Positive selection of self-antigen-specific CD8+ T cells by hematopoietic cells
Abstract
In contrast to thymic epithelial cells, which induce the positive selection of conventional CD8+ T cells, hematopoietic cells (HCs) select innate CD8+ T cells whose Ag specificity is not fully understood. Here we show that CD8+ T cells expressing an H-Y Ag-specific Tg TCR were able to develop in mice in which only HCs expressed MHC class I, when HCs also expressed the H-Y Ag. These HC-selected self-specific CD8+ T cells resemble innate CD8+ T cells in WT mice in terms of the expression of memory markers and effector functions, but are phenotypically distinct from the thymus-independent CD8+ T-cell population. The peripheral maintenance of H-Y-specific CD8+ T cells required presentation of the self-Ag and IL-15 on HCs. HC-selected CD8+ T cells in mice lacking the Tg TCR also showed these features. Furthermore, by using MHC class I tetramers with a male Ag peptide, we found that self-Ag-specific CD8+ T cells in TCR non-Tg mice could develop via HC-induced positive selection, supporting results obtained from H-Y TCR Tg mice. These findings indicate the presence of self-specific CD8+ T cells that are positively selected by HCs in the peripheral T-cell repertoire.
Introduction
Generally, developing thymocytes undergo positive selection by interacting with MHC molecules on cortical thymic epithelial cells (cTECs). However, subsets of T cells can develop even when cTECs do not express MHC molecules, and are thus positively selected by hematopoietic cells (HCs). For instance, NKT cells are known to be selected by CD1, a nonclassical MHC class Ib molecule, on DP thymocytes 1. HC-induced positive selection of MHC class Ib-restricted CD8+ T cells has also been demonstrated 2, 3. 2C TCR Tg CD8+ T cells, which are selected by Kb and react with allogeneic Ld molecules, also developed in BM chimera mice in which cTECs do not express MHC class I 4. However, it was later indicated that 2C TCR also reacted with MHC class Ib molecules 5. Thus, HC-induced positive selection might not simply be an inefficient pathway of T-cell development but preferentially generate MHC class Ib-restricted T cells. However, since it was shown that a small but significant number of MHC class Ia-restricted CD8+ T cells were selected by HCs 3, 6, there could be other or additional conditions that permit HC-induced selection. Interestingly, recognition of self-Ags has been suggested for some HC-selected T cells, such as H2-M3-restricted CD8+ T cells as well as NKT cells 7, 8.
A substantial proportion of mature T cells in naive mice resemble memory T cells not only in the expression pattern of surface molecules but also in the effector functions. These memory phenotype (MP) T cells might include bona fide memory T cells, but exist even in naive germ-free mice 9. Actually, naive CD8+ T cells that have undergone lymphopenia-induced proliferation are indistinguishable from MP CD8 T cells 10. In addition, innate CD8+ T cells, which are distinct from conventional CD8+ T cells in that they can develop independently of Tec family kinases bypassing the naive state, also express memory markers in situ 11. Interestingly, innate CD8+ T cells could develop in the absence of MHC class I molecules on cTECs, while HC-selected CD8+ T cells express memory markers 3, 11. Expression of memory markers on CD8+ T cells in MHC class Ia (Kb and Db)-deficient mice was also reported 12-14, although it was later demonstrated that HC-induced development rather than class Ib-restriction determines the expression of memory markers 15. Therefore, innate MP CD8+ T cells and HC-selected CD8+ T cells likely represent the same cell populations.
