NF-κB factors control the induction of NFATc1 in B lymphocytes
Abstract
In peripheral lymphocytes, the transcription factors (TFs) NF-κB, NFAT, and AP-1 are the prime targets of signals that emerge from immune receptors. Upon activation, these TFs induce gene networks that orchestrate the growth, expansion, and effector function of peripheral lymphocytes. NFAT and NF-κB factors share several properties, such as a similar mode of induction and architecture in their DNA-binding domain, and there is a subgroup of κB-like DNA promoter motifs that are bound by both types of TFs. However, unlike NFAT and AP-1 factors that interact and collaborate in binding to DNA, NFAT, and NF-κB seem neither to interact nor to collaborate. We show here that NF-κB1/p50 and c-Rel, the most prominent NF-κB proteins in BCR-induced splenic B cells, control the induction of NFATc1/αA, a prominent short NFATc1 isoform. In part, this is mediated through two composite κB/NFAT-binding sites in the inducible Nfatc1 P1 promoter that directs the induction of NFATc1/αA by BCR signals. In concert with coreceptor signals that induce NF-κB factors, BCR signaling induces a persistent generation of NFATc1/αA. These data suggest a tight connection between NFATc1 and NF-κB induction in B lymphocytes contributing to the effector function of peripheral B cells.
Introduction
Upon Ag contact, the induction of resting peripheral lymphocytes leads to networks of signaling events that emerge from their immune receptors. Primary targets of these signals are members of NF-κB, NFAT, and AP-1/ATF/CREB families of transcription factors (TFs). Upon activation they bind to the promoters, enhancers, and further regulatory elements of numerous genes, control their expression and, thereby, orchestrate gene expression programs that control the growth, proliferation, and effector function of peripheral lymphocytes.
One of the best-characterized lymphocyte-specific promoters is the promoter of human and murine Il2 genes. Within the Il2 promoter of approximately 300 bp, there are several noncanonical sequence motifs for the binding of NF-κB and AP-1/ATF/CREB factors, and composite NFAT + AP-1 sites to which both factors can bind with high affinity 1. The distal NFAT-binding site from the Il2 promoter (the ARRE2 2, or distal purine box, Pu-bd 3, that is often referred as the “prototypical” NFAT site), consists of the NFAT “core motif” 5′ T/AGGAAAA 3′ and a noncanonical TRE (TPA-responsive element), i.e. an AP-1/ATF-binding motif, located 10 bp downstream. Such composite NFAT + AP-1 sites are typical for the promoters/enhancers of many genes induced in peripheral T lymphocytes by immune receptor signals 4.
There are two noncanonical NF-κB-binding motifs within the Il2 promoter 1. One of them was described as a CD28-responsive element 5-7. It belongs to a set of κB motifs that were identified as optimal c-Rel-binding motifs 8 but to which both NF-κB and NFAT factors can bind. However, unlike the cooperative binding of NFAT and AP-1/ATF factors to DNA, NFAT and NF-κB factors contact identical nucleotides and do not mutually enhance their DNA binding. NFATs form homodimers at these κB/NFAT-binding motifs in the human IL8 promoter 9, the HIV-LTR 10, and other promoters. The ability to bind to similar DNA sequence motifs is reflected in the DNA-binding domains of both factors. While they share less than 20% sequence similarity, the Rel domain of NF-κB1/p50 and the Rel similarity domain (RSD; also designated as or RHR, Rel homology region) of NFAT factors share a similar configuration 11-13.
Among the four “genuine” members of NFAT factor family that are activated by Ca++/calcineurin signals, the three factors NFATc1 (also designated as NFAT2 or NFATc), NFATc2 (NFAT1, NFATp), and NFATc3 (NFAT4, NFATx) are expressed in lymphocytes. Upon immune receptor stimulation, they are rapidly transported from cytoplasm to the nucleus and control the expression of many genes 14-16. In addition to this rapid induction, immune receptor signals enhance the expression of Nfatc1 gene and lead to the predominant synthesis of NFATc1/αA, a short NFATc1 isoform which lacks a C-terminal domain of approximately 250 aa typical for most of other NFAT proteins. NFATc1/αA is the most prominent NFAT factor in activated lymphocytes and—contrary to other NFAT proteins – can protect peripheral T and B cells from early cell death upon immune receptor stimulation 17.
