Conditioned pain modulation and pain sensitivity in functional somatic disorders: The DanFunD study
Funding information
This work was supported by grants from the Lundbeck Foundation [grant number R155-2013-14070] and TrygFonden [grant number 7-11-0213]. TG-N is a part of the Center for Neuroplasticity and Pain (CNAP) supported by the Danish National Research Foundation (DNRF121).
Abstract
Background
Disrupted pain regulation has been proposed as a component in functional somatic disorders (FSD). The objective of this study was to examine a general population sample, encompassing three delimitations of FSD while assessing pain sensitivity and conditioning pain modulation (CPM).
Methods
Pressure pain thresholds (PPTs) at the tibialis and trapezius muscles were recorded at baseline. During cold pressor stimulation of the hand, the tibialis PPTs were re-assessed and the difference from baseline measures defined the CPM effect. Participants (n = 2,198, 53% females) were randomly selected from the adult Danish population. FSD was established by self-reported symptom questionnaires.
Results
With a few exceptions, only weak associations were seen between PPTs and CPM in cases with FSD (p > .1). A high PPT was associated with lower odds of having multi-organ bodily distress syndrome (ORPPT trapezius: 0.66, 95% CI: 0.49–0.88, p = .005), with the symptom profile characterized by all symptoms (ORPPT trapezius: 0.72, 95% CI: 0.58–0.90, p = .003 and ORPPT tibialis: 0.75, 95% CI: 0.62–0.91, p = .004), and with multiple chemical sensitivity (ORPPT trapezius: 0.81, 95% CI: 0.67–0.97, p = .022). High CPM was associated with high odds of having irritable bowel (ORCPM relative: 1.22, 95% CI: 1.04–1.43, p = .013 and ORCPM absolute = 2.66, 95% CI: 1.07–6.45, p = .033).
Conclusion
However, only PPT measured over the trapezius muscle were still significant after correction for multiple testing for the symptom profile characterized by all symptoms. Findings from this study do not support altered pain regulation in questionnaire-based FSD which is in contrast with the existing presumption. Further epidemiological studies in this field are needed.
Significance
Disrupted pain regulation as measured by abnormal pain thresholds has been hypothesized as a central mechanism in Functional Somatic Disorders (FSD). The hypothesis has been raised in clinical setting where patients presented subjective and objective features of hypersensitivity. The present population-based study does not support this notion. This points to the importance of further studies into the underlying pathophysiology mechanisms of FSD.
1 INTRODUCTION
Functional somatic disorders (FSD) are frequent in all medical settings and characterized by persistent physical symptoms that cannot be explained by other somatic or psychiatric conditions (Burton et al., 2020). Research into the pathophysiological mechanisms is challenged by the very nature of these disorders, with symptoms that tend to cluster across multiple organ systems. Pain is a frequent symptom and is included in the delimitation of most FSD, which has made the study of pain an obvious target in the pursuit of a better understanding of the mechanisms triggering these conditions (Bourke et al., 2015; den Boer et al., 2019). A narrative review including a broad range of studies suggests that abnormal pain regulation within the central nervous system may be contributing to the unexplained pain in FSD (Bourke et al., 2015).
Pain thresholds to, for example heat or pressure can be used to assess increased pain sensitivity (Arendt-Nielsen, 2015; Fischer, 1987; Woolf, 2011). In general, studies have shown hypersensitivity and lower pain thresholds in FSD; however, most studies have been performed in selected groups of patients with the majority of studies including participants fulfilling the criteria for fibromyalgia (FM) (Desmeules et al., 2003; Gormsen et al., 2012; Jespersen et al., 2007; Petzke et al., 2003), irritable bowel syndrome (IBS) (Jarrett et al., 2016; Piché et al., 2010; Stabell et al., 2013; Zhou et al., 2010; Zhou et al., 2010), and to a lesser extent whiplash-associated disorders (WAD) (Curatolo et al., 2001; Ng et al., 2014), chronic fatigue syndrome (CFS) (Meeus, Nijs et al., 2010; Meeus, Roussel et al., 2010; Nijs et al., 2012), multiple chemical sensitivity (MCS) (Tran et al., 2013), and multiple FSD (Gerhardt et al., 2017).
The conditioned pain modulation (CPM) paradigm is considered a reliable measure of descending pain modulatory system efficacy (Fernandes et al., 2019; Lewis et al., 2012; Petersen, McPhee et al., 2019; Yarnitsky et al., 2010, 2015). CPM assessment involves the application of a conditioning pain stimulus to one extremity with simultaneous assessment of pain sensitivity at another extremity using a test stimulus (Kennedy et al., 2016; Yarnitsky et al., 2010, 2015). CPM has been assessed in FSD, with the majority of studies including participants fulfilling the criteria for FM (Gerhardt et al., 2017; Lannersten & Kosek, 2010; O'Brien et al., 2018; Potvin & Marchand, 2016) and IBS (Albusoda et al., 2018; Jarrett et al., 2016), and to a lesser extent in WAD (Daenen et al., 2013; Ng et al., 2014) and CFS (Meeus et al., 2008, 2015), Hence, decreased CPM has overall been demonstrated across FSD. However, most CPM studies are based on small highly selected groups (i.e. <100) applying different methodologies, and many studies have only included female participants, which induces a great risk of selection bias. Hence, these methodological shortcomings call for a replication of existing findings.
