Differential compartmentalization of memory B cells versus plasma cells in salmonid fish
Abstract
The disposition of teleost memory and plasma cells (PCs) has essentially been un-explored. As the organization of the teleost immune system differs significantly from that of mammals (i.e. no bone marrow or lymph nodes, hematopoietic anterior kidney), this disposition could be essential in understanding how comparable functions are achieved. To address this question, the primary and secondary antibody-secreting cell, B memory cell, and antibody responses to T-independent and T-dependent antigens were analyzed in trout. Although the TI and TD antibody responses did not differ substantively from one another, the secondary responses to both were significantly prolonged compared with the primary responses. Logarithmic increases in titer and affinity were achieved for both antigens during the primary, with only modest increases during the secondary response. Antibody-secreting cells, both PCs and plasmablasts, quantitatively paralleled antibody production, with antibody-secreting cells skewing to the hematopoietic anterior kidney for both antigens. However, the enhanced antigen-inducible response of immune fish (indicative of the memory pool) skewed to the peripheral blood and spleen. This pattern of memory versus PC disposition parallels that observed in mammals even though the organization of the respective immune systems differs considerably.
Introduction
Teleost memory has been defined by the development of a differentially heightened responsive state to antigen, albeit lacking in a number of mammalian features. Primary immunization can, thus, lead to increased immunogen sensitivity 1, enhanced in vivo 2-6 and in vitro 6-8 antibody responsiveness, expansion of antigen-sensitive precursor pools 9 and, most importantly, anamestic responses to pathogens 4, 5, 10, 11 and immunoprophylaxis 4, 12-14. Anamestic features absent from teleost responses include isotype switching 15 (although they possess multiple isotypes 16), relatively modest somatic mutation 17-19 and affinity maturation 20-22 as compared with the IgG responses of mammals. Intriguingly, characteristics of teleost memory B cells (MBCs) 9 parallel those associated with a class of mammalian MBCs. Recent evidence reveals that human MBCs can include unswitched, mutated IgM+ cells 23; nonmutated, CD27+IgM+ cells 24, those induced by T-independent (TI) antigens 25, 26, preimmune diversified repertoires 27 (as has recently been posed for the catfish 19), or arise independently of GCs 27, 28. Together, these previous studies prompt a renewed focus on the critical elements of μ specific memory, both in teleosts and mammals. For example, perhaps the requisites for exceptionally high-titered, extensively mutated, high-affinity responses in mammals are simply a requisite for employing a bivalent antibody (such as IgG) which would not be as avid as an IgM. Also, as the teleost system does not employ bivalent antibodies in its secondary responses, these species may serve as uncomplicated models for the analysis of the capabilities of mammalian μ memory, without the interplay of competing bivalent antibodies and their corresponding B-cell populations.
The commonalities between mammalian and teleost MBCs could suggest the potentially unique value of the teleost as a biomedical model. Parallel examination of mammalian and teleost memory may reveal evolutionarily universal mechanisms of memory generation. Tantalizing possibilities of such parallel features have been observed with long-lived plasma cell (LLPC) distributions in mammals 29-31 and teleosts 32-34, which home to their respective hematopoietic tissues, even though the organs themselves are different (i.e. bone marrow versus anterior kidney). One unexplored facet, however, has been the observation that the two distinct cellular arms of mammalian memory, the MBCs and LLPCs, possess distinctly different regionalities, with MBCs centered in peripheral tissues 35-37, and LLPCs within the primary lymphoid tissues 29-31. As teleosts possess neither bone marrow nor lymph nodes, the question arises whether comparable regional patterns of dissemination occur in the teleost as well and, if so, whether distinct functional differences are imparted to these different organizational divisions. Further, rigorous examination of teleost memory, its induction, and maintenance may reveal the critical mechanisms which transcend phylogeny or, alternatively, suggest reasons for their divergence.
