Langerhans cells and viral immunity†
Abbreviations
CLRC-type lectin receptors
LCLangerhans cells
VZVvaricella-zoster virus
Abstract
Langerhans cells (LC) are a unique dendritic cell subset that are located in mucosal stratified squamous epithelium and skin epidermis. Their location is ideally suited for their function as antigen presenting cells that capture invading viruses and induce anti-viral immunity. However, it is becoming evident that the interaction between LC and viruses can result in different responses, depending on the virus and the receptors involved. Here we will discuss the recent data on the similarities and differences in roles of LC in viral immunity to and infection with HIV, herpes simplex and varicella–zoster virus. Although all three viruses interact with LC during initial infection, the effects can be quite different, reflecting differences in biology and pathogenesis.
Biology of LC and interactions with viruses
The resident epidermal dendritic cells (DC), Langerhans cells (LC), form a tight network with each other and with the surrounding keratinocytes via E-cadherin in the skin or mucosal stratified squamous epithelium. In the anogenital region they are distributed throughout the anogenital skin and mucosa including vagina, ectocervix as well as male foreskin. In stratified squamous epithelium, LC overlie interstitial or dermal DC 1. LC can repopulate from local and blood-borne precursors (reviewed by Kaplan et al. in this issue 2). Similar to other myeloid DC, LC are able to bridge innate and adaptive immunity. They are able to interact directly with microorganisms at the periphery to produce effector cytokines and initiate or restimulate activation of T and B lymphocytes through antigen presentation. LC express different pattern recognition receptors such as C-type lectin receptors (CLR) and Toll-like receptors (TLR), to bind and capture pathogens. These interactions result in activation of intracellular signalling pathways leading to LC activation and cytokine induction. The CLR expressed by skin DC differ by site: Langerin by epidermal LC and DC-SIGN and mannose receptor by dermal DC. LC like other migratory DC are in an immature, highly endocytic state while sessile in the skin/mucosa. However after pathogen binding to TLR and uptake, LC become mature and migratory, downregulating their endocytic and antigen processing capacity. Mature LC migrate to the lymph nodes where they are directly or indirectly involved in presentation of pathogen-derived antigens on MHC Class I and II to T cells, resulting in activation of antigen-specific T cells 2-4. Recent studies have raised the possibility that LC may have an immunosuppressive role 5-7. Kaplan et al. 2 showed that contact hypersensitivity was amplified on LC ablation, while Cumberbatch et al. 5 found defective LC mobilisation in patients with psoriasis. Immunosuppression rather than stimulation could be explained, in part, by the maturational process associated with LC migration during steady-state conditions. Jiang et al. 6 demonstrated that DC maturation induced by disrupting E-cadherin interactions led to a regulatory-type T-cell response. Since E-cadherin is involved in LC–keratinocyte interactions in skin, they argued that steady-state LC migration should result in a form of LC maturation that is tolerogenic in nature. Consistent with this suggestion, Waithman et al. 7 formally showed that presentation of skin-derived self-antigen by lymph node-resident mature LC during the steady-state, directly led to deletional T-cell tolerance. Also consistent with immunoregulation, the TLR expression profile by LC with low expression of TLR-2 and -4 necessary for bacterial recognition results in selective unresponsiveness of LC to bacteria compared with viruses and may contribute to tolerance to bacterial commensals 8, 9. In some cases dermal DC may play the immunostimulatory role previously attributed to LC 10-12. Recently, a distinct subset of dermal DC was identified in mouse that expresses langerin and this subset might be important for immunostimulation 13-15 although so far, little is known about their function and whether a similar subset exists in humans.
LC probably play a pivotal role in certain virus infections. LC are ideally located to interact with viruses that infect humans through skin or mucosa such as HIV, the herpesviruses, including herpes simplex virus (HSV) and varicella–zoster virus (VZV), and poxviruses such as vaccinia and human papillomavirus. However, there are two sides to these virus–LC interactions: capture, degradation or infection of LC by viruses and the induction of innate and adaptive immune responses to viruses by LC. Here we will discuss the overlapping differences and similarities in these effects after infection of LC by HIV-1, HSV and VZV all of which naturally infect LC in the skin and mucosa.