The objective of this large population-based study was to examine a large random sample of the adult general population, encompassing three different delimitations of FSD while assessing pain sensitivity and CPM. It was hypothesised that cases of various FSD would have altered PPTs and CPM compared with controls.
2 METHODS
2.1 Study population
The present study is based on the Danish Study of Functional Disorders (DanFunD), a large population-based cohort, initiated to unravel the epidemiology of FSD and the world's first larger coordinated epidemiological study focusing exclusively on FSD (Dantoft et al., 2017).
All invited individuals were randomly drawn from the Danish Civil Registration system. Inclusion criteria were age 18–71 years and living in 10 municipalities in the south-western part of suburban Copenhagen. Exclusion criteria: not born in Denmark or not being a Danish citizen and pregnancy. The sub-study on pain was initiated in November 2012, and pain assessment was terminated by the end of 2013. Altogether 7,942 persons were invited and 2,198 participated (27.7%). Participants were fasting at the time of testing, that is no food or drinks after 11 p.m. prior to the day of testing. If a participant was scheduled for a time after 12.30 p.m., a small meal no later than 6 hr prior to testing was allowed. Demographic and questionnaire data were collected before testing. Information about the use of pain medication, both prescribed and over-the-counter drugs, was registered on the day of testing. The study was approved by the Ethical Committee of Copenhagen County (H-3-2012-015) and all participants gave written informed consent. The study was performed according to the principles of the Helsinki Declaration.
2.2 Assessment of pain
Participants were asked to lie down on a bed in a quiet room with the head elevated. Verbal information about the pain testing procedure was provided by the member of the staff performing the test. The experimental pain test procedure was as follows: (a) PPTs were assessed with a pressure algometer (SBMEDIC type II; Somedic Senselab AB) over the tibialis anterior muscle 10 cm distal to the apex patellae on the non-dominant side and over the upper trapezius muscle 10 cm from the acromion in direct line with the neck at the non-dominant side, (b) 2 min of cold pressor stimulation to the non-dominant hand in a circulating water bath (maximum 3°C), and (c) PPTs were re-assessed over the tibialis anterior muscle during the cold pressor test. There were 20 s between each PPT assessment. The mean value of three PPTs recordings defined the final PPT. Changes in PPTs from baseline to reassessments during the conditioning cold pressor stimulation were considered to reflect the CPM effect (Yarnitsky et al., 2010, 2015).
2.3 Case definitions of FSD
All case definitions were based on self-reported symptom questionnaires with symptoms specific for defining a range of various FSD (Dantoft et al., 2017). The questionnaires assessed symptoms experienced within 12 months prior to answering the questionnaires. We applied post hoc diagnostic criteria on the symptom questionnaires for defining the FSD. Previous research has repeatedly suggested that the delimitations of the various FSD are inconsistent; several diagnostic criteria have been used for defining each of them, and most of these are consensus-based and present with a huge symptom overlap. This has led some researchers to propose that the huge range of different FSD diagnoses are manifestations of the same condition or a family of closely related conditions rather than being different entities (Burton et al., 2020; Wessely et al., 1999; Wessely & White, 2004). In this study, FSD are therefore approached with three different delimitations, all based on frequent and bothersome symptoms. The delimitations included both the mono- and multi-systemic types of FSD, enabling studies of associations with the various pain measures within different theoretical approaches (Petersen, Ørnbøl et al., 2021; Petersen., Schröder et al., 2021):
The first delimitation constituted the most common groups of specialty-specific functional somatic syndromes (FSS): Irritable bowel (IB) (Kay and Jørgensen, 1996), chronic widespread pain (CWP) (White et al., 1999), chronic fatigue (CF) (Chalder et al., 1993), WAD (Kasch et al., 2008), and MCS (Dantoft et al., 2021).
The second delimitation constituted the unifying diagnosis of Bodily Distress Syndrome (BDS) (Budtz-Lilly et al., 2015). BDS constitutes a single/oligo-organ type with four subtypes (cardiopulmonary, gastrointestinal, musculoskeletal, general symptoms) and the multi-organ type.
We further identified a group of participants who did not fulfil any of the criteria of either FSS or BDS but still reported musculoskeletal pain symptoms to be either (a) 'somewhat', 'quite a bit', or 'a lot' bothering (Budtz-Lilly et al., 2015), or (b) 'often' or 'almost constantly' bothering (White et al., 1999), a 'non-FSD pain group'.