We have reported unique features of self-Ag-specific CD8+ T cells that develop in mice expressing Tg TCR specific for an H-Y Ag peptide on Kb (H-Y TCR Tg) 16. Despite negative selection in the thymus, there are a large number of TCR Tg CD8+ T cells in the periphery of male mice. These self-specific CD8+ T cells do not react with male APCs and are thus not “self-reactive,” but do respond to anti-TCR mAbs or an increased dose of the Ag peptide stimulation 17. Interestingly, they resemble innate CD8+ T cells in many aspects, such as the expression of memory markers, IL-15-dependency, and in situ equipment of effector functions, supporting the hypothesis that innate MP CD8+ T cells in WT mice include self-specific CD8+ T cells 18, 19. In fact, H-Y TCR Tg CD8+ T cells can develop in male athymic mice, indicating that cTECs are not an absolute requirement for their development, although the absolute number was far lower in male athymic mice than those in euthymic mice 20. In addition, it is unclear if such self-specific CD8+ T cells are selected by HC in euthymic conditions. It has only been shown that HCs fail to induce positive selection of H-Y TCR Tg CD8+ T cells in female mice 4, 21.
In the present study, we found that the self-specific TCR Tg CD8+ T cells were positively selected by HCs. By using soluble MHC class Ia molecules loaded with male Ag peptides, we also found that self-Ag-specific CD8+ T cells in TCR non-Tg mice could develop via HC-induced selection. Thus, our data provide further evidence for self-specificity of the innate HC-selected T-cell repertoire.
Results
Positive selection of self-specific TCR Tg CD8+ T cells by HCs
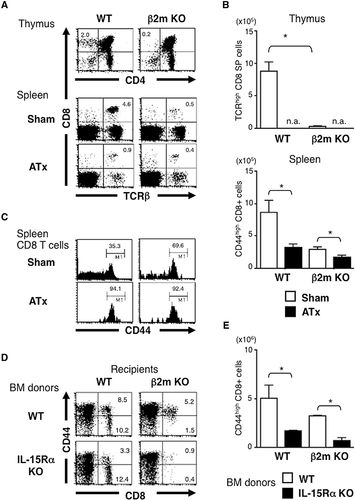
Male or female C57BL/6 (B6) mice or β2m KO mice were lethally irradiated and transferred with CD8+ cell-depleted BM cells (BMCs) from male or female H-Y TCR Tg mice. In the thymus of female WT recipients reconstituted with BMCs from female Tg mice, development of CD8+ SP thymocytes, the majority of which expressed Tg TCR, was observed (Fig. 1A and data not shown). On the other hand, the thymi of female recipients reconstituted with male BMCs showed a developmental arrest of thymocytes at the DN stage similar to the thymi of male recipients. Thymocyte development was arrested at the DP stage in β2m KO recipients reconstituted with female BMCs, indicating a requirement of MHC class I molecules on non-HCs for the positive selection of H-Y TCR Tg thymocytes. Thus, H-Y Ag-specific CD8+ T cells efficiently developed only in female recipients reconstituted with female BMCs (Fig. 1B).
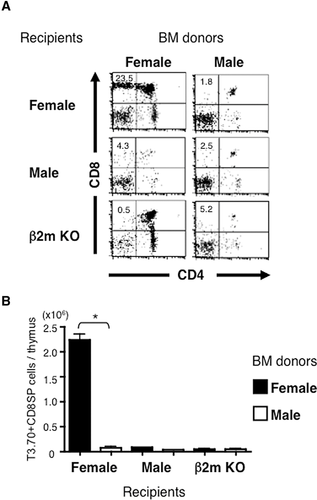
Despite the negative selection in the thymus, CD8+ T cells expressing Tg TCR (T3.70+) were abundantly found in the spleens of either male or female recipients reconstituted with male BMCs (Fig. 2A), although the expression level of CD8 was lower than those developed in female recipients transferred with female BMCs. Notably, there were also a large number of T3.70+CD8+ T cells in β2m KO mice reconstituted with male but not female BMCs. The absolute number of CD8+ T cells expressing Tg TCR in β2m KO recipients with male BMC was comparable with or even greater than that in WT recipients (Fig. 2B). Thus, self-specific CD8+ T cells can develop depending on MHC class I molecules expressed on HCs. We observed a similar pattern of T-cell development by transferring whole T-cell-depleted BMCs in Rag2-deficient H-Y TCR Tg mice (Supporting Information Fig. 1). Most T3.70+CD8+ T cells express CD8αβ but not CD8αα similar to those in unmanipulated male H-Y TCR Tg mice.