Results
Primary and secondary antibody responses to TI and TD forms of antigen
Examination of the responses to TNP-LPS (TI) and TNP-keyhole limpet hemocyanin (KLH) (T-dependent, TD) revealed striking similarities between the responses (Fig. 1). The TNP-LPS response peaked earlier (week 16) with average titers of approximately 50 000 units/mL and TNP-KLH titers peaked between weeks 16 and 25 averaging 10 000 units/mL. Both these responses subsided to approximately 2 000 units/mL (TNP-LPS) and 5 000 units (TNP-KLH) by week 44. Upon rechallenge it was found that both antigens produced a higher maximal response than observed in the primary (∼100 000 units/mL for TNP-LPS and ∼50 000 units/mL for TNP-KLH) and this response persisted significantly longer with higher responses still occurring at 46 weeks post challenge (week 92 overall).
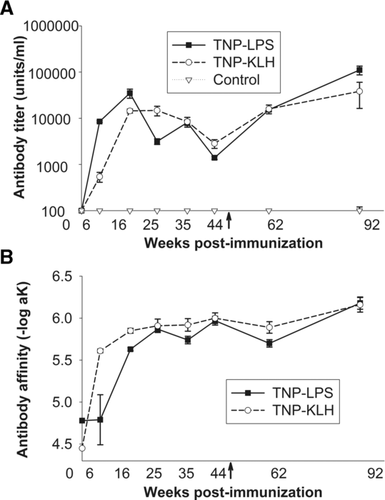
Although affinity maturation to the TNP-KLH response was slightly more rapid than that observed for the TNP-LPS response (Fig. 1B), both responses achieved approximately the same average affinity of 6.0 (−log aK) by week 44 of the primary response. This represents a 20–50-fold increase in affinity over the course of the primary response. This level of affinity was maintained and marginally increased (6.2) by week 46 of the secondary response
Antibody-secreting cell (ASC) production and distribution
The spleen appeared to harbor the highest number of ASCs during the primary response, with the anterior kidney becoming more dominant during the secondary response (Fig. 2A and C). These data were expressed as ASCs induced per 106 leukocytes (Fig. 2A, C and E) and as total ASCs per whole tissue (Fig. 2B, D and F). The latter metric is important as, for example, the entire blood volume can harbor up to 15 times the number of total leukocytes as can be found in the spleen and up to four times the number within the anterior kidney. However, at all time points, and for both immunized groups, the anterior kidney was the primary contributor to the total number of in vivo ASCs (Fig. 2B and D), with a mean of 89.6% of the entire ASC response over all time points. The mean splenic contribution was approximately 9.1%, while the blood only contributed 1.3%. Although, the secondary in vivo anterior kidney response to TNP-KLH followed approximately the same kinetics as in the primary response, the TNP-LPS response attained much higher numbers (∼250 000 versus 20 000 ASCs per anterior kidney, Fig. 2B versus D).

Plasma cell (PC) production and distribution
In order to ascertain the relative PC response that can occur in these tissues, leukocytes were also cultured for 7 days without antigen, and in the presence of HU. This technique permitted enumeration of non-replicating, antigen-insensitive, PCs 32, 38 (Fig. 3A–D).

The antibody titers (Fig. 1A) mirrored the anterior kidney PC as well as ASC responses (Fig. 2A–D), with both primary and secondary TNP-LPS challenges providing more robust responses (Fig. 1A and 2). With the ASCs in general, the bulk of the PC activity was found within the anterior kidney.
MBC production and distribution
The TI and TD induction of MBCs was assessed by comparison of in vitro challenge of PBLs, splenocytes, and anterior kidney lymphocytes from naive or primed individuals as previously performed in teleosts and mammals 1, 7, 39, 40. By week 44 post TNP-LPS immunization, the MBC response had increased approximately 18-fold in the blood and spleen over naïe (control) individuals, while those within the anterior kidney only increased eightfold (Fig. 4A and B versus Fig. 4E and F). After a secondary TNP-LPS booster, a 200-fold increase in the responsiveness had occurred (week 92; Fig. 4).