Studies of the function of LC are difficult because of the low proportion of LC in epithelial tissues, the difficulty in isolating LC from these tissues and the absence of an exact in vitro model. LC can be isolated from human skin in vitro by two main methods, emigration from split skin explants or enzymatic (trypsin/collagenase) digestion and flow sorting or MACS bead technology. The latter offers better preservation of the immature phenotype but is labour intensive, technically difficult and care must be taken that the trypsin treatment does not cleave key binding epitopes from cell-surface proteins such as CD4. Therefore many preliminary studies have been conducted in human model DC in vitro or LC in murine models in vivo.
LC interactions with important human viruses
HIV and LC
There has been much debate about the mechanisms of HIV infection and entry via the female genital tract, including the importance of epithelial breaches or microabrasions and the cell types involved 16-18. This has become of increasing importance because of the recent problems in vaccine development against HIV-1 19, and therefore there is a need for alternative methods to prevent HIV-1 transmission. An important alternative are the vaginal microbicides to contain spread of the virus. However, the failure of recent clinical trials of detergent and polyanion microbicides to protect against transmission shows that our knowledge about the mechanism of HIV-1 transmission is still insufficient 20. A role for LC has been long advocated as they are the major HIV infectable constitutive cell type in the anogenital stratified squamous epithelium. Several in vivo and ex vivo data support a role for LC infection in HIV-1 transmission 21, 22. Vaginal simian immunodeficiency virus (SIV) infection of Rhesus macaques resulted in infected LC beneath the vaginal epithelium within the first day of infection 22. Infection of biopsies of human cervical and skin of primate foreskin tissue explants show that LC can be infected 23, 24. Topical infection of human vaginal epithelial explants with HIV strongly suggested that LC and non-activated T-lymphocytes are the major cell types expressing HIV antigen in and emigrating from the explants and that they are often associated during emigration with HIV antigen concentrated at their contact region 21. The latter suggests that LC are transferring HIV to CD4 lymphocytes. LC may also provide a mode of intracellular storage while transporting HIV to CD4 lymphocytes in the submucosal lymphoid tissue and thence to draining lymph nodes 16. Recent data suggest that LC may have an anti-viral function by capturing HIV-1 for degradation and thus initially impairing HIV-1 transmission 25. This anti-viral function is dependent on viral load and LC phenotype, strongly suggesting that the role of LC in HIV-1 transmission is also likely to be influenced by local conditions such as viral load, stage of the menstrual cycle, state of vaginal mucus and/or inflammation and co-infections.
DC subsets in HIV-1 transmission
Monocyte-derived DC have been used in several studies to investigate the mechanism of HIV-1 transmission, since primary DC are difficult to isolate from human tissues in sufficient amounts. Such DC more closely resemble lamina propria DC, particularly in the types of CLR expressed in the endocervix. Although similar mechanisms were assumed to be involved in LC transmission of HIV-1, it is becoming evident that LC might have a more complicated role in transmission 25. Monocyte-derived DC capture HIV through the CLR DC-SIGN 26 and, to lesser degrees, mannose receptor and heparin sulphate proteoglycan syndecan-3 27. HIV-1 is either endocytosed or transferred directly to CD4 and CCR5 resulting in viral–cell membrane fusion and infection. Immature DC and LC express high levels of CCR5 but on maturation CCR5 is downregulated and CXCR4 upregulated resulting in reduced infectability by R5 (CCR5 using) viral strains, those strains shown to be predominant during sexual transmission. These data imply that immature DC and LC in contrast to their mature forms are more important in HIV-1 transmission. Viral endocytosis leads to partial acid-proteolytic degradation in the late endosome, although some virus may be retained in its infectious state for longer periods 27, 28. The captured HIV-1 can be transferred to T cells and this process is called trans-infection or first phase transfer 26. However, HIV entry via fusion leads to de novo productive infection first detectable at 24 h and