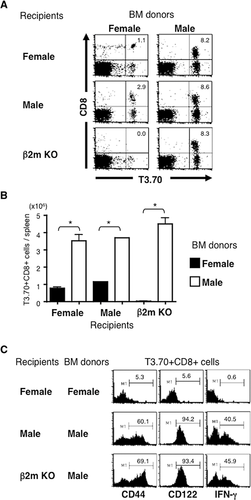
We and others have shown that H-Y TCR Tg CD8+ T cells developed in male mice resemble innate MP CD8 T cells 18. Here we found that the majority of T3.70+CD8+ T cells developed in β2m KO mice reconstituted with BMCs from male H-Y TCR Tg mice expressed CD44 and CD122. They produced IFN-γ after brief ex vivo stimulation with PMA and ionomycin (Fig. 1C), similar to those developed in WT recipients. Therefore, HC-selected T3.70+CD8+ T cells are functionally indistinguishable from T3.70+CD8+ T cells in unmanipulated male H-Y TCR Tg mice.
Maintenance of self-specific CD8+ T cells requires co-expression of self-Ag and IL-15 on HCs
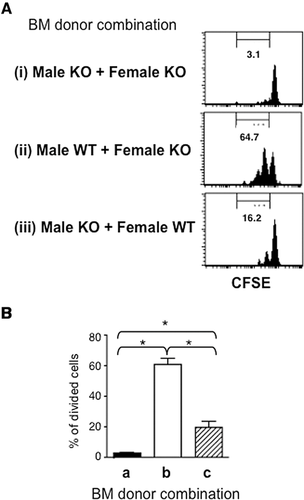
IL-15 induces homeostatic proliferation of T3.70+CD8+ T cells in male H-Y TCR Tg mice as well as MP CD8+ T cells in WT mice 19. Expression of self-Ag is also required for the homeostatic proliferation of male H-Y TCR Tg T cells 22. IL-15 exerts in vivo biological activity by means of transpresentation by IL-15Rα expressed on HCs 23. Therefore, it is of interest to examine whether the maintenance of T3.70+CD8+ T cells requires the male Ag expressed on the same HCs that express IL-15Rα. β2m KO mice were lethally irradiated and transferred with combinations of BMCs from male or female mice with or without IL-15Rα. Two months later, CFSE-labeled CD8+ T cells from male Tg mice were injected into the mixed BM chimera mice and were subsequently assessed for cell division (Fig. 3). Homeostatic proliferation of T3.70+CD8+ T cells was hardly detected in the BM chimera mice that lack IL-15Rα. However, T3.70+CD8+ T cells that had undergone homeostatic proliferation were clearly detected in the BM chimera mice with IL-15Rα-expressing male Ag-presenting cells, but were significantly reduced when male Ag and IL-15Rα were not expressed on the same cells. Thus, HCs induce not only development but also maintenance of the self-specific CD8+ T cells by concomitant presentation of the self-Ag and IL-15.
HC-selected CD8 T cells are not identical to thymus-independent CD8 T cells
HC-induced selection implies that the expression of MHC on TECs is not required for their development. However, it is unclear whether HC-induced development represents thymus-independent development (i.e., extrathymic development). So, we compared the development of H-Y TCR Tg T cells between euthymic and athymic β2m KO mice. Although development of T3.70+CD8+ T cells was clearly seen in athymic β2m KO mice as well as in athymic WT mice, their cell number was much lower than that of euthymic counterparts (Fig. 4A and B). It is worth noting that T3.70+CD8+ T cells in athymic mice had higher proportion of CD44high cells producing IFN-γ (Fig. 4C). Thus, there were quantitative and qualitative differences between HC-selected CD8+ T cells and thymus-independent CD8+ T cells, even if they expressed the same TCR.