Initial priming with TNP-KLH markedly increased the memory response capacity in blood and spleen (∼100–200-fold over naive fish), with only a relatively modest, 20-fold, increase in the anterior kidney (Fig. 4C and D). Unlike the T-independent booster, a secondary boost with the TD antigen did not achieve a further increase over the primary response. The net effect of the two challenges with the two different antigens resulted in the same level of MBCs at the end of secondary response period.
Unique B-cell differentiative capacities of blood, spleen, and anterior kidney
The staggered addition of HU to antigen-stimulated cultures permitted analysis of plasmablast versus PC differentiation from resting MBCs. Virtually all induced ASC activity from PBLs was terminated within 24 hours of HU addition, indicating that virtually all of the induced ASCs were plasmablasts and remained so throughout culture (Fig. 5A, D, G and J). Peripheral blood cultures without antigen produced no ASCs. A small pulse of antigen-induced, non-replicating ASCs was witnessed in most cultures with a peak expression at approximately day 4. Although typically achieving approximately 5% or less of the total response on day 4, approximately 20% of the response at this time point was observed in one culture set (Fig. 5A). The magnitude of this minor response was not impacted by the timing of HU addition, indicating that this pool of antigen-induced ASCs does not undergo plasmablast-like proliferation. We have tentatively termed the precursor of these ASCs a transitional or preplasma cell (Fig. 6, Stage IV; rationale provided in the Discussion). Importantly, as HU does not halt this ASC activity, yet the population slowly diminishes over the culture period, these ASCs appear to be short-lived plasma cells (SLPCs).
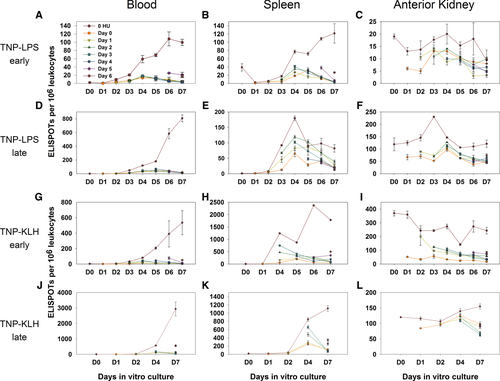
The antigen-induced splenic response was fairly comparable to the PBL response, except for the relative magnitude and kinetics of the preplasma cell population. Again, no responses occurred without the addition of antigen. Early after immunization (Fig. 5B and H) the splenic plasmablast population appears comparable to that found in the PBLs; however, with significantly more SLPCs peaking at day 4 (Fig. 5E and K). Like the population within the blood, this population was not impacted by the timing of HU addition, and consistently diminished over time post day 4.
Anterior kidney ASCs, although typically not as numerous as those found within the peripheral blood and spleen (Fig. 5C, F, I and L) were (i) present from culture inception, (ii) HU resistant at all times, and (iii) did not require antigen for induction. This is indicative of LLPCs.
Discussion
The kinetics and magnitude of both TI and TD antibody responses (Fig. 1A) mirrored the corresponding total ASC and PC responses (Fig. 2 and 3). However, precise quantitative comparisons between antibody titers and ASCs cannot be made as (i) the secretion rate of the ASCs (composed of PCs and plasmablasts) increases over time postimmunization 41, and (ii) high-affinity antibodies, which dominate the late immune response 20, possess more prolonged half-lives 42, 43 thus making such comparisons problematic. Indeed, based on these previous studies 41-43, one should expect that fewer ASCs would give rise to higher antibody titers as the response matures.
Two features of these antibody responses; however, were particularly notable. First, the kinetics, magnitude, and affinity of the TI and TD responses were quite similar. Both responses exhibited a relatively short primary phase versus a prolonged secondary and both maintained the high affinity achieved late in the primary (by week 16), even as the titers began to wane (week 25–44). This heightened affinity continued into the secondary, with even a slight increase (∼from −log aK 5.9 to 6.2), late in the secondary. This kinetics would indicate that although T-cell help may be required for the TD response 44 it remains fairly comparable to the TI response. Second, these observations also suggest that the antigen-sensitive precursor pool had changed with the bulk of the responding antigen-sensitive precursors being of higher affinity than those in the naive fish, while the pool of secondary ASCs are either longer lived (e.g. LLPCs) or result from an extended period of PB replication.