We show here that NF-κB factors contribute to the induction of NFATc1/αA upon stimulation through the BCR. While upon primary BCR stimulation of splenic B cells from NF-κB1-deficient mice, a mild reduction in nuclear occurrence of preformed NFATc1 protein was observed upon secondary stimulation NFATc1/αA induction was abolished. A strong defect in NFATc1/αA induction was also observed in B cells from c-Rel-deficient mice. NF-κB p50/c-Rel heterodimers bind to two κB-like sequence motifs within the inducible P1 promoter of Nfatc1 gene and, thereby, affect the induction of NFATc1/αA.
Results
NFATc1 ablation affects the formation of NF-κB complexes in splenic B cells
For the optimal induction of the Nfatc1 gene, splenic B cells have to be stimulated through their BCR by α-IgM whereas stimulation with α-CD40, LPS, or CpG – all of which induce NF-κB factors – exerts only a marginal effect on Nfatc1 induction 18. Compared to NFATc1 induction, α-IgM-mediated BCR signals exert a poor and transient effect on the induction of nuclear NF-κB complexes in splenic B cells whereas α-CD40, LPS, or CpG signals induce strongly and persistently nuclear NF-κB complexes (Supporting Information Fig. 1A and B). In EMSA supershift experiments we observed predominantly NF-κB1/p50 protein as component of faster migrating (p50/p50 homodimeric) NF-κB complexes and c-Rel as the main, albeit not sole component of slower migrating (c-Rel/p50 heterodimeric) NF-κB complexes upon α-IgM stimulation for 8 h (Fig. 1A).
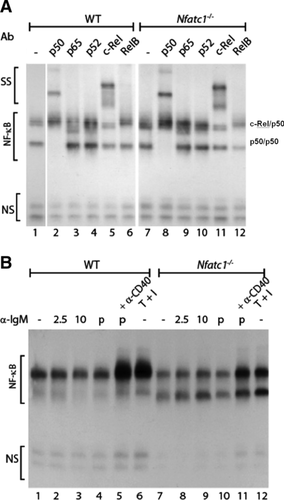
At the RNA level, BCR stimulation by α-IgM led to a three- to fourfold increase in c-Rel transcripts whereas the transcripts of all other Rel members remained almost unaffected (Supporting Information Fig. 2). In contrast, α-CD40 stimulation of splenic B cells resulted in a four- to fivefold increase in RNA reads of Nfkb2 gene encoding p52 whereas only a slight increase was detected for RelB transcripts upon α-CD40 stimulation for 24 h (Supporting Information Fig. 2). Inactivation of the Nfatc1 gene in BM B cells from Nfatc1 fl/fl x mb1-cre mice did neither affect the transcripts of κB genes (results not shown) nor of occurrence of nuclear κB proteins (Fig. 1A).

Whereas NFATc1 ablation did not affect the expression of individual κB proteins, it influenced the formation of nuclear NF-κB complexes. In WT B cells that were strongly activated by “pulsed” α-IgM+α-CD40 or T + I treatment, the predominant formation of active, heterodimeric NF-κB complexes is observed, while more “inactive” p50/p50 homodimeric complexes are seen in Nfatc1−/− B cells (compare lanes 5 + 6 with lanes 11 + 12 in Fig. 1B and see Supporting Information Fig. 3). These findings indicate that NFATc1 supports the formation of NF-κB complexes but does not markedly affect the expression of NF-κB members.