The third delimitation constituted eight data-driven symptom profiles (SP) that were developed in a previous study within DanFunD using latent class analysis (Eliasen et al., 2018). In the present study, five of the eight SPs are included: SP class one is characterized by no symptoms (used as control group in the present study), SP classes 5–7 are characterized by multiple symptoms, and SP class eight characterized by all symptoms (class 5–8 have been suggested to be those resembling manifestations of FSD (Eliasen et al., 2018; Petersen, Ørnbøl et al., 2021; Petersen., Schröder et al., 2021) and were therefore used as case delimitations in the present study).
2.4 Mental distress assessment
Eight items from the Symptom-Check-List-90-R (SCL-90-R) (Derogatis, 1992) were included as a measure of mental distress including anxiety and depression (SCL-8) (Fink et al., 2004; Fink, Ørnbøl, Huyse, et al., 2004). The Danish translation of the SCL-90-R has been psychometrically evaluated producing normative data in the Danish general population (Olsen et al., 2004).
2.5 Statistics
Statistical analyses were performed using Stata version 16.0 for Windows (StataCorp., 2019). Descriptive statistics are presented as mean and standard deviations (SD) or as medians and interquartile ranges (IQR) depending on the distribution of the continuous variables. For categorical variables, frequencies with percentages are shown.
We aimed to test whether participants with altered, that is lower levels of PPT and CMP, had higher odds of fulfilling some of the FSD case criteria. Therefore, a number of logistic regression analyses were applied including all FSD groups, the non-FSD pain group and controls as the primary outcome variables, and pain parameters (PPTs and CPM) as primary explanatory variable. Other explanatory variables which were controlled for in the analyses were (prioritized order): (a) sex, (b) age, (c) use of pain medication and (d) mental distress. As the CPM effect is relative to the baseline PPT value, baseline PPT was controlled for as the primary explanatory variable in the CPM analyses. It was necessary to make these prioritizations of the explanatory variables in order to prevent overfitting in the analyses as the number of cases in some of the primary outcome variables was low (e.g. 19 multi-organ BDS cases).
Initially, the primary (continuous) explanatory variables were modelled using restricted cubic splines with five knots at the 5th, 27.5th, 50th, 72.5th and 95th percentiles according to the recommendations by Harrell (2015) to avoid the strong assumption of a linear effect on the log odds of the outcome. We then tested whether there were any deviations from linearity using a χ2-test (p < .05) (results not shown).
Associations between the FSD groups and the pain parameters were reported as odds ratios (OR) with confidence intervals (CI). For analysis showing a linear relationship between the primary outcome and the primary explanatory variable, estimates were shown as OR comparing participants who differed 100 kPa on the PPT measures, 50 kPa on the CPM absolute measure (baseline minus during conditioning), and 20% on the CPM relative measure (baseline divided by during). These units constituted a priori defined units, established with the aim of obtaining a true difference in pain measures between cases and controls if such difference existed. Hence, the units were based on the descriptive data and on clinical experience. Analyses showing a non-linear relationship between the primary outcome and primary explanatory variable are presented as OR comparing the 10%, 25%, 50%, 75% and 90% percentiles with reference values according to a previous study in the same sample by Skovbjerg et al., (2017): PPT tibialis = 512 kPa, PPT trapezius = 446 kPa and CPM absolute = 178. As a secondary analysis, we used a dichotomized version of the CPM relative, where an increase in PPT below 20% after a cold stimulus was considered pathological. This approach was inspired by another study that, however, used another test paradigm: That study used cuff pressure pain threshold as test stimulus and a conditioning stimulus that included cuff pain where the current study used PPT as test stimulus and a cold pressor test as conditioning stimulus (Vaegter & Graven-Nielsen, 2016).
Correction of multiple testing was performed with Bonferroni correction with the critical significance level set at 0.05 and 12 tests. Therefore, a significance level ≤0.004 indicated rejection of the null hypothesis of no difference. To illustrate the effect of the continuous variables modelled by restricted cubic splines we used the user written Stata command -xbrcspline- (StataCorp., 2019).
3 RESULTS
Following the recommendations from The American Statistical Association, we avoid the words ‘significance’ in the statistical sense, and have deliberately left it out of this result section (Wasserstein et al., 2019; Yaddanapudi, 2016).
3.1 Sample characteristics
Of the 2,198 participants completing the pain tests, 53% were women, median age 53 (IQR: 43–62) years. Descriptive characteristics of the study participants are shown in Tables 1 and 2. The raw numbers suggested a tendency towards lower PPTs, higher sensitivity towards the cold pressor test, and increased VAS scores in the FSD groups than in the control groups. FSD groups also had a tendency to have a higher intake of pain medication and higher scores on mental distress. The CPM response varied across the study groups.