with a plateau in vitro at 72 h. Recent studies suggest this de novo produced virus can re-enter the same or adjacent DC by endocytosis and the latter can also be infected de novo, thus mixing up the initial two distinct phases of viral endocytic degradation and de novo infection. Contact between DC that have captured HIV with activate or non-activated CD4 lymphocytes leads to efficient transfer of virus to lymphocytes in two distinct phases. The DC and CD4 lymphocyte form a ‘infectious synapse’ at the contact region 29, 30, which is sealed and protected from antibody entry by interaction of the adhesion molecules LFA-1 and ICAM-1 (CD54) 31, 32. CD4 and CCR5 are concentrated at the enclosed lymphocyte membrane. HIV-containing endosomes are diverted to the contact region, fuse with the cell membrane dumping their load of residual HIV into the synapse. Thus the amount of virus transferred declines over 12 h because of the endocytic degradation 28. Conversely transfer of de novo produced virus which is also diverted to the contact region and in filopodia surrounding CD4 lymphocytes increases over 24–72 h 33. Thus, DC capture HIV-1 which is transferred to T cells, without prior infection of DC, or DC become infected and de novo produced HIV-1 is transmitted to T cells. Both phases might be important at different stages of HIV-1 transmission. There is debate about the relative importance of these two phases in HIV transfer but recent evidence suggests de novo infection to be more important 34, especially in LC 23, 33.
In LC the existence of these two stages is less clear. Several studies have demonstrated that LC infection with R5-tropic HIV-1 is essential to its role in transmission 34-37. However, recently it was shown that mature CD34+-derived LC-like cells can transmit HIV-1 without infection 38. LC are susceptible to de novo infection with R5 but not X4 strain HIV and such infection can result in transfer of R5 strains to CD4 lymphocytes 34. Recent data suggest that the CLR Langerin on LC captures HIV-1 for internalisation into LC-specific vesicular organelle Birbeck granules for degradation, thereby preventing cis-transfer to CD4/CCR5, thus differing from DC-SIGN on monocyte-derived DC. Thus, Langerin binding effectively inhibited de novo infection of LC and subsequent transfer to CD4 lymphocytes, an effect which could be overcome by inhibition of Langerin or by high concentrations of HIV which saturated Langerin function 25. The critical role of LC in anti-viral defence can explain differences in HIV-1 susceptibility, as the risk of acquiring HIV-1 is severely increased by inflammation, ulceration and co-infection with sexually transmitted diseases. These conditions might modulate HIV binding by Langerin by decreasing its expression due to LC maturation and ulceration might also allow access to DC-SIGN expressing dermal/interstitial DC.
Several studies have been reported with HIV infection of abraded skin explants, obtained through blistering where CCR5 inhibitors but not mannan blocked HIV uptake and apparently transfer to exogenously added T cells, leading the authors to conclude that Langerin played no role in HIV transfer. These data suggest that HIV does not bind to Langerin on LC isolated in this way, or does not result in cis-transfer to CD4/CCR5 (and therefore enhanced infection), unlike DC-SIGN on MoDC 34.
Although LC may be the first DC in the body to encounter HIV, a role for this interaction in stimulating or impairing the anti-HIV immune response has not yet been shown. However, it is likely that HIV-containing DC may present antigen to specific CD4 lymphocytes as well as transfer HIV to them, thus resulting in their death. There is strong evidence for selective depletion of HIV-specific CD4 lymphocytes 39, 40 and this could be a key mechanism, but is yet to be proven. HIV infection markedly affects the function of CD4 lymphocytes even before inducing lysis or apoptosis. HIV infection of DC also affects their function in more subtle ways. Recent studies show that when a substantial proportion of DC or LC are infected they undergo partial maturation and enhanced migration 35, 41, 42. The partial maturation is sufficient to enhance T-cell stimulation and might also enhance viral transfer, thus affecting both viral production and anti-viral immunity. Further investigation of HIV modulation of DC and LC gene expression should demonstrate other effects on DC function affecting anti-viral immunity, especially TLR expression and cytokine and chemokine secretion.