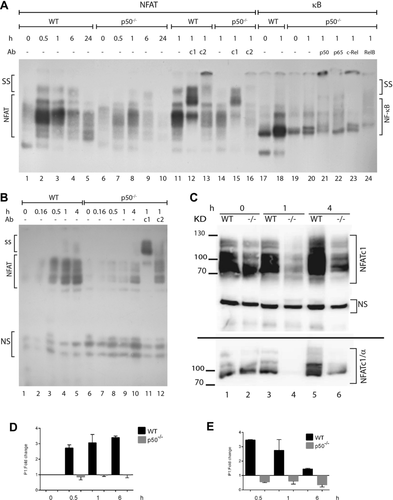
NFATc1 deficiency affects NF-κB complex formation in chicken DT40 B lymphoma cells
To investigate the effect of NFATc1 ablation on NF-κB in another type of B cells, we inactivated the NFATC1 gene in chicken DT40 B cells and studied the generation of nuclear NF-κB complexes. When we tested in EMSAs the occurrence of nuclear NF-κB complexes in NFATC1−/− DT40 cells stimulated with α-IgM for 2 h, we observed a marked decrease in complex formation compared to WT DT40 cells. In particular, the formation of slowly migrating heterodimeric “activating” NF-κB complexes was found to be impaired (compare lanes 5–8 with lanes 1–4 in Fig. 2A).
Numerous signals leading to NF-κB activation in lymphocytes are mediated through the activation of protein kinase Iκκβ 19. Therefore, we investigated the effect of NFATC1 inactivation on the induction of Iκκβ activity in kinase assays using protein extracts from WT and NFATC1−/− DT40 cells. While we did not see any difference in Iκκβ activity between WT and NFATC1−/− cells stimulated with T + I, we observed a distinct weaker Iκκβ induction in NFATC1−/− cells compared to WT cells stimulated through their BCR by the α−IgM Ab M4 for 10–30 min (or by a hybridoma supernatant for 10 min; Fig. 2B). This suggests that signaling events upstream of Iκκβ are controlled by NFATc1.
Nuclear NFATc1 complexes upon secondary BCR stimulation are not formed in Nfκb1−/−/p50−/− mice
Compared to the drastic effect of inactivation of Rela gene on embryonic development 20, inactivation of Nfkb1 gene exerted only a mild, if any effect on embryogenesis but affected strongly the activation and function of peripheral lymphocytes, in particular of B cells 21, 22. When we compared the NFATc1 induction by T + I in splenic B cells from Nfkb1−/− mice with that in B cells from WT mice, we observed a mild decrease in formation of nuclear NFAT complexes upon primary induction by T + I for 0.5–24 h (Fig. 3A) but a very strong defect upon secondary stimulation (Fig. 3B). This might be due to a comparable amount of preformed cytosolic NFATc1 protein in splenic B cells from WT and p50-deficient mice, as detected in immunoblots of preparations from whole cellular proteins (Supporting Information Fig. 4). Upon secondary stimulation of B cells induced first by α-IgM for 24 h with T + I for 10 min to 4 h a drastic drop in NFATc1 levels was detected, both for the induction of nuclear NFATc1 complexes in EMSAs using nuclear protein from Nfkb1−/− B cells and in western blots using whole cellular protein (Fig. 3B and C). This is in line with the strong decrease in P1 promoter-directed induction of NFATc1 RNA levels, as observed in RT-PCR assays. Already upon primary stimulation of splenic B by T + I for 0.5–6 h resulting in a three- to fourfold increase in P1-directed RNA transcripts in WT cells, a complete defect in P1-directed RNA induction was detected (Fig. 3D). And upon secondary stimulation—following the treatment of B cells by α-IgM for 24 h—a further decrease in P1-directed transcripts was observed (Fig. 3E). These findings demonstrate that p50 is necessary for the induction of P1 activity in B lymphocytes.