Analysis of ASC activity revealed a relatively heavier involvement of the spleen during the primary response, than in the secondary. Past studies 32, 33 and the HU analysis with this study (Fig. 5) would suggest that, earlier in the response the spleen is also more active with plasmablast activity, while the anterior kidney is more active late in the response with PCs. Therefore, the persistent antibody response during the secondary response may be due to anterior kidney LLPCs. This would indicate that, as in mammals 29-31, PCs are preferentially distributed and maintained in the hematopoietic tissues.
The distribution and relative size of the memory pools were determined by in vitro antigen induction of cells from primed fish 1, 7, 39, 40. In contrast to the ASC distribution (PCs and PBs), MBCs were significantly skewed to the periphery (Fig. 4), particularly the blood. The spleen; however, may play a slightly more dominant role, on a per cell basis, during the primary response. This distribution also mirrors the mammalian MBC distribution, wherein MBCs preferentially reside in the periphery as opposed to central lymphoid tissues 35-37.
The differentiative potential of antigen-inducible cells (including MBCs) derived from the blood, spleen and anterior kidney was explored via HU pulsing in the presence or absence of antigen (Fig. 5). This analysis revealed striking differences between all three tissues and provides a basis for modeling the mechanics of the terminal, antigen-dependent phase of teleost B-cell differentiation (Fig. 6). Virtually all antigen-inducible cells within the blood appear to be resting B cells that are only capable of progressing to the plasmablast stage of differentiation upon antigenic stimulation, as virtually all ASCs were proliferating (HU-sensitive). As the TI and TD responses were sixfold and fivefold greater, respectively, than observed with naive PBLs (Fig. 5), we believe that the bulk of these responses are primarily derived from resting memory cells. Also, as the introduction of HU at any time point immediately terminates all ASC activity, the bulk of these cells never differentiate to PCs during culture. Thus, although PBL culture is sufficient to support early plasmablast differentiation in vitro, it cannot support differentiation to PCs.
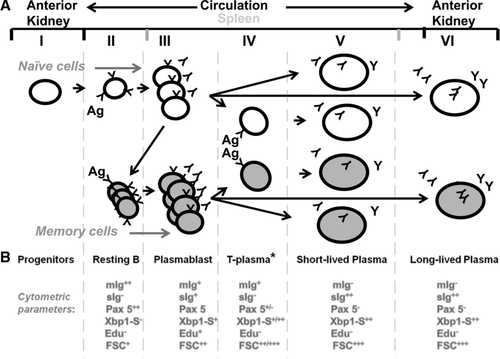
The spleen presents a distinctively different profile wherein, although a significant proportion of splenic ASCs are antigen-induced plasmablasts, up to 85% differentiate into HU-resistant (plasma) cells (Fig. 5E and K; day 4). Further, as early HU addition does not impair the production of a large portion of these PCs, it appears that the precursors of these PCs had previously differentiated beyond the proliferative, plasmablast stage (Fig. 6, Stage III) yet uniquely require antigen for terminal PC differentiation. As this precursor functionally appears poised between the plasmablast and PC stage of differentiation, we suggest that it may be a preplasma or transitional PC (T-plasma cell) as previously described 45. This T-plasma cell cytometrically appears to be a nonproliferative intermediary between the plasmablast and the mature PC, which also contains high levels of sIg as in the mature PC. However, the T-plasma cell also contains higher Pax-5 than the mature PC. Thus, it is possibly transitional to the mature PC (Fig. 6, Stages V and VI). Barr et al. have suggested that these distinct stages of PC differentiation may offer greater adaptability in the teleost response 45. This transitional cell also appears to possess characteristics of the putative murine preplasma memory cell 46, which, as this nomenclature suggests, could adjoin two distinct poles of plasmablast differentiation, the memory cell and the PC. This memory cell is thought to differentiate from the more classical B220+ memory cell 47 such that, upon antigen reexposure, it undergoes expedited proliferation/differentiation to the PC stage 48, 49. Further, like the trout T-plasma cell, it also appears to have a significant presence in the spleen and bone marrow, but not in the blood. Determination of the magnitude of its presence in the anterior kidney is problematic; however, as the presence of many LLPCs within the anterior kidney would confound one's ability to accurately parse its precise lineage. The trout T-plasma cell also differs significantly from the mammalian pre-plasma memory cell in that it is totally resistant to HU (i.e. it does not pass through any proliferative phase) prior to becoming a PC.
