of 15 L/min, make-up gas flow of 0.23 L/min, Ni-cones, MicroMist nebulizer with an Argon flow of 1.00 L/min, Scott-type spray chamber cooled to 2°C. The following mass-to-charge ratios (m/z) were separated (Q1 → Q3) using a He flow of 3 mL/min in the collision reaction cell (CRC) for kinetic energy discrimination: Mn (55 → 55), Fe (56 → 56); Cu (63 → 63) and Zn (66 → 66). Calibration ranges were Mn: 0.02–15 μg/L; Fe, Zn: 0.4–300 μg/L and Cu: 0.1–75 μg/L. Quality of results was assured by EU certified reference material ERM®-BB 422 (fish muscle) recovery. Limit of detection and quantification (LOD and LOQ, respectively) were calculated based on the average concentration of at least four technical blanks +3σ for LOD or + 10σ for LOQ.
3 RESULTS AND DISCUSSION
3.1 Assay Description
Representing the final step of every major DNA repair pathway, information on DNA ligation specifically, as an alternative to whole DNA repair pathway analyses, might provide valuable insights for the research field of genomic stability. Past investigations of mammalian DNA ligases all involved substrates labelled with radioactive phosphorous isotopes 32P (Audebert et al., 2004; Chen & Yu, 2019; Parsons & Dianov, 2012; Zhao et al., 2020). With current advances in molecular sciences however, handling of radioactive substances with ensuing safety concerns, increased requirements for personal and laboratory equipment, is no longer necessary. That is why this publication presents a method for quantification of DNA ligation efficiency in murine tissues established based on fluorescently labelled oligonucleotides.
Protein extracts are prepared from murine tissues and filtered through 10 kDa molecular weight cut-off filters. In the presence of cofactors ATP and Mg the obtained extracts are incubated with hairpin-structured oligonucleotides, designed with a nick in their DNA backbone. This enables the DNA ligases contained in tissue extracts to repair that nick. In a subsequent denaturing electrophoresis ligated oligonucleotides are separated from unrepaired ones and can be easily visualized because of their fluorescent labelling. From the ratio of ligated to unrepaired oligonucleotides DNA ligation efficiency can be deduced. A schematic illustration of the assay principle can be found in Figure 1.
3.2 Assay establishment
While the brain in general is of increasing interest in DNA repair syndromes, the cerebellum seems to be especially vulnerable to impaired BER and single-strand break repair (Li et al., 2022; Narciso et al., 2016). Therefore, the nonradioactive assay for determination of DNA ligation efficiency in murine tissue samples was first established in cerebellum tissue. It is based on a cleavage assay originally established in peripheral blood mononuclear and A549 cells by Hamann et al. (2009). A549 cell extracts were concentrated using cellulose membrane filter units with a cutoff at 10 kDa. Therefore, ligation efficiencies in untreated cerebellar extracts were compared with extracts purified and concentrated by similar centrifugal filter units. Since potentially inhibiting components of the tissue extracts were not identified, molecular weight cut-offs at 10 and 30 kDa were tested. It was shown that indeed, ligation occurred at highest efficiencies in cerebellum tissue as well when 10 kDa cutoff filters were employed (Figure 2a).
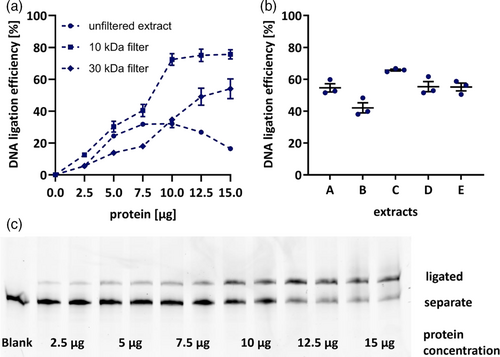
Subsequently, titration of applied protein concentrations was performed, aiming for optimal method sensitivity to changes in ligation efficiency. A representative polyacrylamide gel is depicted in Figure 2c. For cerebellar tissue extracts 7.5 μg of protein were chosen to ensure detection of inhibition as well as induction of DNA ligation capacities (Figure 2a). Saturation of DNA ligases was observed at concentrations of 10 μg and higher in filtered extracts, possibly due to substrate availability, while in unfiltered extracts even decreases were observed for high protein concentrations (Figure 2a). This might be a result of non-specific inhibition, exerted by unidentified factors which are also applied in higher concentrations with application of more protein. Apparently, this effect is halted by filtration. (Figure 2b). To assess method reproducibility, ligation efficiency in five protein extracts was measured on three separate days which yielded consistent results with deviations of 2%–10% (Figure 2c).
