Bone morphogenetic protein 4 inhibits liposaccharide-induced inflammation in the airway
Abstract
Bone morphogenetic protein 4 (BMP4) is a multifunctional growth factor that belongs to the TGF-β superfamily. The role of BMP4 in lung diseases is not fully understood. Here, we demonstrate that BMP4 was upregulated in lungs undergoing lipopolysaccharide (LPS)-induced inflammation, and in airway epithelial cells treated with LPS or TNF-α. BMP4 mutant (BMP4+/−) mice presented with more severe lung inflammation in response to LPS or Pseudomonas aeruginosa, and lower bacterial load compared with that in BMP4+/+ mice. Knockdown of BMP4 by siRNA increased LPS and TNF-α-induced IL-8 expression in 16HBE human airway epithelial cells and in primary human bronchial epithelial cells. Similarly, peritoneal macrophages from BMP4+/− mice produced greater levels of TNF-α and keratinocyte chemoattractant (KC) upon LPS treatment compared with cells from BMP4+/+ mice. Administration of exogenous BMP4 attenuated the upregulation of TNF-α, IL-8, or KC induced by LPS and/or TNF-α in airway epithelial cells, and peritoneal macrophages. Finally, partial deficiency of BMP4 in BMP4+/− mice protected the animals from restrictive lung function reduction upon chronic LPS exposure. These results indicate that BMP4 plays an important anti-inflammatory role, controlling the strength and facilitating the resolution of acute lung inflammation; yet, BMP4 also contributes to lung function impairment during chronic lung inflammation.
Introduction
Inflammation is a complex response that can be both defensive and harmful to the body. Although the primary role of inflammation is to eliminate the detrimental factors in tissues, the out-of-controlled inflammatory responses will lead to tissue damage that is even more harmful than the primary disease condition. Establishment of inflammatory cascades includes the release of both pro- and anti-inflammatory mediators and the maturation, activation, and migration of the responding inflammatory cells 1. The excessive inflammation in the lung causes lung injury that includes reduced alveolar-capillary barrier function, increased pulmonary vascular permeability, leukocyte infiltration into the alveolar space, etc. 2. If the inflammatory factors cannot be cleared out timely, it makes the acute inflammation turn into chronic condition 3.
Accumulating evidence indicates that transforming growth factor β (TGF-β) plays a determining role in regulating both innate and acquired immunity 4. TGF-β1 null mice exhibit widespread lethal inflammation in tissue, primarily in the heart and lung 5. In addition, TGF-β1 stimulates fibroblast proliferation, promoting airway remodeling during chronic lung inflammation associated with asthma and chronic obstructive pulmonary disease (COPD) 6. Bone morphogenetic proteins (BMPs) are multifunctional growth factors that belong to TGF-β superfamily members 7. Owing to their chemotactic activity on fibroblasts, myocytes, and inflammatory cells, BMPs were also considered to influence inflammatory processes in adults 8. Bone morphogenetic protein 4 (BMP4), a member of the BMPs family, is primarily expressed in airway epithelial cells and plays a key role in the early stage of lung development 9. BMP4 was demonstrated being upregulated in ovabumin-induced inflammation in mouse lung 8. However, the role of BMP4 in lung inflammation, particularly in lung innate immunity is unknown.
In this study, we hypothesized that BMP4 might participate in regulating the innate immune responses to bacterial infection. We examined the lung BMP4 expression during LPS-induced inflammation. Moreover, we compared the LPS-induced and Pseudomonas aeruginosa (PA) induced airway inflammation and pulmonary bacterial clearance capacity in BMP4 WT and heterozygous mutant mice. The involvement of airway epithelial cells and macrophages was also investigated to verify the role of BMP4 in the immune responses to LPS. Finally, we investigated whether or not BMP4 affects LPS-induced chronic lung inflammation and lung function in mice.
Results
Inhalation of LPS increases BMP4 expression in mouse lung
Lung inflammation was induced by i.n. inhalation of LPS at 7.5 μg/mouse, and evaluated at 12, 24, and 48 h post-LPS challenge. In LPS-treated mice, remarkable increases of total cell and neutrophil counts (Fig. 1A), TNF-α (Fig. 1B), and keratinocyte chemoattractant (KC; Fig. 1C) levels in BALF were observed at all three time points. The peak increases of total cell and neutrophil counts (Fig. 1A) and KC (Fig. 1C) in BALF were observed at 24 h post-LPS challenge, whereas TNF-α was peaked at 12 h, followed by remarkable drop but remaining significant increases at 24 and 48 h (Fig. 1B).