| Covariates |
Controlsa (n = 202) |
CWP (n = 104) |
IB (n = 66) |
MCS (n = 48) |
CF (n = 192) |
WAD (n = 33) |
Single-organ BDS (n = 314) |
Multi-organ BDS (n = 19) |
Pain groupb (n = 729) |
|---|---|---|---|---|---|---|---|---|---|
| Female; n (%) | 94 (46.2) | 77 (74.0) | 45 (68.2) | 37 (77.1) | 135 (70.3) | 19 (57.6) | 205 (65.3) | 16 (84.2) | 376 (51.6) |
| Age; median (IQR) | 52 (43–62) | 57 (50–65) | 49.5 (31–61) | 53 (47–62) | 49 (39–59) | 49 (43–56) | 55 (46–62) | 48 (40–53) | 54 (44–63) |
| PPT tibialis; mean (SD) | 566.3 (249.8) | 485.9 (247.0) | 483.4 (218.9) | 492.4 (258.0) | 492.9 (238.1) | 565.8 (326.3) | 523.73 (250.3) | 449.6 (199.2) | 543.5 (235.6) |
| PPT trapezius; mean (SD) | 522.4 (224.5) | 423.6 (215.5) | 448.1 (237.9) | 408.9 (204.2) | 430.3 (217.1) | 519.5 (263.4) | 458 (215.8) | 364 (195.2) | 474.2 (207.6) |
| CPM absolute; mean (SD) | 202.9 (154.7) | 169.9 (152.1) | 226.4 (170.3) | 171.2 (180.7) | 178.6 (157.5) | 199.0 (139.9) | 200.9 (168.1) | 162.9 (130.1) | 198.1 (157.5) |
| CPM relative; | 41.9 (35.6) | 43.7 (45.4) | 63.8 (59.3) | 40.7 (37.1) | 40.4 (36.7) | 47.9 (42.4) | 46.2 (42.5) | 39.7 (39.3) | 42.0 (34.6) |
| VAS score; mean (SD) | 6.0 (2.2) | 6.8 (2.6) | 7.1 (2.3) | 6.9 (2.1) | 7.1 (2.9) | 6.9 (2.3) | 6.8 (2.3) | 7.9 (2.1) | 6.4 (2.2) |
| Seconds cold pressor test; mean (SD) | 96.3 (31.9) | 74.2 (39.9) | 78.0 (37.9) | 81.0 (35.4) | 83.5 (35.8) | 84.4 (37.6) | 82.1 (36.7) | 70.9 (38.0) | 88.3 (34.2) |
| Pain medication; n(%) | 3 (1.5) | 11 (10.6) | 3 (4.5) | 1 (2.1) | 11 (5.7) | 1 (3.0) | 19 (6.1) | 4 (21.1) | 19 (2.6) |
| Mental distress; median (IQR) | 0 (0–2) | 3 (0–7) | 5 (2–9) | 3 (1.5–9) | 6 (2–11) | 2 (0–7) | 3 (1–8) | 11 (5–16) | 1 (0–3) |
| Work status; n (%) | |||||||||
| Employed | 145 (71.8) | 50 (48.1) | 39 (59.1) | 34 (70.8) | 117 (60.9) | 29 (87.9) | 191 (60.8) | 11 (57.9) | 497 (68.2) |
| Previously employed | 50 (24.8) | 49 (47.1) | 23 (34.9) | 13 (27.1) | 66 (34.4) | 4 (12.1) | 112 (35.7) | 8 (42.1) | 202 (27.7) |
| Never been employed | 6 (3.0) | 1 (1.0) | 4 (6.1) | 0 (0) | 6 (3.1) | 0 (0) | 6 (1.9) | 0 (0) | 15 (2.1) |
| Marital status; n (%) | |||||||||
| Married | 132 (65.4) | 80 (76.9) | 38 (57.6) | 31 (64.6) | 104 (54.2) | 25 (75.8) | 186 (59.2) | 10 (52.6) | 478 (65.6) |
| Unmarried | 47 (23.3) | 13 (12.5) | 19 (28.8) | 9 (18.8) | 64 (33.3) | 6 (24.2) | 70 (22.3) | 7 (36.8) | 148 (20.3) |
| Divorced/separated | 17 (8.4) | 6 (5.8) | 8 (12.1) | 5 (10.4) | 18 (9.4) | 0 (0) | 44 (14.0) | 1 (5.3) | 77 (10.6) |
| Widowed | 6 (3.0) | 4 (3.9) | 1 (1.5) | 3 (6.3) | 5 (2.6) | 0 (0) | 12 (3.8) | 1 (5.3) | 22 (3.0) |
Note
- Participants may fall within more categories of FSS and BDS therefore n does not equal the total sample size of 2,198.
- Abbreviations: BDS, bodily distress syndrome; CF, chronic fatigue; CPM, conditioned pain modulation; CWP, chronic widespread pain; IBS, irritable bowel syndrome; IQR, interquartile range; MCS, multiple chemical sensitivity; PPT, pressure pain threshold; SD, standard deviation; WAD, whiplash associated disorders.
- a Participants without FSS or BDS and without musculoskeletal pain symptoms qualifying for being in the pain group.