Ablation of c-Rel abolishes any NFATc1 induction
Similar to the inactivation of Nfkb1 gene, ablation of c-Rel did not lead to gross abnormalities in embryonal development and in differentiation of hematopoietic cells but in defects of B-cell function 23, 24. In particular, the growth of BCR-activated peripheral Rel−/− B cells during the G1 phase of cell cycle and their promotion to progress from the G1 to S phase were strongly affected 24. Freshly prepared splenic B cells from c-Rel-deficient mice did not show any formation of nuclear NFATc1 complexes (Fig. 4A). The complexes that we observed in EMSAs using an NFAT consensus probe and nuclear protein from Rel−/− B cells consisted mainly of NFATc2 but not NFATc1 (see lanes 5–8 in Fig. 4A). While in immunoblots with whole cell proteins one NFATc1 band was detected in nonstimulated c-Rel−/− B cells, no NFATc1 induction was observed upon α-IgM or α-IgM+α-CD40 treatment in c-Rel−/− B cells (Fig. 4B). By mapping of P1-directed NFATc1/α transcripts in RT-PCR assays, a similar suppression of NFATc1/α induction was detected at the transcriptional level (Fig. 4C).
Two composite κB/NFAT sites in the Nfatc1 P1 promoter
Within the 800 bp of Nfatc1 P1 promoter that are highly conserved between mouse and human, there are two composite κB/NFAT motifs located around the nucleotide positions –90 and –720, respectively (Fig. 5A). These motifs share the NFAT core binding motif T/AGGAAA with numerous other sites to which both NFAT and NF-κB factors can bind, and with the sequence 5′-NGGRN(A/T)TTCC-3′ that was identified as optimal c-Rel-binding site 8. When these κB/NFAT sites were used as probes in EMSAs with nuclear protein from in vitro-induced GC B cells (iGCs) which—due to their stimulation by CD40 25—express high levels of NF-κB, we detected the formation of typical κB complexes that were indistinguishable from complexes generated with a κB consensus site (Fig. 5B and not shown). This conclusion is supported by EMSA competition experiments in which competition with a 50-fold molar excess of a κB consensus site, but not with a site mutated within the κB motif, led to disappearance of NF-κB complexes (Fig. 5C). However, in EMSAs with nuclear proteins from splenic B cells that contain less NF-κB proteins than iGCs, a very poor formation of p50 homodimers was observed with the two κB/NFAT sites, compared to a κB consensus site (compare lanes 1–8 with lanes 9–14 in Fig. 5D).

To demonstrate the binding of p50 and c-Rel to the P1 promoter in vivo, by using κB-specific Abs in ChIP (chromatin immune precipitation) assays, we precipitated P1 chromatin from unstimulated splenic B cells or cells stimulated by T + I for 4 h. In PCRs with P1-specific primers, we observed a weak binding of both p50 and c-Rel to the chromatin of P1 promoter in unstimulated B cells, but a marked six- to tenfold increase upon B-cell induction (Fig. 5E and F). This shows that BCR signals enhance the binding of p50 and c-Rel to the Nfatc1 P1 promoter.
To determine the functional relevance of factor binding to the κB/NFAT sites, we introduced point mutations into the sites (Fig. 5A) that abolished the binding of NF-κB and NFAT, and cloned the mutated promoter in front of a luciferase reporter gene. Cotransfections of P1-directed constructs with a vector expressing c-Rel into 293 HEK cells revealed a two- to threefold increase in luciferase activity upon T + I induction and a further increase by cotransfecting c-Rel. By using P1-luciferase constructs that were mutated in either the proximal κB/NFAT-90 or the distal κB/NFAT–720 motif, or in both motifs, we observed a strong decrease in luciferase induction, and c-Rel had no effect on the construct bearing mutations in both κB/NFAT sites (Fig. 6A).