3.3 Trace elements and DNA ligation
It is well known that a balanced trace element homeostasis is crucial for a variety of processes in functioning organisms, including DNA repair. Several trace elements, such as copper (Cu), manganese (Mn), selenium (Se), and zinc (Zn) contribute to preservation of genomic integrity by being part of antioxidant enzymes, due to anti-inflammatory properties or by direct interaction with DNA repair proteins (Hosseinimehr, 2015; Wandt et al., 2021). However, under conditions of impaired homeostasis, overloads may arise which can be detrimental due to chemical properties of the respective elements. This is especially true for the metallic trace elements Cu, iron (Fe), and Zn. When exceeding physiological concentrations and not adequately sequestered by binding proteins, Cu and Fe are prone to increase the cellular burden of oxidative stress due to ROS formation. Additionally, all three metals were shown to directly affect multiple enzymes of DNA repair (Li et al., 2009; Wandt et al., 2021). Therefore, the present assay was applied to assess the effects of Cu, Fe, and Zn on DNA ligation. In order to put added concentrations into context, the amounts of Cu, Fe, Mn, and Zn in tissue extracts were measured by ICP-MS/MS and can be found in Table 1. It could be shown that all three metals, Cu, Fe and Zn, inhibit DNA ligation efficiency when added prior to substrate incubation, although to different extents. Employing the established assay, it could be shown, that Cu concentrations of about 9.5 μM, corresponding to 0.88 ng Cu/μg protein, reduce DNA ligation efficiency to about 50% (Figure 3a). Fe and Zn on the other hand, both inhibit DNA ligation to 50% efficiency at molar concentrations of around 85 μM (Figure 3b,c), which corresponds to 6.33 ng Fe/μg protein and 7.41 ng Zn/μg protein, respectively. A representative polyacrylamide gel visualizing inhibition of DNA ligation efficiency by Zn is shown in Figure 3d. In relation to basal concentrations in protein extracts large variations in inhibition potency are revealed. For Fe, a 23-fold increase prevents 50% of DNA ligation whereas a 59-fold increase is necessary for Zn. Cu contrarily already exerts a reduction of 50% DNA ligation efficiency when added in concentrations exceeding basal levels about 11 times.
| Mn | Fe | Cu | Zn | |
|---|---|---|---|---|
| Concentration [ng/mg tissue] | 0.146 ± 0.009 | 10.18 ± 0.63 | 2.66 ± 0.21 | 4.70 ± 0.29 |
| Concentration [ng/μg protein] | 0.004 ± 0.001 | 0.271 ± 0.011 | 0.071 ± 0.003 | 0.125 ± 0.002 |
| IC50 [μM] | 85 | 9.5 | 85 | |
| IC50 [ng/μg protein] | - | 7.41 | 0.805 | 6.33 |
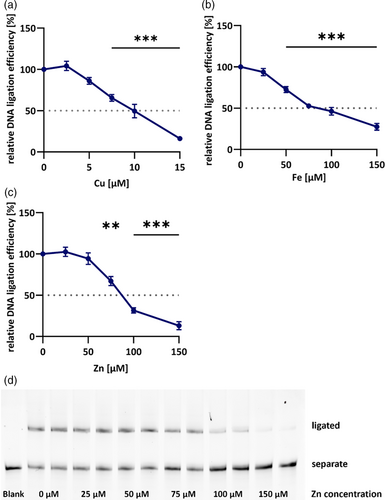
These inhibitory properties have already been shown for Fe and Zn before, even though at different molar concentrations (Hegde et al., 2010; Li et al., 2009). Unfortunately, the method description by Li et al. does not allow for direct comparison with present results. Inhibition by Zn seems to be common for a variety of enzymes in general and likely functions as regulatory mechanism (Maret, 2013). One obvious explanation for the strong inhibition of DNA ligation by Cu and Fe is the formation of free radicals if not sufficiently buffered. Those are prone to damage cellular macromolecules, including proteins, which might disrupt DNA ligase function. However, Li et al. (2009) showed that the generation of oxidative stress cannot be the sole reason for the observed effect due to the lack of amelioration observed after addition of radical scavenging substances. Furthermore, Zn is not redox-active, indicating specific protein damage impairing catalytic function. One possible mode of action could be the replacement of Mg2+ in the active center of DNA ligases. Even though Mg2+ is added in excess to the experimental system, Zn2+ is known to replace the cofactor due to its higher affinity for binding pockets of proteins (Foster et al., 2014). This preference is based on the Irving-Williams series, according to which also Cu2+ and Fe2+ show higher preferences to bind proteins compared with Mg2+ (Irving & Williams, 1948). In a physiological environment the effect of this series is controlled by several metalloproteins, sequestering excess metals and keeping concentrations of free metals in strictly regulated limits. However, in this experimental setup, metals were added in vitro to the tissue extracts, prohibiting cellular adaptation to elevated metal contents. Apart from occupying the active center, another possible mechanism explaining the strong inhibitory character of Cu might be its interaction with Zn finger domains which DNA ligase III possesses (Tomkinson & Della-Maria, 2013). It has already been shown for a variety of proteins that despite their high Zn specificity, Cu is thermodynamically favored in Zn finger domains due to its affinity to thiol groups of cysteines (Doku et al., 2013; Dragone et al., 2022; Shimberg et al., 2017; Yuan et al., 2017). Although results regarding domain configuration and protein folding differ, all literature detected impairments in DNA binding or recognition upon Zn displacement by Cu (Doku et al., 2013; Dragone et al., 2022; Shimberg et al., 2017; Yuan et al., 2017). So far, this has not been observed for DNA ligases but for other DNA repair proteins, such as XPA which is essential for DNA damage recognition in nucleotide excision repair (Asmuss et al., 2000).