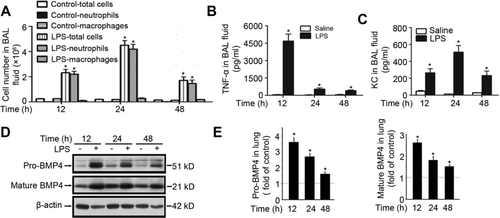
Next, we examined pro-BMP4 and mature BMP4 protein expression in lungs from saline control and LPS-treated mice. As shown in Figure 1D and E, the levels of both pro-BMP4 and mature BMP4 in LPS-treated mouse lung were markedly increased when determined at 12, 24, and 48 h, with the maximal increases observed at 12 h, followed by reduced increases at 24 h, and further reduced increases at 48 h post-LPS challenge.
LPS and TNF-α stimulate BMP4 expression in airway epithelial cells
To understand the mechanism of increased BMP4 expression during acute lung inflammation, we examined the effects of LPS and TNF-α on BMP4 expression in airway epithelial cells that are known to be the major sites of BMP4 production in the lung 10. As shown in Figure 2A, treatment of LPS (1 or 5 μg/mL) for 24 h dose-dependently increased BMP4 mRNA expression and mature protein secretion in 16HBE cells, with significant increases seen at 5μg/mL of LPS treatment. When cells were treated with 5 μg/mL LPS for various times from 3 to 24 h, the levels of secreted mature BMP4 in 16HBE-cultured medium were found increased as early as 6 h; further incubation to 12 and 24 h did not augment the degree of LPS stimulation (Fig. 2B). Similar to the effects of LPS, treatment of TNF-α (25-100 ng/mL) for 24 h dose-dependently increased BMP4 mRNA expression and mature protein secretion in 16HBE cells, with significant increases seen at 50 ng/mL of TNF-α (Fig. 2C). When cells were treated with 50 ng/mL TNF-α for various times from 3 to 24 h, the level of secreted mature BMP4 in 16HBE-cultured medium was also found increased as early as 6 h; again, further incubation to 12 and 24 h did not raise the degree of TNF-α stimulation (Fig. 2D). In primary mouse tracheal surface epithelial (MTSE) cells, treatments with LPS (1 or 5 μg/mL) for 24 h dose-dependently increased BMP4 secretion with significant increase seen with 5μg/mL of LPS (Fig. 2E).
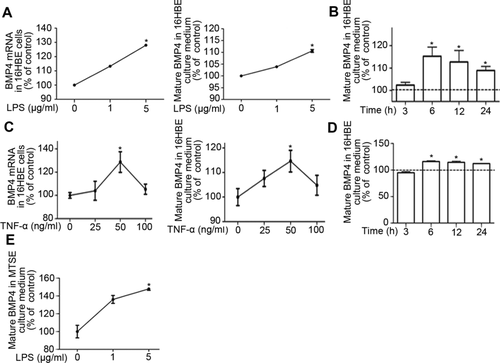
BMP4 mutant mice exhibit impairment in resolution of lung inflammation after LPS challenge
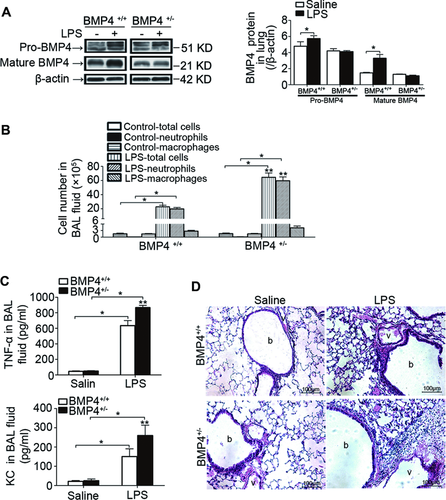
To determine the role of upregulated BMP4 in LPS-induced acute lung inflammation, we utilized BMP4 WT (BMP4+/+) and heterozygous mutant (BMP4+/−) mice. Treatment of LPS increased both pro-BMP4 and mature BMP4 protein expression in lungs from BMP4+/+ mice; these increases were absent in BMP4+/− mice (Fig. 3A). In conjunction with the diminished increases of BMP4 expression, BMP4+/− mice exhibited enhanced lung inflammation as indicated by greater total cell and neutrophil counts (Fig. 3B), higher TNF-α and KC levels in BALF (Fig. 3C), and increased inflammatory cell infiltration in lung tissue (Fig. 3D) when observed at 48 h post-LPS challenge as compared with the BMP4+/+ mice. These results suggest that BMP4 acts as an anti-inflammatory factor and plays an essential role in resolution of LPS-induced acute lung inflammation. In this study, we selected the time point of 48 h for assessments, as the LPS-induced respective changes in BMP4 WT mice were all reduced comparing to their peak changes at 12 or 24 h, which indicates the acute lung inflammation were subjected to the process of resolution at 48 h post-LPS challenge (Fig. 1).