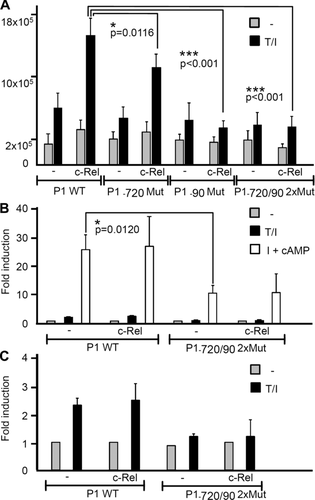
Upon transfection into El4 thymoma cells, we also observed a marked decrease in the activity of P1 promoter mutated in both κB/NFAT motifs (at –720 and –90) compared to the nonmutated promoter, and cotransfection of c-Rel was without effect. However, c-Rel exerted only a minor, if any positive effect on the induction of WT promoter by T + I or I + cAMP signals which exert a strong stimulatory effect on P1 in El4 cells 26 (Fig. 6B). Interestingly, for the mutated P1 promoter, any T + I-mediated induction was abolished (Fig. 6C) while its induction by I + cAMP signals was weaker but not abolished (Fig. 6B). We assume that in El4 cells due to their high constitutive CN activity and NFATc1 levels 27 c-Rel exerts only a minor effect on P1 activity in these cells. A similar situation seems to exist in effector lymphocytes upon induction (see Discussion).
NFAT and/or NF-κB binding to composite κB/NFAT sites
In Figure 7A, we present a compilation of composite κB/NFAT motifs from numerous promoters that are active in lymphocytes. One of those elements, the κB-like site from the Myb promoter, was used in EMSAs of Figure 2A for the analysis of NF-κB/NFAT binding in nuclear protein from chicken DT40 cells showing strong NF-κB binding upon T + I induction, and weak NF-κB binding upon ablation of NFATc1 in DT40 cells. But when human NFATc1/αA was overexpressed in DT40 cells deficient for NFATc1 (Fig. 7B) instead of NF-κB binding, we observed the predominant binding of NFATc1 to the κB/NFAT probe. This is demonstrated in lane 4 of Figure 7B in which adding an Ab specific for NFATc1 resulted in a supershift of complexes generated with the Myb κB/NFAT site. In contrast, only traces of endogenous NF-κB1 were found in this complex (Fig. 7B).

In order to see whether like the κB/NFAT sites from the HIV-LTR, the Myb site favors the formation of NFAT dimers, we performed EMSAs with bacterially expressed GST-NFATc1-RSD protein containing the DNA-binding domain of human NFATc1. In these assays, the composite Myb κB/NFAT site formed complexes very similar to those with the HIV-1 LTR κB/NFAT site, whereas the complexes formed at a similar site from the c-IAP2 promoter resembled both the HIV-1 LTR κB/NFAT and the canonical distal NFAT (+AP-1) site from the Il2 promoter (Fig. 7D). These findings suggest that both sites, the Myb and c-IAP2 sites, can be bound by NFAT homodimers, as the κB/NFAT sites in the HIV-1 LTR 10.
Discussion
The data in this study show that ablation of NF-κB proteins c-Rel or NF-κB1/p50 exerts a strong suppressive effect on the BCR-mediated induction of Nfatc1 gene in splenic B cells. This leads to the loss of induction of NFATc1/αA, a short NFATc1 isoform that represents the most prominent NFATc1 protein in effector lymphocytes upon immune receptor stimulation 17. Since NFATc1/αA supports the proliferation and survival of lymphocytes, one may assume that the loss of its induction contributes to the phenotype of mice deficient for one or both NF-κB factors 28.
Over the last 20 years, mouse lines deficient for either p50 or c-Rel, or for both have been studied in detail (reviewed in 29, 30). Albeit both p50 and c-Rel are expressed early in development and in numerous cell types, the most striking effect of their ablation was detected on B-cell activation and function. While Nfκb1−/− and Rel−/− B cells showed specific defects in their proliferation capacity and apoptosis, B cells deficient for both κB proteins exhibited an acceleration of these defects resulting in impaired B-cell function, as in a complete loss of germinal center formation and decrease in Ab production 28. The results reflect individual properties for p50 and c-Rel, as it had been demonstrated at the molecular level for the neutralization of the proapoptotic Bcl-2 member Bim by specific phosphorylation cascades which help B cells to survive 31.