Even though concentrations required to inhibit 50% of DNA ligation exceed physiological levels by far, metal inhibition studies might still prove useful in the context of pathophysiological conditions, such as neurodegenerative diseases where disturbed homeostases of Cu, Fe, and Zn are common (Górska et al., 2023).
The example of Alzheimer's disease, one of the currently most common neurodegenerative diseases, shows not only absolute concentrations but also distribution of elements to be altered in affected brains. Fe concentrations are increased to varying extents in different brain areas of Alzheimer's disease patients and correlate with cognitive decline (Ayton et al., 2020). For Cu on the other hand, absolute levels decrease up to 18 % compared to healthy controls. Instead, labile Cu concentrations are increased up to 25 % in patients, indicating possible involvement of oxidative stress (James et al., 2012). Similarly, also for Zn conclusions cannot be drawn from absolute element levels alone, which can be increased up to 30 % in certain regions of diseased brains, but rather from its association and accumulation within amyloid plaques (Danscher et al., 1997; Miller et al., 2006).
In the present study inhibition of DNA ligation by trace elements was investigated in cerebellum tissue and related to its physiological element contents. For other tissues, fold changes of applied amounts necessary to inhibit DNA ligation might vary due to differences in inherent trace element concentrations. While Cu concentrations range similar in cerebellar and hepatic tissue of adult male mice, higher concentrations of Fe and Zn are found in liver tissue, possibly affecting sensitivity of DNA ligation to variations in trace element status (Friese et al., 2024; Wandt, Winkelbeiner, Lossow, et al., 2021).
3.4 Tissue limitations
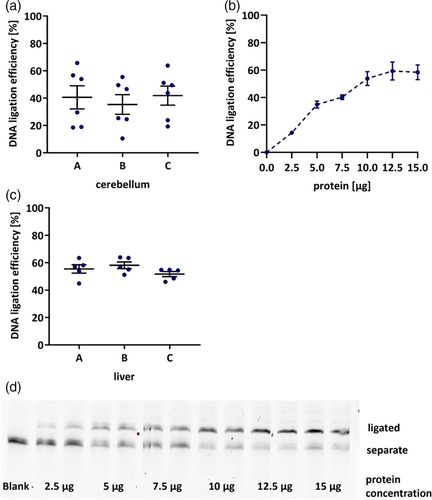
For further validation of method precision and repeatability, intra- and interday variability was aimed to be determined. Accordingly, the cerebellum of three animals was aliquoted and extracts were prepared from each piece. While repeated measurements of one extract yielded stable results (Figure 2c), large variations in ligation efficiency were observed between extracts of different pieces (Figure 2d). This might be based on the particular anatomical structure of the cerebellum, as there are for example, strong differences between gray and white matter in addition to the characteristic layering. Both affect the distribution of cell soma where most metabolic processes as well as DNA repair take place (Geden et al., 2021). Consequently, cerebellum tissue might need to be homogenized for robust assessment of DNA ligation efficiency. However, due to the small tissue volume in mouse experiments and depending on the requirements of other endpoints performed in the respective tissue, this might not be feasible in every experiment.
Representing an additional indispensable organ for research on DNA repair, in further experiments hepatic tissue was investigated. As widely known, its relevance in the field of genomic stability research is mainly based on the physiological location of the liver as primary entry site for compounds absorbed from food into systemic circulation, and finally its metabolic capacity. In contrast, for liver tissue these limitations do not apply. Homogenization can be easily performed in a mortar under liquid nitrogen. After filtration through 10 kDa cutoff filters ligation efficiencies range slightly lower than those observed in cerebellum (Figure 2e). So far, DNA ligation capacity in liver and brain tissue has not been compared, yet. Moderately higher rates in cerebellum tissue of about 15 % might be explained by the postmitotic status of neurons, emphasising the need for successful DNA repair, whereas hepatocytes could rely more on cell renewal due enabled by high cell turnover. A representative polyacrylamide gel image illustrating dependency of DNA ligation efficiency on protein content can be found in Figure 4d. In accordance with ICH guidelines on bioanalytical method validation (European Medicines Agency, 2022), repeated extract preparation and measurements from three mice yielded intraday variations of about 13% while interday measurements varied around 14% (Table 2).