DC subsets in innate and adaptive immunity to HSV
In human mucocutaneous herpes simplex, HSV infection is typically confined to the stratified squamous epithelium but not to dermis nor lamina propria 43. The cell-mediated immune response to HSV is of major importance in control and clearance of recurrent infection in recurrent oral or genital herpes 44. In skin the immunoreactive cells responsible for controlling the transmitted HSV include the normal constituents of the squamous epidermis, keratinocytes and LC, and infiltrating cells. In particular HSV-specific CD4 and CD8 T lymphocytes play a central role in controlling primary and recurrent HSV infections in humans and in primary disease in murine models (where human specimens are difficult to obtain); in recovery from infection as well as in restricting HSV reactivation and spread in the nervous system 45-48. In human recurrent disease, there is a temporal sequence of cellular infiltration, first predominantly monocyte/macrophages and CD4 lymphocytes and later predominantly CD8 lymphocytes, as shown by immunohistochemistry and direct T-cell cloning from lesions biopsied serially 43.
HSV infection of epidermal keratinocytes induces the secretion of a sequence of chemokines and cytokines, which is reflected in the whole lesion in vivo i.e. firstly interferon (IFN)-α and β chemokines and then interleukin (IL)-12 followed by IL-1 and IL-6 49. The β chemokines may assist in chemotaxis of monocytes, CD4 and CD8 lymphocytes into lesions. IFN-α and IL-12 may entrain Th1 patterns of cytokine response from HSV antigen stimulated CD4 (and CD8) lymphocytes, especially IFN-γ. Interferons alpha and gamma synergise to inhibit infection of keratinocytes after transmission from axon termini (see below). HSV-1 or -2 downregulate MHC class I expression by epidermal keratinocytes via the viral protein ICP47 interaction with cellular translocator associated proteins (TAP) in the endoplasmic reticulum 50, 51. This is reversed by IFN-γ mainly secreted by CD4 lymphocytes infiltrating the lesion. The CD8 lymphocytes recognise the infected keratinocytes after MHC I is restored on their surface by this IFN-γ 43, 47, 52. IFN-γ also stimulates MHC class II expression on keratinocytes in the lesion, allowing recognition by CD4+ lymphocytes. The later CD8 lymphocyte infiltrate appears to correlate with virus eradication from the skin.
The epidermal LC are the obvious primary DC likely to be initially involved in HSV antigen uptake, although the involvement of dermal DC in the upper region of the dermis has not been excluded. Indeed, recently we have demonstrated HSV structural antigens within these cells during recurrent herpes simplex (L. Bosnjak et al. unpublished data). One of the earliest studies on the role of LC in anti-HSV responses showed that depletion of LC from murine skin prior to footpad HSV-1 infection led to increased HSV virulence 53. Subsequently, LC were reported to accumulate in the draining lymph nodes after subcutaneous administration of HSV-1, their numbers peaking at three days after infection 54. Lymphocytes isolated from the lymph nodes of HSV-1-immunised mice proliferated in vitro in response to HSV antigen, but these responses were abolished if LC were depleted from skin prior to HSV infection 54. DC subsets have also been shown to be superior in initiating specific T-cell proliferative responses to inactivated HSV 55. Thus earlier studies suggested that depletion of LC from skin, after HSV-1 infection of mice via the footpad, led to increased HSV virulence 56. However, others and we have recently shown that LC are not the cells presenting HSV antigen to CD8+ T cells in lymph nodes. After epidermal scarification with the virus, it was observed that CD8+ DC present HSV antigens to CD8+ T cells in draining lymph nodes 24 h after infection 57, and after vaginal inoculation of HSV, dermal DC were demonstrated to present HSV antigen to CD4+ T cells 12. The apparent paradox of initial HSV antigen uptake by LC but presentation by another DC subtype may be explained by transfer of the antigens from one DC subtype to another 57.