- b Participants reporting musculoskeletal pain symptoms but did not fulfil the study criteria for FSS or BDS.
| Covariates |
SP 1 (n = 1,117) |
SP 5 (n = 137) |
SP 6 (n = 75) |
SP 7 (n = 52) |
SP 8 (n = 38) |
|---|---|---|---|---|---|
| No symptoms | MS + GS | MS + GI + Fatigue | CP + GI + GS | All | |
| Female; n (%) | 502 (44.9) | 94 (68.9) | 51 (68.0) | 38 (73.1) | 29 (76.3) |
| Age; median (IQR) | 53 (43–63) | 56 (47–62) | 57 (50–66) | 48 (26.5–56) | 50.5 (44–58) |
| PPT tibialis; mean (SD) | 574.1 (246.7) | 533.2 (267.0) | 492.4 (209.8) | 501.0 (223.6) | 410.8 (208.9) |
| PPT trapezius; mean (SD) | 502.2 (217.3) | 460.5 (238.9) | 452.2 (217.9) | 442.0 (163.4) | 362.9 (223.1) |
| CPM absolute; mean (SD) | 190.3 (157.3) | 188.8 (177.6) | 189.8 (152.8) | 203.4 (196.4) | 157.6 (131.4) |
| CPM relative; | 39.4 (35.6) | 40.9 (36.4) | 46.6 (46.4) | 47.4 (47.1) | 48.6 (48.1) |
| VAS score; mean (SD) | 6.0 (2.3) | 6.9 (2.4) | 7.0 (2.2) | 6.8 (2.4) | 7.4 (2.4) |
| Seconds cold pressor test; mean (SD) | 95.1 (32.0) | 77.5 (37.3) | 76.8 (37.6) | 90.5 (33.6) | 70.8 (37.3) |
| Pain medication; n(%) | 10 (0.9) | 9 (6.6) | 4 (5.3) | 1 (1.9) | 6 (15.8) |
| Mental distress; median (IQR) | 1 (0–2) | 4 (1–8) | 3 (1–7) | 7 (3–11) | 10 (6–16) |
| Work status; n (%) | |||||
| Employed | 828 (74.1) | 82 (59.9) | 42 (56.0) | 30 (57.7) | 20 (52.6) |
| Previously employed | 257 (23.0) | 49 (35.8) | 32 (42.7) | 18 (34.6) | 17 (44.7) |
| Never been employed | 20 (1.8) | 3 (2.2) | 0 (0) | 3 (5.8) | 0 (0) |
| Marital status; n (%) | |||||
| Married | 751 (67.2) | 91 (66.4) | 52 (69.3) | 23 (44.2) | 25 (65.8) |
| Unmarried | 215 (19.3) | 25 (18.3) | 11 (14.7) | 21 (40.4) | 8 (21.1) |
| Divorced/separated | 102 (9.1) | 16 (11.7) | 10 (13.3) | 7 (13.5) | 3 (7.9) |
| Widowed | 41 (3.7) | 4 (2.9) | 2 (2.7) | 1 (1.9) | 2 (5.3) |
- Abbreviations: CP, cardiopulmonary; CPM, conditioned pain modulation; GI, gastrointestinal; GS, general symptoms; IQR, interquartile range; MS, musculoskeletal; PPT, pressure pain threshold; SD, standard deviation; SP, symptom profile.
3.2 Pressure pain thresholds
Generally, weak associations (p > .3) were found between the five FSS delimitations and the non-FSD pain group with PPT measured over both the tibialis anterior and trapezius muscles (Table 3 and Figure 1). An association was seen between MCS and PPT measured over the trapezius muscle (OR: 0.81, 95% CI: 0.67–0.97, p = .022, n = 48), that is comparing two participants differing 100 points in PPT measured over the trapezius muscle (independently of value on PPT), the participant with the highest PPT score had around 20% lower odds of fulfilling the criteria of MCS. Almost the same picture was seen for BDS, however, an association was seen between multi-organ BDS and PPT measured over the trapezius muscle. Comparing two participants differing 100 points in PPT measured over the trapezius muscle (independently of value on PPT), the participant with the highest PPT score had around 30% lower odds of fulfilling the criteria of multi-organ BDS (OR: 0.66, 95% CI: 0.49–0.88, p = .005, n = 19) (Table 3). None of the associations between the five FSS, BDS and the PPT measures were still significant after adjusting for multiple testing.