PA-infected BMP4+/− mice have greater lung inflammation but lower bacterial load
To understand the functional significance of the increased lung BMP4 expression during LPS-induced lung inflammation, we compared the PA clearance capacity in BMP4+/+ and BMP4+/− mouse lungs. The pulmonary clearance of bacteria was evaluated by enumerating CFU of lung homogenates at 12 or 24 h post-PA infection. As seen in Figure 4A, inhalation of PA at 1 × 105 CFU/mouse and 1 × 106 CFU/mouse for 12 h resulted in approximately 1.9 × 104 CFU/mouse and 4.3 × 105 CFU/mouse left in BMP4+/+ mouse lungs, indicating partial clearance of the bacterial. Comparing to BMP4+/+ mice, the PA clearance at both dosages was greater in BMP4+/− mice. When inhaled with 1 × 105 CFU/mouse of PA, extension of the infection time to 24 h greatly reduced the CFU counts of PA to about 15% comparing to that at 12 h in BMP4+/+ mouse lungs (Fig. 5B). At both 12 and 24 h post-PA inhalation, BMP4+/− mice showed greater PA clearance in the lung comparing to BMP4+/+ mice (Fig. 5B).
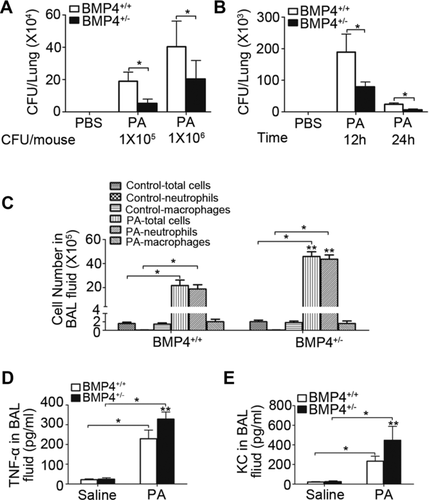
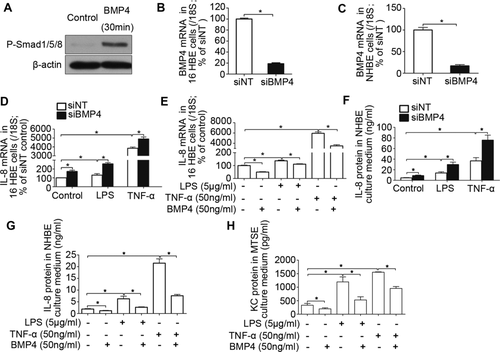
Next, we examined if the enhanced PA clearance in BMP4+/− mice was associated with exaggerated lung inflammation. As shown in Figure 4C–E, infection of PA (1 × 105 CFU/mouse) for 12 h caused significant inflammation in BMP4+/+ mouse lung, which was manifested by remarkably increased total cell and neutrophil counts (Fig. 4C), elevated levels of TNF-α (Fig. 4D) and KC (Fig. 4E) in BALF. Comparing to BMP4+/+ mice, BMP4+/− mice exhibited enhanced inflammation in the lung with greater numbers of total cells and neutrophils, higher TNF-α and KC levels in BALF (Fig. 4C–E). These results suggest that BMP4 diminution in BMP4+/− mice enhances bacterial clearance likely as a consequence of uncompromised innate immunity in the lung.
BMP4 activates Smad signaling and inhibits cytokine production in airway epithelial cells
Since LPS causes more severe lung inflammation in BMP4 heterozygous mutant mice, we therefore tested the possibility whether BMP4 inhibits proinflammatory cytokine expression in airway epithelial cells. Exposure to BMP4 led to rapid canonical activation of mothers against decapentaplegic homologs (Smad)/5/8 in 16HBE cells, indicating BMP4 signaling was active in bronchial epithelial cells (Fig. 5A). Exposure to BMP4-specific siRNA (siBMP4) in 16HBE cells resulted in approximately 85% reduction of BMP4 mRNA comparing to nontargeting siRNA (siNT) exposed cells, indicating efficient knockdown (Fig. 5B). In 16HBE cells exposed to siNT, LPS (5μg/mL) and TNF-α (50 ng/mL) treatment caused about 1.3- and 38.5-fold induction of IL-8 mRNA expression; knockdown of BMP4 increased the basal, LPS, and TNF-α-induced IL-8 mRNA expression for about 72, 98, and 27%, respectively, comparing to siNT-treated cells (Fig. 5D). Conversely, pretreatment with exogenous BMP4 (50 ng/mL) not only attenuated the basal expression of IL-8 mRNA, but also the one induced by LPS and TNF-α in 16HBE cells (Fig. 5E).
