Dual inhibition of proteasomal and lysosomal proteolysis ameliorates autoimmune central nervous system inflammation†
Abbreviations
CatScathepsin S
DALVSdansyl-Ahx3L3VS
Iiinvariant chain
LHVSN-morpholinourea-leucine-homophenylalanine-phenyl-vinylsulfone
MOGmyelin oligodendrocyte protein
p.i.post immunization
Abstract
Multiple sclerosis (MS) is a detrimental disease of the central nervous system (CNS) leading to long-term disability. In the course of animal models of multiple sclerosis (experimental autoimmune encephalomyelitis), we find enhanced activity of proteasome subunits b1i, b2, b2i and b5 in the CNS. We demonstrate that pharmacological inhibition of the proteasome by bortezomib ameliorates experimental autoimmune encephalomyelitis in mice and rats in prophylactic and therapeutic treatment with reduced numbers of T-cells secreting proinflammatory cytokines. The anti-inflammatory effect of proteasome inhibition was accompanied by reduced NF-jB activity in the CNS and lymphoid organs. The combined inhibition of proteasomes and lysosomal proteases involved in major histocompatibility complex II antigen presentation further improved therapeutic efficacy. We suggest proteasome inhibition alone or in combination with inhibition of lysosomal proteases as a novel therapeutic strategy against inflammation-induced neurodegeneration in the CNS. We demonstrate the impact of the proteasome and lysosomal proteases on development of autoimmunity.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS), which is characterized by perivascular inflammation, demyelination and axonal damage in the CNS 1. Experimental autoimmune encephalomyelitis (EAE) serves as the animal model of MS and can be induced in susceptible rodent strains by active and passive immunization with myelin antigens 2. Proteases have currently evolved as a new class of targets for treating autoimmune diseases and a variety of compounds that inhibit protease activity have become available. The activation of self-reactive CD4+ T cells by (auto) antigens presented on major histocompatibility complex (MHC) II molecules is crucial in disease initiation in EAE and possibly MS 3. One approach to interfere with this mechanism is to inhibit this autoimmune response by blocking proteolysis of the antigen or loading of the antigenic peptides onto the MHC II molecules. Several proteases are involved in MHC II-associated antigen processing, among them Cathepsin S (CatS) 4. CatS has emerged as a prime target for disrupting antigen presentation because it controls the proteolysis of MHC II-associated invariant chain (Ii), which is a prerequisite for peptide loading of MHC II. The CatS-selective inhibitor N-morpholinourea-leucine-homophenylalanine-phenyl-vinylsulfone (LHVS) 5 has been shown to block both li processing and antigen presentation in vitro and in vivo by inhibition of the protease 6-10.
The proteasome is a large proteolytic complex, which is abundant in the nucleus and cytosol of eukaryotic cells and responsible for the degradation of redundant and misfolded as well as a variety of key regulatory proteins and involved in a high number of neurodegenerative diseases 11, 12. The 26S proteasome consists of a central 20S core, containing the catalytically active threonine residues and two 19S regulatory complexes. It contains three different active sites, named β1, β2 and β5, responsible for postglutamyl-, tryptic- or chymotrypsin-like proteolytic activity. Upon stimulation with interferon (IFN)-γ, the production of immunoproteasomes is stimulated, in which active beta subunits are replaced with β1i, β2i and β5i subunits, displaying different substrate-specificities.
Apart from providing peptide cargo for MHC I, the proteasome also controls the activity of NF-κB, which is involved in the pathogenesis of many autoimmune inflammatory diseases 13. NF-κB regulates essential proinflammatory genes that play important roles in the pathogenesis of EAE and MS 14-16. NF-κB is sequestered in the cytoplasm by the inhibitory protein IκB. Upon exposure of cells to various stimuli, IKK enzymes phosphorylate IκB, leading to its degradation by the proteasome and release of NF-κB 17, 18. NF-κB consequently moves to the nucleus where it binds to specific DNA sequences and controls the transcription of proinflammatory genes.
Proteasome inhibitors have been shown to be effective drugs for the treatment of multiple myeloma and certain lymphomas 19, 20. In addition to their anticancer properties, they modulate inflammatory and immune responses by affecting antigen processing, apoptosis, cell cycle, co-stimulation, adhesion and chemotaxis 21, 22. Specifically bortezomib, a boronic acid dipeptide with selective activity as a proteasome inhibitor, has been shown to inhibit NF-κB activity in malignant cells 20, 23. Inhibitors of the proteasome decrease NF-κB activation and have anti-inflammatory activity in vivo 24, 25. So far the role of the proteasome and its inhibition has not been analyzed in greater detail in EAE and MS.