In view of the difficulty in obtaining sufficient numbers of immature human LC, monocyte-derived DC have been used as a model. Both immature and mature monocyte-derived DC express the HSV receptors nectin-1, nectin-2, and HVEM and can be infected with HSV-1 and HSV-2, but only immature monocyte-derived DC produce virus although at tenfold lower levels than most cell lines. Similar to HIV-1, the C-type lectin DC-SIGN functions as an attachment receptor on immature DC that enhances DC infection 58. Mature DC are non-productively/abortively infected 59, 60. However, mature DC are not usually found at the cutaneous sites of HSV infection, and therefore studies examining immature DC are more relevant.
HSV infection results in asynchronous downregulation of the key costimulatory molecules CD40, CD80, CD83, and CD86, preventing proper maturation of the DC, whereas, exposure to UV-inactivated HSV results in upregulation of these molecules 59, 61. Furthermore, HSV-infected DC are resistant to further maturation stimuli supplied by LPS, TNF-α or CD40L 62. Interestingly, MHC class I is not downregulated on infected monocyte-derived DC, unlike most other infected cells 59. The inability of HSV-1 ICP47 to interfere with MHC class I expression is most likely the consequence of the innately high levels of translocator associated proteins TAP in DC. The most striking effects were seen with CD40 and CD54 with a 1–2 log decrease in the surface expression within 12 h of infection. Secretion of IL-12, one of the major Th1 cytokines, by DC is dependent on CD40–CD40L interaction 63, 64. It can be predicted that downregulation of CD40 expression on HSV-1 infected DC may lead to decreased production of IL-12 upon contact with T cells. In fact, HSV-1-infected DC fail to produce IL-12 in response to LPS and CD40L 62.
The observed downmodulation of CD54 on the surface of immature DC may also have a powerful impact on generation of anti-HSV immunity. Virus-mediated downmodulation of CD40 and CD54 may favour viral spread, which is mainly controlled by Th1 cytokine-induced T cells 65, 66.
In most other cell types, HSV-1 produces anti-apoptotic effects, whereas in DC, both HSV-1 and HSV-2 induce apoptosis progressively throughout the cell sheet over 24 h 59, 67. Furthermore, HSV-2 induces apoptosis more rapidly than HSV-1. Similar results are also seen in murine bone marrow-derived DC. Although apoptosis is well-documented as a global cellular response to viral infection (including HSV), these results are somewhat surprising as both HSV-1 and -2 encode several gene products that effectively interfere with this pathway 68-74. Nevertheless HSV-1 also induces apoptosis in monocytes and T cells 75-77.
These apoptotic cells can then be phagocytosed by uninfected bystander cells, and the HSV antigens contained within these apoptotic cells are then cross-presented (on MHC class I) to CD8+ T-cell clones 67. Other viral antigens have also been shown to be cross-presented in vitro including vaccinia virus, canarypox virus, HIV and HCMV, amongst others 78-83. This cross-presentation suggests a mechanism for the antigen transfer between DC subtypes observed in murine models and suggests that the immunoevasive mechanisms of costimulatory molecule downregulation and apoptosis of DC by HSV can be counteracted by uptake by bystander DC.
Preliminary experiments with immature LC in vitro show a similar downregulation of costimulatory molecules (see below). Whether bystander LC or subjacent upper dermal DC respond in human infection in a fashion similar to that in the murine models remains to be elucidated. In addition, uninfected immature monocyte-derived DC undergo partial maturation after uptake of inactivated HSV.
These murine and human data suggest a complex in vivo scenario in acute HSV infection (Fig. 1). Initial infection of epidermal cells will result in infection of resident LC as they come into direct contact with the incoming virus. Such infected LC may resist further maturation stimuli provided by TNF-α and IL-1β from infected keratinocytes and may undergo apoptosis at the site of infection or the dermis as they migrate towards the lymphatics. Thus, apoptotic LC fragments may be phagocytosed by bystander uninfected LC or dermal DC for cross-presentation. Murine studies suggest that at least some of this transfer also occurs within the draining lymph node as the CD8+ DC population responsible for the activation of HSV-specific CD8+ T cells resides in lymph nodes 57, 84. In addition, it remains possible that virus or viral antigen may ‘leak’ into the lymphoid compartment, allowing uptake by (or even infection) of lymph node-resident DC—although attenuation of T-cell priming by inhibiting skin DC migration argues that this may not be a dominant mechanism of lymph node access 57. More sensitive PCR techniques will allow further clarification. While not disputing the above hypothesis, other studies indicate that the antigen transfer may also occur adjacent to the site of infection and subsequently activate CD4+ T lymphocytes in the lymph node 12. Therefore, there may be different locations of antigen uptake/transfer to DC for stimulation of CD4+ or CD8+ T lymphocytes.