Several lines of evidence suggest a close connection between c-Rel and NFATc1 expression and function in B cells. One example is the important role that both factors play in controlling BCR-mediated proliferation. While c-Rel and NFATc1 appear to be less or unimportant in the control of LPS/TLR-4-mediated proliferation, their ablation strongly impaired the proliferation of splenic B cells upon α-IgM stimulation 18, 32. Moreover, the κB/NFAT-binding motifs in the promoters of numerous inducible genes, including the Nfatc1 P1 promoter, correspond to DNA sites for optimal c-Rel binding (Fig. 6A) 8, and one may assume that the binding of either c-Rel or NFATc1 to these sites is of functional relevance for the fate of peripheral B cells.
Contrary to the numerous studies on the contacts between NFATs and AP-1-like factors 33, there are only a few reports on direct interactions between NF-κB and NFAT proteins. One of them deals with the CD154/CD40LG promoter whose activity in human large B-cell lymphoma cells is under the control of NF-κB and NFAT factors. There are several composite κB/NFAT motifs within the CD40LG promoter, and in EMSAs using such κB/NFAT motifs as probes, the generation of NFATc1/DNA and c-Rel/DNA complexes was reported 34. Since, however, in conventional EMSAs, the DNA probe is in excess, these data reflect the generation of individual NFATc1/DNA or c-Rel/DNA complexes, but not of (heterodimer) complexes that contain NFATc1 as well as c-Rel. The authors also show data on the coprecipitation of NFATc1 by an Ab specific for c-Rel from large B-cell lymphoma cells in which both factors were overexpressed 34. A similar strategy, i.e. viral overexpressing of NFATc1 in neonatal cardiomyocytes followed by immune precipitation, was used to illustrate an interaction between NFATc1 and p65/RelA, and modulating RelA activity in cardiomyocytes affected the NFAT-mediated gene activity 35. While these data suggest interplay between NFATc1 and NF-κB factors, they do not prove (i) whether under physiological conditions—i.e. without overexpressing one or both factors—NFAT and NF-κB factors indeed interact. In addition, they do not show (ii) whether protein–protein interactions between both factors affect their DNA binding and, thereby, the activity of genes controlled by NFAT and NF-κB.
The results of our study favor a different scenario of interplay between NF-κB and NFAT factors in controlling B-cell function. Our data show that the induction of NFATc1 is controlled by NF-κB factors, in particular by c-Rel that binds together with p50 to the Nfatc1 P1 promoter (Fig. 5). The data suggest that the composite κB/NFAT-binding sites found in the promoters of numerous inducible genes are either bound by NFAT (homo-) or NF-κB (c-Rel/p50 hetero-) dimers that contact similar nucleotides at the NFAT T/AGGAAA core motif. Depending on the concentrations of both factors in the cell nucleus, these promoters that contain often multiple copies of “genuine” NFAT (and κB) and complex κB/NFAT sites are controlled either by both factors, or predominantly by NFAT. Examples for such promoters are the Nfatc1 P1 and Il2 promoters. The former contains two composite κB/NFAT sites and two high-affinity NFAT-binding sites in tandem (Fig. 5A), the latter two high-affinity NFAT-binding sites, including the ARRE2/Pu-bd, one κB site and at least one composite κB/NFAT site, the CD28-responsive element 1. We assume that the primary activation of both promoters is controlled by NF-κB and NFAT factors that are strongly induced by immune receptor and costimulatory signals, whereas upon secondary stimulation, NFAT factors play the major role in activating both promoters. Detailed studies on the induction of genes directed by promoters with composite κB/NFAT sites in vivo will help to evaluate this view on the inducible gene expression in effector B (and T) lymphocytes upon antigenic stimulation by NFAT and NF-κB factors.