Moreover, we examined the effects of BMP4 on LPS and TNF-α-induced IL-8 release in primary normal human bronchial epithelial (NHBE) cells, which are considered to be more relevant to cells under physiological condition. Nucleofection delivery of siBMP4 resulted in about 83% reduction of BMP4 mRNA comparing to siNT-treated cells (Fig. 5C). Exposure to LPS and TNF-α greatly increased IL-8 releases in NHBE cells exposed to siNT; knockdown of BMP4 by siBMP4 enhanced the basal IL-8 release, as well as the IL-8 releases induced by LPS and TNF-α comparing to cells treated with siNT (Fig. 5F). In contrast to the results obtained with siBMP4, pretreatment with exogenous BMP4 (50 ng/mL) attenuated the basal IL-8 release, and the one induced by both LPS and TNF-α in NHBE cells (Fig. 5G). Similar to NHBE, we found pretreatment with BMP4 inhibited the basal and LPS-induced and TNF-α-induced KC releases in primary MTSE cells (Fig. 5H).
BMP4 inhibits LPS-induced proinflammatory cytokine production in mouse peritoneal macrophages
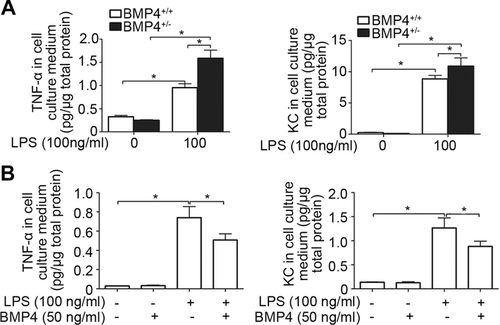
Besides epithelial cells, macrophages is also critical for airway innate immunity by producing proinflammatory cytokines, such as TNF-α and KC. To understand the impacts of BMP4 on macrophages-mediated inflammation, we isolated the primary peritoneal macrophages from BMP4+/+ and BMP4+/− littermate mice and exposed them to LPS (100 ng/mL). As shown in Figure 6A, the basal TNF-α and KC releases in macrophages from BMP4+/+ and BMP4+/− mice were not different. LPS exposure stimulated TNF-α and KC releases in macrophages from both strains of mice; however, their increases in cells from BMP4+/− mice were significantly greater than that from BMP4+/+ mice (Fig. 6A). Contrary to the above results obtained from BMP4 mutant mice in vivo, pretreatment with exogenous BMP4 (50 ng/mL) inhibited LPS-induced (100 ng/mL) TNF-α and KC releases in primary peritoneal macrophages from BMP4+/+ mice (Fig. 6B).
BMP4 deficiency protects against chronic LPS exposure-induced lung function reduction in mice
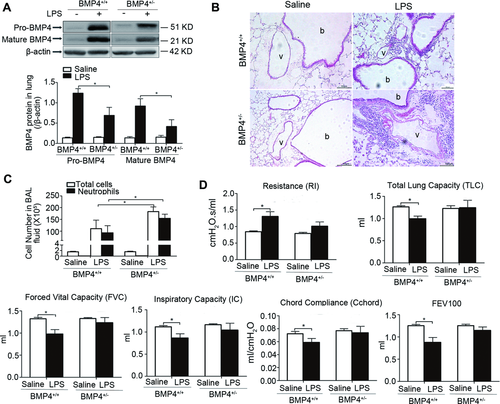
Next, we examined if BMP4 participates in regulation of lung inflammation and function associated with chronic injury induced by repetitive LPS challenge. Treatment of LPS for 4 weeks increased pro-BMP4 and mature BMP4 protein expression in lung from both BMP4+/+ and BMP4+/− mice; however, the extent of increase in BMP4+/− mice was drastically lower than that in BMP4+/+ mice (Fig. 7A). In conjunction with these changes, chronic LPS treatment caused significant inflammation in lung from both BMP4+/+ and BMP4+/− mice, the inflammation in BMP4+/− mouse lung was more severe as reflected by greater interstitial inflammatory cell infiltration in lung tissue (Fig. 7B) and greater total and neutrophil cell counts in BALF (Fig. 7C) comparing to that in BMP4+/+ mice. In contrast, BMP4+/+ mice treated with LPS exhibited restrictive lung function reduction manifested by increased resistance, decreased total lung capacity (TLC), forced vital capacity, inspiratory capacity, chord compliance, and forced expiratory volumes at 100 ms, whereas BMP4+/− mice was protected against LPS-induced lung function decline (Fig. 7A–F).