| Liver 1 | Liver 2 | Liver 3 | |
|---|---|---|---|
| Intraday variation (RSD) | 15.4% | 15.1% | 7.6% |
| Interday variation (RSD) | 12.2% | 18.8% | 10.7% |
3.5 Assay application
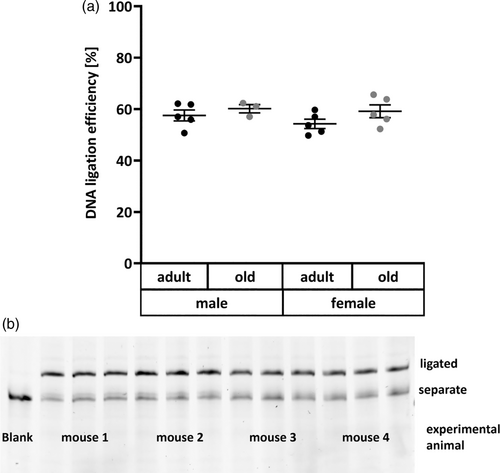
After confirming its validity in liver tissue, the DNA ligation assay was applied to a mouse experiment comprising 10 adult and 8 old untreated animals of both sexes. As several DNA repair pathways, especially BER, are reported to be downregulated with age (Lebel et al., 2011), contrary to protein concentrations used for assay establishment, 10 μg of protein were employed here to maximize sensitivity for detection of decreased ligation efficiency. However, this hypothesis was not confirmed in the present experiment. Neither age nor sex showed significant impact on DNA ligation efficiency in the liver (Figure 4a). Those relatively constant levels of DNA ligation efficiency are also represented by an exemplary polyacrylamide gel image in Figure 5b. So far, it has not been explicitly studied how activities of DNA ligases are affected by the aging process. However, opposing results exist regarding the impact of aging on genomic stability, as extensively reviewed by Lebel et al. (2011). While research on DNA damage and DNA repair in relation to age has diminished in the last years, in accordance with the “Hallmarks of Aging” (López-Otín et al., 2013), several studies have reported accumulation of DNA lesions and decreased BER repair capacity with age (Cabelof et al., 2002; Intano et al., 2003; Mikkelsen et al., 2009; Zahn et al., 2000). Contrary to those results, other animal experiments yielded no substantial differences in DNA strand break levels (Wandt et al., 2021; Winkelbeiner et al., 2020), 8-oxoguanine concentrations (Anson et al., 1999; Wandt et al., 2021) or protein levels of DNA ligases I and III (Intano et al., 2003) between liver tissue of young and old mice. Similarly, also BER incision activity, both nonspecific as well as towards 8-oxoguanine, is also reported as unchanged with age (De Souza-Pinto et al., 2001; Wandt et al., 2021; Winkelbeiner et al., 2020) or even increased with age (Gorniak et al., 2013). This list highlights the large discrepancies existing in available data and the need for further investigation of the impact of aging on genomic stability employing suitable methods.
-
3.6 Assay limitations
While efficiently assessing total DNA ligation based on substrate repair, this assay is not suitable for specifically determining activity of isolated DNA ligase isoforms. Nevertheless, due to the oligonucleotide design which resembles a BER intermediate, the assay is quite likely to rather represent ligase I and III activity than ligase IV which is predominantly involved in double-strand break repair (Sallmyr et al., 2020). Furthermore, even though essential cofactors such as ATP and Mg were added to the reaction mix, DNA ligases do not function independently, but rather are part of a complex machinery. This means that also other proteins which are not controlled in this assay setup may limit the DNA ligation outcome. Therefore, the established methods should be regarded as assessing ligating activity of tissue extracts rather than specific ligation activity.
4 CONCLUSION
It could be shown that the present method based on fluorescently labeled oligonucleotides is suitable for determination of DNA ligation efficiency in homogenized murine liver tissue. Cu, Fe and Zn in concentrations surpassing physiological amounts were revealed to inhibit DNA ligation. However, neither age nor sex seem to impact DNA ligation activity in mouse liver.
AUTHOR CONTRIBUTIONS
SF was responsible for most of data curation, formal analysis, and writing of the original draft. TH contributed by performing ICP-MS/MS analyses. FE provided aid with assay setup and troubleshooting. TS contributed by project administration and funding acquisition. All authors supported writing, review, and editing.
ACKNOWLEDGMENTS
The authors would like to thank Christiane Ott (German Institute for Human Nutrition) for enable assay establishment by providing access to murine tissues. Further, the authors would like to thank the German Research Foundation (DFG) research unit TraceAge (FOR 2558) for financial support. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