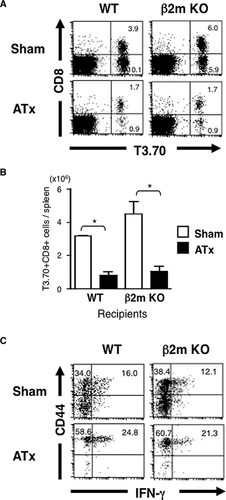
Similar developmental requirements of HC-selected CD8+ T cells in WT and H-Y TCR Tg mice
There is a possibility that CD8+ T cells in male H-Y TCR Tg mice are Tg idiosyncrasies. We here examined whether HC-induced CD8+ T cells in TCR non-Tg mice behave differently from those in H-Y TCR Tg mice. It was revealed that the number of CD8+ T cells developed in athymic mice was less than that developed in euthymic β2m KO mice, and that CD8+ T cells developed in athymic mice had higher portion of CD44high cells (Fig. 5A–C). Most of these HC-induced T cells in the spleen expressed CD8αβ but not CD8αα (data not shown). We also examined IL-15 dependency of HC-induced CD8+ T cells in TCR non-Tg mice. We found few CD8+ T cells developed in β2m KO recipients when the donor HCs lack the expression of IL-15Rα, while the lack of IL-15Rα did not significantly affect the number of CD44lo naive CD8+ T cells in WT recipients (Fig. 5D and E, data not shown). Thus, HC-induced CD8+ T cells in H-Y TCR Tg mice and TCR non-Tg mice shared similarities in these aspects.
HC positively selected self-Ag-specific CD8+ T cells in normal mice
It was reported that rare Ag-specific CD8+ T cells in naive TCR non-Tg mice can be detected by magnetic bead-based enrichment technique using a tetramer of MHC class I molecules loaded with Ag peptides 24. We applied this technique to examine Ag specificity of HC-selected CD8+ T cells in naive TCR non-Tg mice. We first analyzed the number of OVA257–264 peptide-specific CD8+ T cells in naive B6 mice, and confirmed it comparable to that reported previously (157 ± 51 cells per spleen, n = 5). We then examined the number of CD8+ T cells specific for a male Ag peptide, Uty246–254, in female B6 mice, which had or had not been primed with male splenocytes. We found that the enrichment technique made it possible to detect male Ag peptide-specific T cells in naive female mice (Fig. 6A). In order to ensure the specificity of tetramer binding, we co-stained splenocytes from female B6 mice with Uty-tetramer and an unrelated tetramer and found they stained different cell populations (Supporting Information Fig. 2). We also confirmed that the tetramer did not stain TCR Tg T cells with irrelevant Ag specificity.
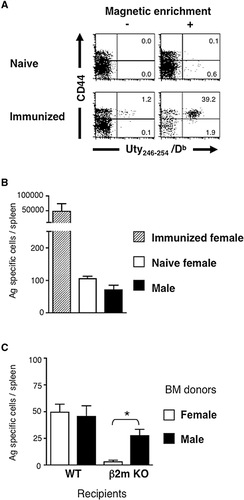
Although somewhat fewer than female mice, Uty peptide-specific CD8+ T cells were also detected in male B6 mice, supporting the relevance of T3.70+CD8+ T cells in male H-Y TCR Tg mice (Fig. 6B). We finally examined the number of Uty246–254 peptide-specific CD8+ T cells developed in BM chimera mice (Fig. 6C). It was revealed that the male Ag-specific CD8+ T cells were able to develop in β2m-deficient recipients when the donor BMC expressed the male Ag. They expressed CD44 as did the majority of CD8+ T cells in these mice (Supporting Information Fig. 2). Few OVA257–264 peptide-specific CD8+ T cells were detected in the β2m KO BM chimera mice (data not shown). Thus, HCs preferentially induced positive selection of self-specific CD8+ T cells also in TCR non-Tg mice.