The anterior kidney is wholly different from the blood and spleen in that the PCs are present even at culture initiation and persist throughout the entire culture period (Fig. 5C, F, I and L), indicating the presence of a stable PC population (e.g. LLPC). Although the LLPCs make up a substantive portion of the ASCs, variable numbers of plasmablasts can be generated in some cultures (i.e. partial diminution of the ASCs with HU). Thus, at times, resting MBCs or naive B cells must be present or transiting this tissue.
Based on these functional analyses and previously reported correlative cytometric profiles 34, 45, 50-52, our model of antigen-dependent trout B cell differentiation (Fig. 6) provides a number of useful correlative associations. For example, the observation of high numbers of resting B cell profiles; small size (FSC+), no secretory Ig (sIg−), high mIg (mIg++), nonproliferating (EdU−), high Pax5 (Pax 5++), low Xbp1-S (Xbp1-S−) within the blood and spleen (Fig. 6, Stage II) with contrastingly low numbers of the same within the anterior kidney 34, 45, 50-52 parallels the high inducibility seen in the blood and spleen and lower inducibility in the anterior kidney (Fig. 5). Similarly, high numbers of PCs (FSC+++, sIg++, mIg−, Pax5−, Xbp1-S++, Blimp1++, Edu−) within the anterior kidney and relatively low numbers in the periphery also match our functional findings (Fig. 2G–L). Interestingly we also find correlations with respect to the differentiative capacity of the splenic versus peripheral blood B cells. We have demonstrated that PBLs appear only capable of differentiation to a plasmablast, as opposed to splenocytes which can differentiate to SLPCs (Fig. 4). Similarly, Barr et al. 45 find that splenocyte induction demonstrated a greater degree of Xbp1-S expression than can be observed in comparably stimulated PBLs. Thus, both analyses demonstrate that splenic B cells can progress further towards terminal differentiation (Fig. 6, Stage V) than can peripheral blood B cells. With such a broad swath of functional differences and cytometric markers now available for work in trout, fine resolution of the developmental relationships between these mature lineages may be possible. For example, although we cannot positively identify the putative T-plasma cell cytometrically; we may pose likely profiles given the above hypotheses (Fig. 6, Stage V), then attempt isolation of the induced splenic populations and/or note an emergent cytometric profiles that dominate the anti-TNP sIg population at relevant points in time.
Given the above stages of antigen-dependent trout B cell differentiation (Fig. 6), we propose the following temporal/spatial model of the developing teleost B cell response (Fig. 7). As in mammals, the naive B cells first mature within the hematopoietic tissues (teleost anterior kidney) from their pro- and pre-B progenitors. We depict, for the sake of convenience, a portion of the kidney possessing B lymphopoietic (red) stromal tissue and harboring these B-cell progenitors (Fig. 7A). The mature naive B cells then exit from the anterior kidney to the blood and circulate through the periphery. Within the supportive stromal matrix of the spleen (green), antigen induction elicits differentiation though the proliferative plasmablast stage from which memory (Fig. 6, Stage II), T-plasma (Fig. 6, Stage IV), or PCs (Fig. 6, Stages V, VI) may arise. The latter becoming SLPCs within the spleen as its matrix is not supportive of prolonged survival. However, plasmablasts entering the circulation can, like their mammalian counterparts, become LLPCs upon reaching the survival niche stroma of the anterior kidney. The LLPCs then become the primary source of the serum antibodies 32, 33. Although stromal cell support of antigen-induced B-cell differentiation has yet to be demonstrated for trout, splenic and anterior kidney stromal cells have been isolated and demonstrated to foster trout lymphoid, erythroid and myeloid differentiation in vitro 53, 54.