Materials and methods
Mice, isolation, and culture of B cells
Eight- to 12-week-old C57 BL/6 mice were used. Splenic B cells were isolated using a Miltenyi Biotec B-cell isolation kit (mouse; #130-090-862). A total of 5 × 106 cells were cultured in 1 mL X-vivo 15 medium (Lonza) and stimulated with 10 μg/mL α-IgM (F(ab‘)2 fragment of goat anti-mouse IgM; Jackson ImmunoResearch Laboratories), 5 μg/mL α-CD40 (R&D Systems), 10 μg/mL LPS (Sigma-Aldrich), or TPA (T; 10 ng/mL), and ionomycin (I; 0.5 μM). In some assays, B cells were “pulsed” (p) for 30 min (or for 2 h) at 4°C with α-IgM, washed, maintained for 6 h at 37°C and, after adding α-CD40, cultured to 24 h. In vitro-induced GC B cells were generated by cultivating splenic B cells on 40LB 3T3 feeder cells for 4 days in the presence of IL-4 25. Chicken DT40 B cells were cultured at 39.5°C with 5% CO2 using RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 1% chicken serum, 2-mercaptoethanol (50 μM), and l-glutamine (2 mM). Human 293 HEK cells were transfected using a conventional Ca++-phosphate DNA transfection protocol with luciferase reporter genes directed by a −1200 bp WT Nfatc1 P1 promoter fragment, or by fragments mutated in the κB/NFAT motifs (Fig. 6). The cells were left untreated or treated with T + I. Forty-eight hours post transfection luciferase activity was measured. EL4 thymoma cells were transfected by DEAE-dextran and induced as described earlier 26.
The B-cell stage-specific cre lines mb1-cre 36 and Cd23-cre 37 have been described previously. The following mice lines were used: two Nfatc1 fl/fl mouse lines (also designated as Nfat1 fl/fl mice: 38-40), and the Nfκb1−/− 21 and Rel−/− 23 lines. Animal experiments were performed according to project licenses (55.2–2531.01–53/10B), which are approved and controlled by the Regierung von Unterfranken, Würzburg.
EMSAs
Nuclear proteins from splenic B cells or DT40 cells, or bacterially expressed GST-NFATc1 spanning the RSD (aa 418–716) were prepared and used in EMSAs as described 26. For the detection of NFAT and NF-κB binding, the distal NFAT site from the murine Il2 promoter, a NF-κB consensus site or composite κB/NFAT sites were used as probes. In supershift EMSAs, the following Abs were used: NFATc1, BD Pharmingen (#556602); NFATc2, Santa Cruz Biotechnology (#sc-13034); NF-κB1/p50 (#sc-114X); RelA/p65 (#sc-109X); c-Rel (#sc-70X); RelB (#sc-226X); NF-κB2/p52 (#sc-298X).
Immunoblotting
Immunoblots were performed by fractionating whole protein extracts on PAGE-SDS gels followed by detection of NFATc1 using the 7A6 mAb (BD Pharmingen, #556602). For detecting NFATc1/αA (Fig. 3C, lower panel), a pAb raised against the NFATc1α-peptide (ImmunoGlobe) was used. As loading control, filters were stained by Ponceau red. Signals were developed using a chemiluminescence detection system (Thermo Fisher Scientific).
Cloning of the mutated Nfatc1 P1 promoter
The −1155/+275 mouse Nfatc1 P1 promoter was amplified by the primer pair (5′-AACCAAATGTGCCCCAGAAGGAAGG-3′,5′-CAGCGGCGAGCAGCAGGAGGAGGAG-3′) and cloned into the pGL3basic vector as a NheI/XhoI fragment. Mutations were introduced by PCR using the following primer pairs (proximal κB/NFAT site: 5′GGGGGAGGTGTTTTAAAGCTTT AAAAAGGCAG AAGG-3′, 5'-GCCTTTTTAAAGCTTTAAAACACCTCCCCCGGCTC-3′; distal κB/NFAT site: 5- TGGGAATTTAATTACTCCCGCCTGCCAG-3′,5′-CGGGAGTATTAAATTCCCATCCCG CTAAA-3′).