Discussion
Although T cells play central roles in adaptive immune responses, the presence of unconventional T cells, which rather participate in innate immune responses, has been recognized 11. Such innate T cells are characterized by their ability to undergo positive selection by HCs. In this study, we demonstrated that self-specific CD8+ T cells in H-Y TCR Tg mice, which have been shown to share similarities with innate CD8+ T cells in WT mice, were selected by HCs. This not only supports the assumption that innate CD8+ T cells include self-specific T cells but also indicates that MHC class Ia-restricted T cells can be selected by HCs. Innate CD8+ T cells are generally regarded as class Ib-restricted CD8+ T cells. This is at least partly because it is difficult to prove HCs-induced development of MHC class Ia-restricted T cells, as mice lacking MHC class Ib only are not available. Although HCs can induce the development of 2C TCR Tg CD8+ T cells, it is possible that they are selected by MHC class Ib molecules 5. In this regard, H-Y TCR Tg CD8+ T cells are not likely the case, because they failed to be selected by HCs in female mice.
There might be a criticism that H-Y TCR Tg T cells do not represent T cells developed in physiological conditions. In fact, it was shown that the premature Tg TCR expression disturbs the timing of negative selection in the thymus 25. Lineage divergence into γδ T-cell-like cells induced by premature TCR expression was also suggested 26. However, premature TCR expression similarly occurs in the thymocytes of female H-Y TCR Tg mice, although they are not selected by HCs. In addition, premature αβ TCR expression on DN thymocytes in physiological condition was demonstrated 27. Furthermore, not only male CD8+ T cells in H-Y TCR Tg mice, but also HC-selected CD8+ T cells in normal mice resemble γδ T cells in that they belong to innate lymphocytes. Therefore, self-specific CD8+ T cells in H-Y TCR Tg mice may not be just Tg idiosyncrasies but represent innate nonconventional T cells. Yamagata et al. 28 demonstrated agonist-selected development of H-Y TCR Tg T cells, which were induced from female DP thymocytes and expressed CD8αα. Interestingly, such agonist-induced CD8+ T cells showed characteristics of innate immune cells. However, because there are only a few DP thymocytes in male H-Y TCR Tg mice, and most of naturally occurring CD8+ T cells in these mice are CD8αβ, they might represent a different self-specific CD8+ T-cell population with innate functions.
It is notable that male Ag-specific CD8+ T cells were also detected in male normal mice by using peptide-MHC tetramers. Importantly, they developed in the BM chimera mice even when recipients lacked β2m expression, suggesting that HC-induced T-cell development selects self-specific T cells even in physiological conditions. However, it is unclear how strict the discrimination of self and nonself for the HC-induced selection is. Apparently, it is important to examine other systems than the male–female combination, which are, however, not available thus far. It is anticipated that the polyclonal T-cell repertoires that react with a given peptide-MHC complex consist of cells with different TCR affinity. It is known that there is a considerable degree of variation in the avidity to self-MHC molecules among different TCR Tg T cells 29, 30. For instance, H-Y TCR Tg CD8+ T cells in female mice have a low affinity for self-MHC, while OT-1 TCR Tg CD8+ T cells, which recognize OVA peptide in the context of Kb molecules, have a strong affinity for self-MHC. Consistent with this, we observed the development of a small but significant number of OT-I T cells in BM chimera mice in which recipient lacked β2m KO (data not shown). This suggests that the requirements of self-Ag specificity for HC-induced positive selection are relative but not absolute measures. It would be preferable to examine mice with different levels of Ag expression in order to test this possibility.
It has been shown that innate CD8+ T cells in WT mice and the self-specific CD8+ T cells in H-Y TCR Tg mice decrease in the absence of IL-15 19, which is at least partly attributed to their IL-15-dependent maintenance. IL-15 signals lymphocytes by means of transpresentation, which is mediated by HCs, such as DCs. It was also suggested that the maintenance of male H-Y TCR Tg CD8+ T cells required the expression of the male Ag 22. In this study, we found that H-Y TCR Tg CD8+ T cells in male mice require IL-15 and the self-Ag to be presented on the same HCs for their maintenance. In addition, we demonstrated for the first time that HC-induced CD8+ T cells in TCR non-Tg mice also required the expression of IL-15Rα. Thus, HCs play a critical role in the maintenance of HC-induced CD8+ T cells in the periphery.