|
CWP (n = 104) |
IB (n = 66) |
MCS (n = 48) |
CF (n = 192) |
WAD (n = 33) |
Single-organ BDS (n = 314) |
Multi-organ BDS (n = 19) |
Pain groupa (n = 729) |
|
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | ||||||||
| PPT tibialis | 0.98 (0.86–1.12)b | 0.93 (0.81–1.08)c | 0.97 (0.83–1.13)d | 1.04 (0.93–1.16)b | 1.04 (0.89–1.21)e | 1.02 (0.94–1.12)b | 0.78 (0.61–1.01)f | 0.98 (0.91–1.05)b |
| PPT trapezius | Shown in Figure 1 | 0.93 (0.80–1.08)c | 0.81 (0.67–0.97)d | 0.95 (0.84–1.07)b | 1.12 (0.86–1.20)e | 0.93 (0.84–1.02)b | 0.66 (0.49–0.88)f | Shown in Figure 1 |
| CPM absolute | 0.92 (0.84–1.02)c | Shown in Figure 1 | 0.94 (0.84–1.06)e | 0.98 (0.89–1.07)b | 1.0 (0.87–1.13)f | 1.02 (0.96–1.09)b | 0.91 (0.76–1.10)f | 1.0 (0.95–1.05)b |
| CPM relative | 0.97 (0.83–1.13)b | 1.22 (1.04–1.43)d | 0.91 (0.75–1.11)e | 0.94 (0.79–1.10)b | 1.09 (0.89–1.33)f | 1.06 (0.94–1.20)b | 0.97 (0.71–1.31)f | 0.99 (0.89–1.10)b |
| <20% change in CPM PPT | 1.13 (0.57–2.24)b | 0.70 (0.30–1.65)d | 0.53 (0.19–1.45)e | 0.95 (0.49–1.83)b | 1.14 (0.45–2.85)f | 0.62 (0.36–1.07)b | 0.72 (0.45–1.16)f | 0.84 (0.56–1.27)b |
Note
- Odds ratios for differences at PPT 100, CBM absolute 50, and CPM relative 20.
- Analyses on CPM were also adjusted for baseline PPT.
- Abbreviations: BDS, bodily distress syndrome; CF, chronic fatigue; CI, confidence interval; CPM, conditioned pain modulation; CWP, chronic widespread pain; IBS, irritable bowel syndrome; MCS, multiple chemical sensitivity; OR, odds ratio; PPT, pressure pain threshold; WAD, whiplash associated disorders.
- a Participants reporting musculoskeletal pain symptoms but did not fulfil the study criteria for FSS or BDS.
- b Results adjusted for sex, age, intake of pain medication and psychological distress.
- c Because of the small n the analyses were only adjusted for sex, age and pain medication.
- d Because of the small n the analyses were only adjusted for sex and age.
- e Because of the small n the analyses were only adjusted for sex.
- f Because of the small n the shown estimates are unadjusted.
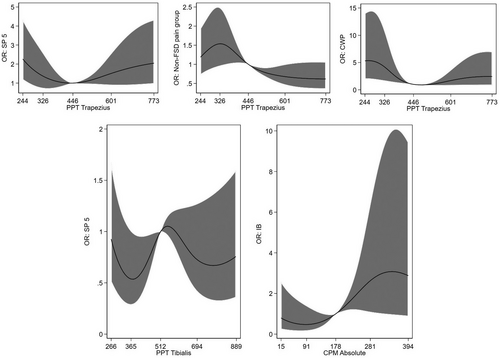
Also, for most of the SP, weak associations (p > .06) were found with PPT measured over both the tibialis and the trapezius muscle; besides from the SP 8, characterized by all symptoms. Comparing two participants differing 100 points in PPT measured over the tibialis and trapezius muscles (independently of value on PPT), the participant with the highest PPT score had 25%–30% lower odds of fulfilling the criteria for SP 8 (OR over the tibialis muscle: 0.75, 95% CI: 0.62–0.91, p = .004, and OR over the trapezius muscle: 0.72, 95% CI: 0.58–0.90, p = .003, n = 38) (Table 4). Hence, only the latter was still significant after multiple testing.
|
SP 5 (n = 137) |
SP 6 (n = 75) |
SP 7 (n = 52) |
SP 8 (n = 38) |
|
|---|---|---|---|---|
| MS + GS | MS + GI + Fatigue | CP + GI + GS | All | |
| OR (95% CI) | ||||
| PPT tibialis | Shown in Figure 1 | 0.89 (0.79–1.01)b | 1.00 (0.86–1.14)c | 0.75 (0.62–0.91)d |
| PPT trapezius | Shown in Figure 1 | 0.91 (0.79–1.03)b | 0.99 (0.85–1.15)c | 0.72 (0.58–0.90) d |
| CPM absolute | 1.04 (0.97–1.11)a | 1.01 (0.93–1.10)b | 1.03 (0.94–1.14)d | 0.93 (0.83–1.05)e |
| CPM relative | 1.04 (0.92–1.16)a | 1.08 (0.96–1.22)b | 1.09 (0.94–1.26)d | 1.13 (0.95–1.34)e |
| <20% change in CPM PPT | 0.7 (0.4–1.3)a | 0.9 (0.5–1.8)b | 0.9 (0.4–2.0)d | 0.8 (0.3–2.1)e |
Note
- Odds ratios for differences at PPT 100, CBM absolute 50, and CPM relative 20. Bold letters indicate rejection of the null hypothesis of no difference after adjusting for multiple testing (p < .004).