Discussion
The present study provides evidence demonstrating that BMP4 plays an important anti-inflammatory role during acute lung inflammation induced by LPS or PA. These evidence include inhalation of LPS upregulated BMP4 expression in mouse lung; BMP4 heterozygous mutant mice exhibited enhanced pulmonary bacterial clearance, yet augmented lung inflammation during infection of PA. Moreover, BMP4 deficiency impaired resolution of acute inflammation in mouse lung. The anti-inflammatory effects of BMP4 were attributable at least in part to its inhibition on the proinflammatory cytokine release from airway epithelial cells and macrophages.
Treatments of LPS and TNF-α increased BMP4 expression in mouse lung and specifically in airway epithelial cells. We do not know how they influenced the expression of BMP4, it is possible that these stimuli upregulate BMP4 expression by activating NF-κB and hypoxia inducible factor 1 (HIF-1) in airway epithelial cells during the innate immune responses. BMP signaling was reported being activated by NF-κB in various systems 11, 12. TNF-α has been shown to increase HIF-1α content in primary inflammatory cells 13, and we recently found HIF-1α could upregulate BMP4 expression by directly binding to the promoter of BMP4 in pulmonary arterial smooth muscle cells (our unpublished data).
Contrary to the anti-inflammatory effects of BMP4 in LPS and PA-induced lung inflammation, there is evidence showing that BMP4 was proinflammatory in the blood vessel wall of systemic circulation, where BMP4 was reported to promote inflammation during the atherogenesis in the lesion-prone areas in arterial region 14. A possible reason for these opposite function of BMP4 in regulating immune responses under different conditions may be due to the cross-talk between BMP family members and other signaling pathways 15, among them the TLR signaling that mediates innate or adaptive immune responses might be a top candidate 16-20. The different pathophysiological environment might also contribute to the differential effects of BMP4 in cells from different location. A similar phenomenon was observed when comparing the role of BMP4 in regulating oxidant production and inflammatory events in cultured human coronary arterial endothelial cells and pulmonary arterial endothelial cells. While treatment of BMP4 was found to increase O2− and H2O2 generation, induce NF-κB activation, upregulate ICAM-1, and induce monocyte adhesiveness to coronary arterial endothelial cells, it failed to induce these adverse effects in pulmonary arterial endothelial cells 21. Additionally, in pulmonary arterial smooth muscle cells, while BMP4 was found to promote proliferation of cells from distal pulmonary arteries, the one from proximal arteries was inhibited 22. In consistency with the results that we observed with LPS-induced inflammation in the airway, BMP4 was found to play anti-inflammatory roles via Smad-dependent activation of myocardin-related transcription factor A in human pulmonary artery smooth muscle cells 23. MRTR-A physically interacts with and block TNF-α-induced NF-κB p65 activity 24.
Both TNF-α and IL-8 (or KC) are important cytokines produced in response to LPS and mediating the incurred acute inflammation. The main sources of them are airway epithelial cells and macrophages that are too major first-line defensive players in innate immunity. Notably, we found that LPS and TNF-α upregulated BMP4 expression in airway epithelial cells, BMP4 in turn attenuated IL-8 or KC upregulation induced by LPS and TNF-α in these cells. Thus, TNF-α is not only a regulator of BMP4 expression, but also a target of its anti-inflammatory effects. It is likely that TNF-α and BMP4 participates in a feedback loop, where TNF-α triggers BMP4 upregulation, BMP4 in turn inhibits further TNF-α expression. Through this negative feedback loop, BMP4 acts as a crucial factor preventing excessive lung inflammation, and facilitating the resolution of inflammation. In airway epithelial cells, BMP4 might interfere with TNF-α-mediated signaling by activating Smad1/5/8 signaling that has been proved in cells beyond airway epithelial cells and to be either dependent or independent of NF-κB 23-26. In consistent with this presumption, we found Smad1/5/8 activation occurred in airway epithelial cells exposed to exogenous BMP4 treatment. Similar to airway epithelial cells, we demonstrated that BMP4 inhibited LPS-induced KC and TNF-α release by macrophages, indicating BMP4 also participates in modulating immune responses of macrophages. It is possible that the inhibitory action of BMP4 on LPS-induced proinflammatory cytokine release in epithelial cells and in macrophages was via a common pathway, as BMP4 could also stimulate Smad1/5/8 phosphorylation in macrophages cell lines, and in primary peritoneal macrophages 27.
PA is a normal flora in the respiratory system. PA infection is usually well controlled and cleared under normal immune system by innate immune responses 28. However, it could become an opportunistic pathogen responsible for pneumonia in patients with immunosuppression or chronic lung diseases. In COPD patients, PA serves as a common risk factor responsible for frequent exacerbations. Combining with other risk factors, such as cigarette smoking, PA-induced exacerbations contribute to the pathogenesis of COPD progression 29. In this study, we demonstrated BMP4-deficient mice exhibited enhanced pulmonary PA clearance, which was associated with elevated inflammation in mouse lung, indicating BMP4 plays an important role in PA infection. The role of BMP4 in COPD exacerbation-associated PA infection and disease progression warrant further investigation.