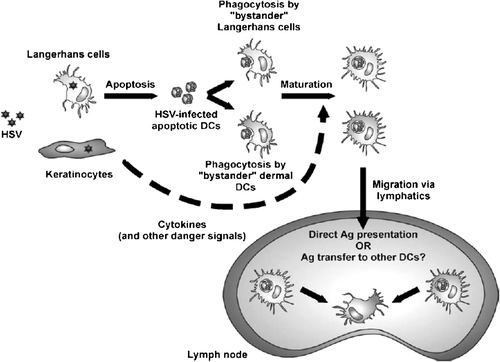
Postulated interactions between HSV and DC in the epidermis and transport of antigen to lymph node. HSV infection of epidermis LC (LC) induces apoptosis followed by uptake by bystander LC or dermal DC moving into the area of inflammation. Cytokines/danger signals released from infected keratinocytes lead to maturation and migration of DC to lymph nodes where they activate CD4+ or CD8+ lymphocytes directly or via intermediate DC. Reproduced with permission from 88.
As these mechanisms are similar to vaccinia virus infection of DC, it is possible that this is a common mechanism shared by epitheliotropic cytopathic viruses that interfere with DC maturation and survival 3. Whatever the mechanism, our results suggest direct cell-to-cell (or DC-to-DC) contact may be required for this exchange to occur.
The role of LC in varicella–zoster disease
Although the pathogenesis of VZV shows many similarities to HSV there are marked differences. VZV infects mainly human cells so that only SCID-Hu murine models with human skin explants can be used. Human varicella and zoster are both dermal as well as epidermal diseases. In biopsies of human varicella or zoster, extensive infection and cytopathic effect of keratinocytes with vesicles containing abundant interferons and other cytokines are observed, like herpes simplex, but LC rapidly emigrate in the early stages of infection (85, 86, and Abendroth et al., unpublished observations]). Whether they are infected or apoptotic is unknown. However, VZV infection of DC does result in apoptosis and passage of the virus to T cells. In contrast to HSV, VZV productively infects both immature and mature monocyte-derived DC but only induces downregulation of co-stimulatory molecules in the latter 87. VZV can be transferred from both DC states to infect CD4 and CD8 lymphocytes, similar to HIV. Whether this is true of LC and occurs via viral synapses is yet to be tested.
Conclusions
Thus, although HIV, VZV and HSV all interact with LC during initial infection of the female genital tract, the effects are quite different reflecting differences in biology and pathogenesis. After LC capture HIV this is followed by degradation or de novo infection and under specific conditions, HIV probably uses LC for transfer to CD4 lymphocytes in the submucosa and subsequent dissemination. HSV infects LC (and probably induces apoptosis), which may facilitate transfer to another type of DC in lymph node responsible for subsequent antigen presentation to CD8 T cells. More generally there are similarities and differences between the effects of VZV and both HIV and HSV in infected DC: HSV and VZV both induce downregulation of maturation markers and apoptosis of maturing DC. After infection DC can transfer both HIV and VZV to CD4 (and CD8) lymphocytes.
Acknowledgements
We would like to acknowledge the intellectual input from Drs Cheryl Jones, Sem Saeland, Allison Abendroth, Stuart Turville, Lidija Bosnjak, Andrew Harman and Heather Donaghy. We would also like to thank Claire Wolczak for her secretarial assistance. A. L. C. and F. R. C. are supported by program grants from the National Health and Medical Research Council of Australia. T. G. is supported by grants from the Dutch Scientific Research program (NWO. 917-46-367; 912-04-025).
Conflict of interest: The authors declare no financial or commercial conflict of interest.