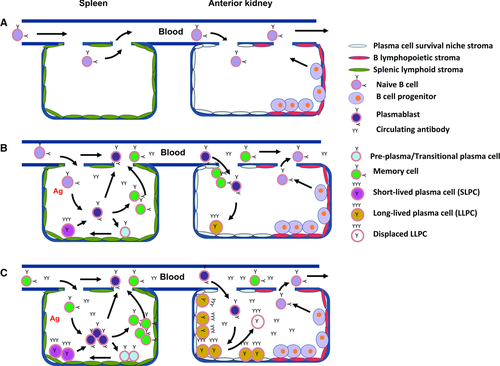
The enhanced responsiveness of the peripheral tissues (blood and spleen) subsequent to priming and postsecondary challenge is due to the proliferative plasmablast activity, which results in the extensive production of memory cells (Fig. 7C). As with naive cell induction, memory cells within the supportive matrices of the spleen produce increased numbers of memory cells, T-plasma cells, and SLPCs. Plasmablasts that now transit to the anterior kidney, upon differentiating to PCs, may displace some portion of the former LLPC population deposited during the primary response.
Materials and methods
Animals
Troutlodge stock of rainbow trout (Oncorhynchus mykiss) was a generous gift from Dr. Greg Wiens of the National Center for Cool and Cold Water Aquaculture, (USDA-ARS), Leetown, WV, USA. The fish were obtained when approximately 50 g and were immunized upon attaining a size approximately 180 g. All fish were maintained in 250 gallon tanks in a recirculation system as previously described 32. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the College of William and Mary.
Antigens and immunization
The immunogens, TNP-LPS; (Escherichia coli 055:B5) and TNP-KLH were prepared as previously described 55, 56, and TNP-BSA 57 was employed as the antigen for antibody affinity, titer determinations, and ELISPOT analyses.
Ninety trout were equally divided into three immunization groups (TNP-LPS, TNP-KLH, and controls). Trout were anesthetized with tricaine (MS-222, Sigma–Aldrich) prior to immunization. Fish in the TNP-LPS group were immunized by intraperitoneal (i.p.) injection with 50 μg TNP-LPS per fish in a total volume of 100 μL (TNP-LPS in PBS emulsified 1:1 with Freund's complete adjuvant (FCA, Sigma-Aldrich)). TNP-KLH fish were immunized with 50 μg per fish in the same manner as the TNP-LPS group. Control fish were challenged with 100 μL of PBS emulsified 1:1 with FCA.
After 1 year, remaining fish in each group (about 12–14 fish per group) were challenged with the same antigen in Freund's Incomplete Adjuvant (Sigma-Aldrich). No antigen was given to control fish.
Assessment of antibody titers and affinities
Anti-TNP antibody titers and affinities were assessed periodically over the entire period of 92 weeks. Titers were determined using the standard antibody titration ELISA and anti-TNP antibody affinities were assessed by a partitioning ELISA-based analysis as previously described 58.
Analysis of ASC responses
ASCs were enumerated using ex vivo ELISPOT analyses as previously described 32 with the following modifications. Briefly, leukocytes from heparinized whole blood, anterior kidney, and spleen were isolated via Histopaque 1077 (Sigma-Aldrich) density centrifugation at a series of time points post primary and secondary immunization. Immediately upon washing the cells, triplicate samples containing 106, 105, and 104 were suspended in 100 μL of RPMI 1640 and deposited into wells of a BioDot Apparatus (BioRad) containing a TNP-BSA coated PVDF membrane. These membranes were prepared by an initial, brief immersion in absolute methanol followed by 2 h incubation in a 100 μg/mL solution ofTNP8-BSA in PBS. The membranes were then blocked in a 0.5% BSA in PBS for 1 h at RT. Development of the ELISPOTs was as described 32.