Similar to what we observed in LPS- and PA-induced acute lung inflammation, BMP4 inhibited the LPS-induced chronic lung inflammation as well. Nevertheless, BMP4+/+ mice exhibited restrictive lung function reduction that was absent in BMP4+/− mice. The reduced lung function in BMP4+/+ mice was likely due to enhanced endothelial and epithelial–mesenchymal transition caused by increased BMP4 expression, which results in increased vascular and airway remodeling 30, 31. BMP4 was found to promote vascular remodeling and development of chronic hypoxia induced pulmonary hypertension in a mouse model 10; it could also drive airway epithelial cell migration and mediate the switch toward an epithelial–mesenchymal transition like phenotype by altering protein expression to facilitate cell migration and wound closure 32, 33. Alternatively, we cannot exclude the possibility that BMP4 might act as a proinflammatory factor as seen in the atheroprone areas of arteries 14, promoting adhesion of monocytes at the site of airway epithelium lesion caused by chronic lung inflammation, thus impairing injury repair and leading to pulmonary dysfunction.
In summary, this is the first study to our knowledge, which demonstrated that BMP4 was upregulated during LPS-induced inflammation and then elicited important anti-inflammatory function by inhibiting proinflammatory cytokine release in lung. In this way, BMP4 may participate in a feedback loop to control the strength of lung inflammation during acute infection, and thus prevent lung injury due to excessive inflammation and facilitate resolution of inflammation. Nevertheless, the increased BMP4 expression may contribute to bacterial colonization and lung function decline during chronic lung inflammation. It will be our future interest to explore how to take advantage of the anti-inflammatory function of BMP4 without impairing bacterial clearance capacity and lung function.
Materials and methods
Animals and LPS treatment
C57BL/6J mice (6–8 weeks old) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). Breeders of BMP4 heterozygous mutant mice (Bmp4+/−) with C57BL/6J background were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). The mice were housed in specific pathogen-free facilities, bred, and genotyped according to the vendor's protocols. All experimental procedures were approved by the Animal Care and Use Committee of Guangzhou Medical University. For acute challenge, BMP4 WT (BMP4+/+) or BMP4+/− mice (male, 6–8 weeks old) were treated with saline or LPS (7.5 μg/mouse in 50 μL saline; L3024; Sigma-Aldrich, St. Louis, MO) by i.n. inhalation, and euthanized at 12, 24, or 48 h thereafter. For chronic LPS exposure, mice (8–10 weeks old) were inhaled with saline or LPS (1.0 μg/mouse in 50 μL saline) once a week for four times, and sacrificed 24 h post the last challenge. Since homozygous mutation of BMP4 (BMP4−/−) is lethal to the embryos, we therefore use heterozygous null (BMP4+/−) mice for experiments. Aside from minor renal, craniofacial, and skeletal phenotypes, BMP4+/− mice appear normal under unchallenged condition 34.
BALF collection, cell counting, and lung histological analysis
Mouse lung was lavaged with 0.8 mL saline/lung for two times. Total cells in the BALF were collected by centrifuging at 5 000 rpm for 5 min, and stained with H&E for differential cell counting. For lung histological analysis, lung lobes were infused via tracheal with 4% paraformaldehyde under 20 cm H2O, xed for 24 h, embedded in paraffin, sectioned at 5 μm thickness, and stained with H&E.
PA infection
The PA infection and assessment of lung bacterial clearance were performed as we described previously 35. Briefly, PA strain PA01 (ATCC, Manassas, VA) was propagated in Luria broth, centrifuged, and resuspended in PBS. The mice were anesthetized by i.p. injection of 50 mg/Kg sodium pentobarbital, inoculated with 1 × 105 or 1 × 106 CFU of PA01 in 50 μL PBS by i.n. inhalation, and were dissected at 12 or 24 h after. The total cells in BALF were counted and the levels of TNF-α and KC in BALF were measured by ELISA. For PA clearance assay, the lungs were homogenized in 10 mL PBS. The bacterial CFU in lung were enumerated on Luria agar plates after overnight incubation under 37°C.
Airway epithelial cell culture and treatments
Human bronchial epithelial cells 16HBE were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), propagated and plated in 6-well culture plates in DMEM supplemented with 10% fetus bovine serum (FBS), 100 U/mL penicillin, and 100ug/mL streptomycin at 37°C.