HC-induced T-cell development might otherwise mean cTEC-independent T-cell development. However, it does not simply mean extrathymic T-cell development, because we found quantitative and qualitative differences between HC-selected and thymus-independent CD8+ T cells. In both TCR Tg and non-Tg conditions, thymus-independent CD8+ T cells have a high portion of CD44high cells. Consistent with our data, Su et al. 14 reported that all class Ib-restricted CD8+ T cells developed in thymectomized mice were CD44high. Thymus-independent class Ib-restricted CD8+ T cells produced IFN-γ in an IL-12- and IL-18-dependent manner during Listeria monocytogenes infection, whereas a large part of MHC class Ib-restricted CD8+ T cells in euthymic mice exhibited bacteria-specific IFN-γ production. Thus, there are different levels of thymus-dependence even for T-cell populations, which do not require MHC on cTECs, although its molecular mechanism is unclear. It is also unknown where the HC-selected T cells develop despite the few CD8+ SP thymocytes (Fig. 1). Furthermore, one may argue that extrathymic development is a nonphysiological pathway of T-cell development, which is switched on only in the absence of the thymus 31. T-cell development via cervical thymus is another concern 32. Nevertheless, the age-related involution of the thymus is a well-known physiological process, and MP T cells in fact predominate in aged animals and humans, although the exact origin of these MP T cells is yet to be determined.
The importance of cTECs in conventional CD8+ T-cell development is associated with the expression of unique Ag processing machinery, the thymoproteasome 33. Thus, a lack of thymoproteasome results in an alteration of the self-peptide repertoire expressed on cTECs. Interestingly, there were various levels of thymoproteasome dependency in positive selection of different CD8+ T-cell clones 34. Female H-Y TCR Tg CD8+ T cells failed to develop in mice lacking thymoproteasome, whereas a small number of OT-I T cells developed, and 2C cells even increased in these mice. This suggests that HCs can substitute for cTECs in positive selection by thymoproteasome-independent self-peptides. This might also be related to differences in the reactivity to the self-MHC complex in the periphery. Because thymoproteasome-independent peptides can be expressed on HCs in the periphery, T cells that have been selected by those peptides might react with the self-MHC complex to induce homeostatic proliferation. However, true self-specific CD8+ T cells, such as H-Y TCR Tg CD8+ T cells in male mice, follow a different rule. They were not affected at all by the absence of cTECs and obviously thymoproteasome as well. Furthermore, they are phenotypically and functionally distinct from conventional CD8+ T cells. Taken together, it is assumed that HC-induced positive selection involves some conventional CD8+ T cells in addition to nonconventional CD8+ T cells, both of which have a relatively high affinity for self-Ags.
Our data added support to the assumption that innate T cells recognize self-Ags, although it is unclear whether there is any advantage of harboring such potentially hazardous self-specific T cells in physiological T-cell repertoires. Further investigation is necessary to understand the roles of self-specific innate CD8+ T cells in immune systems.
Materials and methods
Mice
C57BL/6 (B6) mice were purchased from Charles River Japan, Inc. (Yokohama, Japan). IL-15Rα KO and HY-TCR Tg mice were purchased from Taconic (Germantown, NY), and β2m KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME). HY-TCR Tg mice were crossed with CD45.1-congenic B6 mice and the offspring were used for the experiments. All mice were maintained in specific pathogen-free conditions and used for the experiments at 7–12 weeks of age. The study design was approved by the Committee of Ethics on Animal Experiment at the Faculty of Medicine, Kyushu University. Experiments were carried out under the control of the Guideline for Animal Experiment.