- Abbreviations: CI, confidence interval; CP, cardiopulmonary; CPM, conditioned pain modulation; GI, gastrointestinal; GS, general symptoms; MS, musculoskeletal; OR, odds ratio; PPT, pressure pain threshold; SP, symptom profile.
- a Adjusted for sex, age, intake of pain medication and psychological distress. Analyses on CPM were also adjusted for baseline PPT.
- b Adjusted for sex, age and pain medication.
- c Adjusted for sex and age.
- d Adjusted for sex.
- e Unadjusted estimates.
3.3 Conditioned pain modulation
Generally, weak associations (p > .1) were seen for CPM absolute and all FSD groups (Table 3 and Figure 1), however, participants having a CPM absolute value at 281 (75th percentile) had twice the odds of fulfilling the criteria for IB compared with participants with a CPM absolute value at 178 (ref) (OR: 2.66, 95% CI: 1.07–6.45, p = .033, n = 66) (Figure 1).
The same was seen for CPM relative, where only IB showed an association. Comparing two participants differing 20% in CPM relative (independently of CPM relative value), the participant with the highest CPM relative value had 20% higher odds of fulfilling the criteria for IB (OR = 1.22; 95% CI: 1.04–1.43, p = .013, n = 66) (Table 3).
Weak associations (p > .08) were seen between any of the case groups and having an increase in CPM below 20% after cold stimulus (Tables 3 and 4).
4 DISCUSSION
This is the first large population-based study examining pressure pain sensitivity and CPM in a wide spectrum of FSD. Besides IB, MCS, multi-organ BDS and SP 8 characterized by all symptoms, the results of the experimental pain tests were consistent across the conditions and do altogether not support altered pain regulatory mechanisms as an explanatory factor in FSD.
4.1 Pressure pain sensitivity in FSD
In general, the present data do not support a generalized sensitivity to pressure pain in FSD. However, this does not rule out that it may be a characteristic of some groups. The discrepancies between the current study results and other studies, mainly on selected patient samples, may be explained by the higher FSD severity in patients from specialized clinical settings, which may induce selection bias, compared with participants in the unselected general population-based sample from the current study.
Regarding FM, many studies in PPTs have been performed on smaller clinical samples, especially in women. These studies point to an increased sensitivity to pressure pain in FM compared with a control group (Dadabhoy et al., 2008; Jespersen et al., 2007; Maquet et al., 2004; Petzke et al., 2003; Staud & Rodriguez, 2006) as well as sensitivity towards other pain stimuli (Dadabhoy et al., 2008; Petzke et al., 2003). A previous study on the same sample as in the current study has shown that women generally have lower PPT than men (Skovbjerg et al., 2017). One could therefore argue that the obtained difference in PPT in the mentioned studies of FM is caused by the unselected sample mostly including women and not by the FM. In the current study, we have therefore controlled for sex in the analyses.
General population data on PPTs in FM are sparse, but one study by Wolfe et al., (1995) reported lower pain thresholds in participants with FM, using the upper trapezius muscle as the testing site. This is in contrast to the findings from the current study where we did not find altered PPTs in participants with CWP. The study by Wolfe et al. applied the American College of Rheumatology 1990 criteria for FM (Wolfe et al., 1990) including physical examination. The CWP criteria in the current study was inspired by the same definition, but we did not include any physical examination or validation of diagnoses by diagnostic interviews, which may explain the discrepancies in results.
Regarding pain sensitivity in IBS, a Norwegian population-based study (Stabell et al., 2013) found no convincing increased pressure pain sensitivity. Interestingly, they found increased pain sensitivity towards other pain modalities, that is heat pain and cold pressor pain, which is not in contrast with our study. In clinical studies on IBS, one study reported no differences in PPTs between women with IBS and healthy controls (Chang et al., 2000), whereas increased sensitivity to ischemic pain has been reported in a community sample when compared to a control group (Zhou, Fillingim, Riley, Malarkey, et al., 2010).
Comparison with other epidemiological studies on WAD is limited by the lack of comparable data, but increased sensitivity to pressure pain has been established previously in patients with persistent WAD (Van Oosterwijck et al., 2013). Clinical studies have reported no difference in PPT when comparing MCS patients with controls (Tran et al., 2013), whereas lower PPT have been reported in CFS patients with chronic pain compared with a control group (Meeus, Nijs, et al., 2010; Meeus, Roussel, et al., 2010).
We found tendencies to lower PPT in multi-organ BDS. Sensitivity to pressure pain has not previously been examined in BDS patients, but one study (Kuzminskyte et al., 2010) on patients with multiple functional somatic symptoms found no difference in heat pain thresholds between patients and controls.
4.2 Conditioned pain modulation in FSD
Findings of CPM abnormalities from many clinical studies could not be reproduced in this study. The results were consistent across the FSD groups when compared with the control groups, however, some associations with IB were seen for both CPM absolute and CPM relative.