Primary NHBE cells were purchased from Lonza, Inc. (Allendale, NJ), propagated in BEGM (Lonza, Inc.), and seeded at 2.5 × 105 cells/insert on 12 mm Millicell insert membrane (EMD Millipore, Billerica, MA) coated with human placenta collagen type IV (Sigma). The Millicells were put in 24-well plate containing air–liquid interface (ALI) culture medium (Lonza, Inc.), and switched to ALI culture when transepithelial electrical resistance reached greater than 1 000 Ω cm2 measured with an epithelial Ohm-voltmeter (MER-S00002, Millipore).
MTSE were isolated and cultured as we previously described 36. Briefly, the tracheas from C57BL/6J mice (8–10 weeks old) were removed and infused with 0.5% type XIV pronase (Sigma) to release MTSE cells. Approximately 1 × 105 cells/well were plated on human placenta collagen type IV coated 12 mm Millicell inserts, cultured with DMEM/F-12 medium containing 10 μg/mL insulin, 5 μg/mL transferrin, 25 ng/mL epidermal growth factor, 10−7 M hydrocortisone, 0.1 μg/mL cholera toxin, 1% bovine pituitary extract, 5 × 10−8 M all trans-retinoic acid, 5% FBS, 50 U/mL penicillin, and 50 μg/mL streptomycin. Twenty-four hours after plating, FBS in this medium was replaced with 0.3% BSA. Media were changed every 2 days until the transmembrane resistance reached >1 000 Ω cm2. Media in the apical chamber was then removed to establish an ALI, and the cells were fed only from the bottom chamber.
For drug treatments, the cells were serum starved for 24 h, incubated with LPS (1 or 5 μg/mL; Sigma), TNF-α (50 ng/mL; Sigma), or BMP4 (50 ng/mL; R&D systems, Minneapolis, MN) for indicated time, and then the cell culture medium or cell lysate was collected for subsequent analysis.
Isolation and culture of mouse peritoneal macrophages
Peritoneal macrophages (PMs) from BMP4+/+ and BMP4+/− mice were harvested under aseptic conditions at 3 days after i.p. injection with 3% thioglycollate (Sigma-Aldrich). Red blood cells in PM preparation were lysed in erythrocyte lysis buffer (10 mM Tris-HCl [pH 7.2] containing 150 mM NH4Cl). The resultant PM was seeded in 6-well tissue culture plates at 2.5 × 105 cells/well in DMEM medium containing 10% FBS and the nonadherent cells were removed 24 h after. Then, the cells were cultured for further 24 h in DMEM medium containing 1% FBS before being subjected to 4 h LPS treatment (100 ng/mL). For determining the effects of BMP4 on KC and TNF-α release of PM, PM cells from C57BL/6J mice were treated with LPS (100 ng/mL) for 12 h with or without pretreatment of BMP4 (50 ng/mL) for 1 h.
Small interference RNA transfection
For siRNA treatment, 16HBE cells seeded in 6-well dish were cultured for 24 h, transfected with 1.5 μg BMP4 siRNA (siBMP4; forward: 5′-GGA CUA ACA UGC GGG AUC UUT T-3′; reverse: 5′-AAG AUC CCG CAU GUA GUC CTT-3′) or a siNT using Hiperfect Transfection Reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions. NHBE cells were transfected with siBMP4 or siNT at 1.0 μg siRNA/106 cells using Amaxa NHBE Nucleofector Kit (Lonza, Inc.). Cells treated with siRNA for 48 h were subjected to LPS (5 μg/mL) or TNF-α (50 ng/mL) treatment for 6 h, and then followed by IL-8 or KC measurements.