Plasma cell numbers were determined by the ex vivo suspension of lymphocytes in tissue culture medium (TCM) in the presence of 100 mM hydroxyurea (HU) without antigen. HU is an inhibitor of DNA replication 59, thus enabling the resolution of plasmablasts (HU-sensitive, replicating ASCs) from PCs (HU-insensitive, nonreplicating ASCs) 32, 38. This TCM was modified from 60, basically RPMI 1640 with 1% l-glutamine, was supplemented with 11 μg/mL sodium pyruvate, 10% fetal bovine serum, 50 μg/mL gentamycin, 5 μM 2-mercaptoethanol, 1 × nonessential amino acids, and 10 μg/mL each of adenosine, cytosine, uracil, and guanine. After 7 days of culture, the cells were harvested and ELISPOT analysis performed.
Assessment of antigen inducibility
The remaining leukocytes from each cell preparation were suspended in TCM to a concentration of 1 × 107/mL and 100 μL deposited into individual wells of a 96-well culture cluster plate (Costar) for in vitro culture. These mixtures were then brought to a final concentration of either 1 μg/mL TNP-LPS or without antigen (controls). TNP-LPS was used for all in vitro induction studies as it is capable of eliciting responses from all TNP-specific lymphocytes, whereas the in vitro TNP-KLH responses are significantly influenced by the variable number of auxiliary, helper, and regulatory cells present. The goal of these studies was simply to gauge the relative size of the TNP-specific B-cell pool, regardless of how it was induced. To provide an accurate estimate of antigen-inducible B cells, the number observed in the absence of antigen were subtracted from those produced in the presence of antigen. Production of MBCs was determined by comparing the ability of cells from in vivo primed fish to produce an enhanced response over that seen nonimmunized controls 1, 7, 39, 40. All cultures were harvested and analyzed for TNP-specific ELISPOTS on day 7. Data were expressed both as ASCs/106 leukocytes and as ASCs / whole tissue. The total ASCs per tissue were determined by multiplying the ASCs/106 leukocytes by the total leukocytes per tissue. Total blood volumes were calculated as per Duff et al. 61.
Resolution of in vitro antigen-induced stages of differentiation
The capacity of lymphocytes from each tissue to foster resting naïe/B memory cell differentiation through plasmablast to PC stages was examined via timed, separate HU additions to replicated cultures 32. Aliquots of the above cell preparations were exposed to TNP-LPS alone or with 100 mM HU added on sequential days post culture initiation. Comparison of the non-HU treated to HU treated cultures permitted the resolution of the proportion of antigen-induced B cells undergoing the plasmablast proliferation and differentiation and/or progress to PCs. Cultures were harvested at each day post initiation, through day 7, to determine the proportion of ASCs that were either plasmablasts or PCs at each timepoint.
Acknowledgements
This work was supported by National Institute for Food and Agriculture (NIFA) Grant no 2008–35204-04353. The authors gratefully acknowledge the in depth theoretical and practical advice of Dr. Patty Zwollo, and the critical reviews of Drs. Patty Zwollo, Greg Wiens, and Candace Spier, and those of Ms. Mary Ann Vogelbein and Ms. Ilsa Kaattari. The authors are also appreciative of the generous contribution of trout from Dr. Greg Wiens and the National Center for Cool and Coldwater Aquaculture, ARS, Leetown, WV, USA. VIMS contribution #3167.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- ASC
-
- antibody-secreting cell
-
- FCA
-
- Freund's complete adjuvant
-
- HU
-
- hydroxyurea
-
- LLPC
-
- long-lived plasma cell
-
- MBC
-
- memory B cell
-
- PC
-
- plasma cell
-
- SLPC
-
- short-lived plasma cell
-
- TD
-
- T-dependent
-
- TI
-
- T-independent
-
- TCM
-
- tissue culture medium
-
- TNP-BSA
-
- TNP-bovine serum albumin
-
- TNP-KLH
-
- TNP-keyhole limpet hemocyanin
-
- TNP-LPS
-
- TNP-lipopolysaccharide