As with pain sensitivity, most studies on CPM functioning in FSD have been performed in female FM and IBS patients or small community samples. The majority of these studies suggest differences in the CPM response between patients and controls (Chalaye et al., 2014; Heymen et al., 2010; King et al., 2009; Piché et al., 2011), although negative findings also have been reported (Jarrett et al., 2016). Similar results have been demonstrated for WAD (Daenen et al., 2013), whereas the findings have been negative for MCS (Tran et al., 2013). In CFS, CPM has been studied in combination with FM, and no studies other than the present have included BDS.
One of the present challenges with CPM testing is how to define a 'normal range', that is what constitutes an abnormal response (Kennedy et al., 2016). Whether the observed differences contribute in a clinically meaningful way to the pain in FSD is therefore difficult to establish. Nevertheless, the present study questions the contribution of altered central pain regulatory mechanisms to the symptoms in FSD.
4.3 Strengths and limitations
The current study has several strengths: First, we include a large (n = 2,198) unselected sample from the general population with an almost equal gender distribution. Other studies mostly involve highly selected—often female—patients. This may induce systematical bias regarding somatic or psychological comorbidities in the patient groups under investigation, which may be the primary cause for them to end up in a specialized clinical setting. The population-based study design reduces the risk of selection bias and allows the results to be generalized to other adult populations examining the groups of FSD covered by the DanFunD study. Second, as many different criteria to identify FSD have been proposed (Burton et al., 2020), we included three approaches for defining FSD in our study. Hence, we made an effort to capture the diverse nature of these conditions as both mono- and multi-systematic. Third, we used well-known and validated symptom questionnaires for defining the various FSS and BDS.
However, our study also has some limitations that need to be addressed. First, the delimitation of the FSD groups was based on self-reported symptoms and not on diagnostic interviews, clinical examinations, or existing diagnoses which makes it difficult to establish if the symptoms are part of an FSD or other physical or mental differential diagnoses. This may give rise to some concern when interpreting the findings due to the uncertainties of this approach for case establishment. It is therefore possible that we included cases with milder symptoms than studies based on clinical samples, which may offer some explanation for the negative findings. However, performing diagnostic interviews in such a large sample would be too comprehensive and the use of diagnoses of FSD within somatic practice in Denmark is rather unsystematically and may thereby be unreliable to trust. In our study, a cut-off on symptom severity was made, only including bothering symptoms in the criteria defining FSD cases. Furthermore, we included definitions of multi-systematic conditions (multi-organ BDS and SP 8 characterized by participants presenting all symptoms). We, therefore, argue, in line with previous research from the same study group (Eliasen et al., 2018; Petersen, McPhee et al., 2019; Petersen, Schröder et al., 2019; Petersen, Ørnbøl et al., 2021; Petersen, Schröder et al., 2021) that the cases in our study were not all mild cases but also constituted individuals with symptom patterns of severe FSD. Second, the response rate of 27.7% may be considered low, and even though the risk of selection bias is markedly reduced compared to clinical studies, we cannot completely rule it out. We may therefore have under-estimated the associations between the various pain measures and FSD. However, in another paper, a non-responder analysis showed that responders did not differ from non-responders regarding register-based socioeconomic variables and hospital discharge diagnoses, hence, we believe that the included sample is representative of the originally invited population (Schovsbo, 2021). Third, the prevalence of some of the FSD groups was low, for example only 19 cases of multi-organ BDS. It may therefore be questioned if we had enough power in the statistical analyses. However, the size of most of the CIs was relatively small, indicating a reasonable power. Fourth, we only used pain threshold in order to test pain sensitivity and with one modality, that is pressure, while additional tests, for example supra-threshold stimuli could have been performed. However, participants went through several tests and examinations attending the DanFunD study, and it would have been too comprehensive to put them through more tests, assessing pain perception. Also, it is well-accepted that PPT reflects pain sensitivity (Arendt-Nielsen, 2015), and that it is associated with scores of supra-threshold stimulations (Petrini et al., 2015).
4.4 Conclusions
The present study found overall normal functioning in pain regulatory mechanisms in FSD with only small differences for some of the FSD groups when compared with controls. Findings from the present study, therefore, fail to support the existing presumption that abnormal functioning in pain mechanisms contributes to the pain symptoms in FSD.
ACKNOWLEDGEMENTS
DanFunD steering committee consists of Professor MD DMSc Torben Jørgensen (PI), Professor MD DMSc Per Fink, Senior consultant MD PhD Lene Falgaard Eplov, Professor MD PhD Allan Linneberg, Professor MSc PhD Susanne Brix Pedersen, and MD PhD Michael Eriksen Benros.
CONFLICTS OF INTEREST
The authors declare no competing interests.
AUTHORS' CONTRIBUTIONS
SS, TMD, PF, MEB, ELM and TJ contributed to the conception and design of the study. MWP, SS and JSJ performed the analyses. MWP, SS, JSJ and LKG interpreted the data and drafted the article. TC, TMD, PF, MEB, ELM and TJ contributed to the interpretations of the data. All authors discussed the results and contributed to critically revising the article for important intellectual content. All authors read and approved the final version of the article.