Lung function measurements
Mouse lung function was measured using Forced Pulmonary Maneuver System (Buxco Research Systems, Wilmington, NC, USA) following the manufacturer's protocols. Briefly, mice challenged with saline or LPS were anesthetized by i.p. injection of pentobarbital (50 mg/Kg), tracheostomized, and placed in the body chamber of the system. The average breathing frequency was set to 140 breaths/min. To measure the TLC, inspiratory capacity and forced vital capacity, quasi-static pressure volume maneuver was performed, which inflates the lungs to a standard pressure of +30 cm H2O and then slowly exhales until a negative pressure of −30 cm H2O is reached. The quasi-static compliance was defined as the volume/pressure ratio at 50% of the expiration (Cchord50). With the fast flow volume maneuver, lungs were first inflated to +30 cm H2O (TLC) and immediately afterwards connected to a highly negative pressure in order to enforce expiration until residual volume (RV) at −30 cm H2O. Forced expiratory volumes at 100 ms were recorded during this maneuver. For each test in every single mouse, at least three acceptable maneuvers were conducted to obtain a reliable mean for all numeric parameters.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from lung tissue or cells using Trizol reagent (Invitrogen, Carlsbad, CA). DNA contamination in RNA preparations was removed by TURBO DNA-Free DNase treatments (Ambion, Austin, TX). Reverse-transcription was performed in a 20 μL reaction mixture containing 1 000 ng total RNA using Takara cDNA synthesis kit (Dalian, Liaoning, China). BMP4 cDNA was quantified by real-time PCR on CFX96–C1000 system (Bio-Rad, Hercules, CA) using QuantiTect SYBR Green kit (Qiagen). The primers were: 5′-TGA GCC TTT CCA GCA AGT TT-3′ (forward) and 5′-CTT CCC CGT CTC AGG CAT CA-3′ (reverse) for human BMP4; 5'-AAC TTC TCC ACA ACC CTC TG-3′ (forward) and 5′-TTG GCA GCC TTC CTG ATT TC-3′ (reverse) for human IL-8; and 5′-GCA ATT ATT CCC CAT GAA CG-3′ (forward) and 5′-GGC CTC ACT AAA CCA TCC AA-3′ (reverse) for 18S as internal control. Relative concentration of each transcript was calculated using the Pfaffl method 37. Efficiency for each gene was determined from 5-point serial dilutions of an unknown cDNA sample. The expression of BMP4 or IL-8 was normalized to 18S in the same sample.
Western blotting
Mouse lung tissues and cells were homogenized in RIPA lysis buffer containing 1% protease inhibitor cocktail (Sigma-Aldrich), 5 μM EDTA, and 200 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride to extract the total protein. Equal amount of protein homogenates in samples were separated on SDS-PAGE (Bio-Rad); transferred onto polyvinylidene difluoride membranes (pore size 0.45 μM, Bio-Rad); blotted with mouse monoclonal antibody against BMP4 (1:1 000, EMD Millipore), P-Smad1/5/8, or T-Smad1 (1:500, Cell Signaling Technology, Inc., Danvers, MA) or β-actin (1:3 000, Sigma-Aldrich); and probed with corresponding peroxidase-conjugated secondary antibodies (KPL, Gaithersburg, MD). Signal of bound antibodies was developed using Immun-Star HRP Chemiluminescent kit (Bio-Rad).
ELISA
The protein levels of TNF-α and KC in mouse BALF, IL-8, and secreted BMP4 in cell-cultured media were measured by ELISA. The TNF-α and IL-8 ELISA kits were purchased from eBioscience (San Diego, CA). The capture and detection antibodies for KC measurement were obtained from R&D Systems, Inc. (Minneapolis, MN). The primary and secondary antibodies for BMP4 detection were from EMD Millipore and KPL, respectively. Signal was developed using TMB Substrate Reagent Set (BD Biosciences).
Materials and drugs
Unless otherwise specified, all reagents were obtained from Sigma-Aldrich. Recombinant human BMP4 stock solutions at 50 μg/mL were made in 4 mM HCl containing 0.1% BSA.
Statistical analysis
All experiments were repeated three times and each measurement was performed in triplicates. Statistics were analyzed with ANOVA or two-tailed Student's t-test. If significant F ratios were obtained with ANOVA, pairwise comparison of means was conducted with Bonferroni tests. Data were presented as mean ± SEM. N equals the number of treatment wells or animals. p < 0.05 was considered as significantly different.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81070043, 81071917, 81173112, 81170052 and 81220108001), a Changjiang Scholars and Innovative Research Team in University grant (IRT0961), a Guangdong Natural Science Foundation Team Grant (1035101200300000), the Guangdong Department of Science and Technology of China (2009B050700041 and 2010B031600301), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2014) and the Key Grant for Innovative Research (cxzd1142), a Guangdong Department of Education Research Grant (cxzd1025), Guangzhou Department of Education Yangcheng Scholarships (10A058S and 12A001S), and Guangzhou Department of Education Team Grant for Innovation (13C08). Zhengtu Li performed the experiment, analyzed the data, and drafted the paper; Jian Wang initiated, designed the study, and analyzed the data; Hua Jiang performed the experiment, analyzed the data, and drafted the paper; Yan Wang, Xiaoming Xu, Chenting Zhang, Defu Li, Chuyi Xu, Kedong Zhang, Yafei Qi, Xuefang Gong, and Chun Tang contributed to the experiments; Nanshan Zhong consulted the study; Wenju Lu initiated, designed the study, analyzed the data, and wrote the paper.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- BMP
-
- bone morphogenetic protein
-
- KC
-
- keratinocyte chemoattractant
-
- MTSE
-
- mouse tracheal surface epithelia
-
- NHBE
-
- normal human bronchial epithelial
-
- PA
-
- Pseudomonas aeruginosa
-
- PM
-
- peritoneal macrophages
-
- TLC
-
- total lung capacity