RT-PCR assays
RNA was extracted from splenic B cells using TRIzol reagent (Invitrogen), and RT-PCR assays were performed with primer sequences for detecting Nfatc1 P1 promoter-directed transcripts as described 41.
Chicken NFATC1 gene targeting and expression of human NFATC1/αA
NFATC1 targeting vectors containing segments from the chicken NFATC1 locus were constructed by replacing a ∼3.3 kb genomic fragment encoding exons 4 and 5 with drug-resistance gene cassettes. The targeting vectors were introduced into WT DT40 cells, and cloning of the targeted cells was performed by culturing of cells in the presence of blasticidin, histidinol D, or puromycin as described 42. Full-length cDNA encoding human NFATC1/αA was subcloned into the pcDNA3.1 vector between its BamHI-KpnI sites by PCR using cDNA of human BALM-14 cells as template. The vector was introduced into NFATC1−/− DT40 cells by electroporation.
RNA-Seq assays
RNA from splenic B cells was extracted using Qiagen's RNeasy kit and Illumina's RNA purification beads. RNA-Seq libraries for Next Generation Sequencing were prepared using Illumina's TruSeq RNA Sample Prep Kit V2 following manufacturer's instructions. Barcoded cDNA libraries were sequenced in one lane on an Illumina HiSeq 2000 platform for 50 nucleotides (single end).
IKKß kinase assays
DT40 cells were lysed in TNT-buffer (200 mM NaCl, 20 mM Tris pH 8.0, 1% Triton X-100, protease inhibitor cocktail (Roche), 10 mM NaF, 10 mM β-glycerophosphate, 1 mM DTT). A total of 750–1000 μg of the resulting whole cell extract were subjected to immunoprecipitation with Abs specific for the different IKK subunits (anti-NEMO (IKKγ) Ab: SC-8330, anti-IKK1 Ab: SC-7218, and anti-IKK2 Ab SC-7607, 1 μg each sample). For the kinase reaction, 0.5 μg of a bacterial expressed GST-IκBα (1–53) fusion protein and 10 μCi of 32P-labeled γATP were added per sample followed by incubation for 30 min at 30°C under agitation and the addition of 50 μL of a 50% GSH-sepharose slurry. The samples were washed once with PBS and were subsequently subjected to a SDS-PAGE. The proteins were transferred to a nitrocellulose membrane, and phosphorylation was visualized by autoradiography.
ChIP assays
ChIPs were performed as described previously (Bhattacharyya et al. 18). Chromatin complexes were immunoprecipitated using p50 (#sc-114X) or c-Rel (#sc-70X) specific Abs, or an unrelated Ab as control. Real-time PCRs were performed with the following P1-specific primers: Nfatc1p1(1 F)atgtaaaatcgcaggcttcc and Nfatc1p1(1 R)atttagcgggatgggaattt, Nfatc1p1(2 F)gcctcgcttcacactttttc and Nfatc1p1(2 R)gacgttctaactgcccaacc.
Statistical analysis
Statistical analysis was performed by using Graphpad prism 5.0. Unpaired t-test was performed to calculate the p value. A p value less than 0.05 was considered significant.
Acknowledgments
We are very much indebted to Doris Michel and Ilona Pietrowski for technical support. For mouse lines and reagents we wish to thank Drs. M. Busslinger (Vienna), L. Glimcher (New York), M. Pasparakis (Cologne), A. Rao (San Diego), and M. Reth (Freiburg). We thank Dr. A. Rosenwald for his kind support. The work was supported by the Deutsche Forschungsgemeinschaft, grant TRR52 (to E.S. and A.A.), the Wilhelm-Sander foundation (to E.S. and S.K.-H.), and the Scheel foundation (to E.S. and A.A.).
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
Abbreviations
-
- P1, P2
-
- Nfatc1 promoters
-
- TF
-
- transcription factor
-
- RSD
-
- Rel similarity domain