Antibodies and flow cytometric analysis
Fluorochrome-conjugated mAbs and reagents used for flow cytometric analysis were as follows: FITC-conjugated anti-CD4 (RM4–5) and anti-CD44 (IM7) mAbs, PE-conjugated anti-CD122 (TMβ1), anti-TCRβ (H57–597), anti-IFN-γ (XMG1.2) mAbs, allophycocyanin-conjugated anti-CD8α (53.6.7) mAb purchased from BD Biosciences (San Jose, CA), FITC- or PE-conjugated anti-H-Y TCR (T3.70), PerCP-Cy5.5-conjugated anti-CD45.1 (A20), and CD45.2 (104), PerCP-eFlior710-conjugated anti-MHC class II (M5/114.15.2) mAbs purchased from eBioscience (San Diego, CA). For cell surface staining, a single cell suspension was incubated with an optimal concentration of fluorescent mAbs in HBSS with 0.5% FCS and 0.1% azide (FACS buffer) for 20 min at 4°C. Propidium iodide (1 μg/mL) was added to the cell suspension just before run on a flow cytometer to detect and exclude dead cells from the analysis. Intracellular staining of cytokines was performed after 5 h stimulation of the cells with PMA (25 ng/mL) and ionomycin (1 μg/mL) in the presence of Brefeldin A (10 μg/mL) (Sigma-Aldrich, St. Louis, MO). The BD Cytofix/Cytoperm Kit (BD Biosciences) was used for fixation and permeabilization of the cells. Stained cells were run on a FACSCalibur flow cytometer (BD Biosciences). The data were analyzed using BD CELLQuest software (BD Biosciences).
Generation of BM chimera mice
BMCs were prepared from the femurs and tibias of male or female HY-TCR Tg mice. Red blood cells were lysed with 0.83% ammonium chloride and the remaining mononuclear cells were stained with purified anti-CD8 mAb followed by magnetic depletion using Dynabeads M450 sheep anti-rat IgG (Invitrogen). We routinely depleted more than 95% of CD8+ cells from BMC, and the remaining cells (1 × 106 cells/mouse) were injected i.v. into lethally irradiated (10 Gy) male or female WT B6 mice, and β2m KO mice. Thymectomy had been performed in some recipients 2 weeks before BM transfer, and reconstituted mice were used for the experiments more than 6 weeks after BM transfer.
Adoptive transfer of CFSE-labeled T cells
β2m KO mice were lethally irradiated (10 Gy) and transferred with a mixture (1 × 106 each) of BMCs from male and female IL-15Rα KO mice, male WT and female IL-15Rα KO mice, or male IL-15Rα KO and female WT mice. After 6 weeks, 2 × 106 CFSE-labeled T cells from male HY-TCR Tg mice were injected i.v. into the recipients, whose splenocytes were analyzed 2 weeks later. CFSE labeling was performed by incubating the cells with 1 μM CFSE (Invitrogen Life Technologies, Grand Island, NY, USA) in PBS for 15 min at 37°C.
Magnetic beads-based cell enrichment of Ag-specific T cells
The enrichment of Ag-specific CD8+ T cells by using fluorochrome-conjugated MHC class I tetramer and magnetic beads was performed according to a previous report 24 with some modifications. Splenocytes were stained with 2.4G2 mAb followed by PE-conjugated T-Select H-2Db HY Uty Tetramer or H-2Kb OVA Tetramer (MBL, Nagoya, Japan) in 100 μL of FACS buffer for 60 min at 4°C. Cells were washed twice and resuspended in 90 μL of PBS with 0.5 M EDTA, 0.1% azide, and 10% BSA (MACS running buffer). Cells were stained with 10 μL of anti-PE MACS Microbeads for 20 min rotating at 4°C, washed, and then applied to an autoMACS separator (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Enriched cells were then stained with allophycocyanin-conjugated anti-CD8a mAb. PerCP-Cy5.5-conjugated anti-CD45.2 and eFluor710-conjugated anti-I-A/E mAbs and propidium iodide was also added to exclude the stained cells from the analysis. The absolute number of tetramer-positive cells was calculated from the event count of control samples with a defined number of cells by running them on the flow cytometer at the same time period as the test samples.
Statistics
Statistical significance was calculated by the Student's t-test using Prism software (GraphPad, San Diego, CA). Differences with p-values of <0.05 were considered statistically significant.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for Promotion of Science, and the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases launched as a project commissioned by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
References
Abbreviations
-
- BMC
-
- BM cell
-
- cTEC
-
- cortical thymic epithelial cell
-
- HC
-
- hematopoietic cell
-
- MP
-
- memory phenotype
-
- TEC
-
- thymic epithelial cell