Results
LHVS suppresses EAE
We analyzed the effect of systemic administration of the CatS inhibitor LHVS on the severity of EAE induced by myelin oligodendrocyte protein (MOG) 35–55 peptide in C57BL/6 mice. Mice were injected intraperitoneally (i.p.) with LHVS (25 mg/Kg of LHVS dissolved in 50 μL of DMSO) or vehicle-control and sacrificed at 4, 24 and 48 h post injection. Single cell suspensions of spleen cells were then prepared and incubated with the cysteine protease-specific affinity probe DCG-ON, which irreversibly binds to the active site of active papain-like cysteine proteases. After cell lysis and resolution of the polypeptides by SDS-PAGE, active protease species covalently decorated with the probe can be visualized by Western blotting against the biotin moiety present in DCG-0N. This allows to directly assess the active amounts of the respective protease species. Based on published results, active cathepsins Z, B and S were visualized in spleen cells from untreated control mice in agreement with published data 26. By contrast, active CatS was not visualized 4 h after LHVS treatment (25 mg/Kg) and also the amounts of active CatB and CatZ were significantly reduced in vivo. The activity levels of cysteine proteases slowly increased again 24 h post LHVS treatment and reached normal levels 48 h after treatment (Fig. 1A). When control lysates from spleens of untreated mice were treated with LHVS in vitro, active CatS was selectively eliminated. Thus, LHVS effectively targets active CatS in vivo, but is by no means truly CatS specific under the conditions used here. For subsequent treatment with LHVS in vivo, 25 mg/kg of LHVS or vehicle-control was injected i.p. every 24 h.
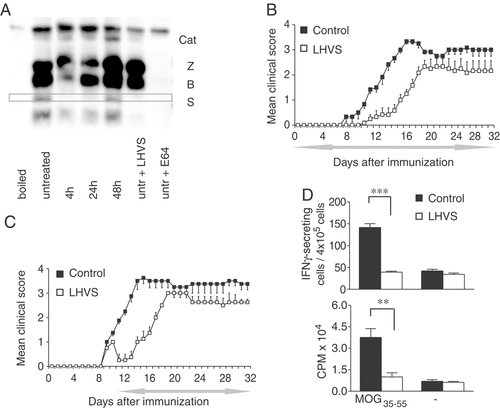
Effect of prophylactic and therapeutic administration of LHVS. (A) Spleen lysates of LHVS-treated (4, 24 and 48 h) or untreated mice were incubated with 10 μM DCG-0N, respectively, for 30 min at RT. Where indicated, cells were pretreated with different inhibitors for 60 min (25 nM LHVS or 25 μM E-64). Proteins were separated by SDS-PAGE on a reducing 12.5% SDS gel and reactive proteins were visualized by streptavidin blotting. (B, C) C57BL/6 mice were injected with MOG35–55 emulsified in CFA and were treated daily i.p. with 50 μL of LHVS (25 mg/Kg) or vehicle-control (DMSO), starting on the day of immunization for a period of 5 wk or (C) therapeutically treated starting 2 days after disease onset; the period of treatment is indicated by the grey left-right arrow. The mean daily score for each group (n=6) is shown. Data are representative of three experiments. (D) Immune responses after daily injections of LHVS or vehicle-control. Spleen cells from MOG-immunized mice were isolated 12 days p.i., cultured with MOG35–55 peptide, proliferative responses and frequencies of Ag-specific IFN-γ producing T cells were measured (mean±SD from four individual mice in each group). Data are representative of two experiments, **p<0.01, ***p<0.001 as compared with vehicle-control-treated group.
In three independent experiments, C57BL/6 mice were vaccinated with MOG35–55 and a clinical score from 0 (healthy) to 5 (moribund or dead) was daily assigned to assess neurological impairment. Daily injections of LHVS or vehicle-control, starting from disease induction (day 0) until the end of the experiment (day 32), resulted in a significant delay in the onset of the disease in treated animals (15.0±1.2 days versus 9.8±0.6 days; p<0.01), and in a significantly lower clinical score ranging from 0 versus 1.0±0.1 on day 11 (p<0.01) to day 19 (1.6±0.2 versus 3.3±0.2, p<0.05; Fig. 1B and Supporting Information Table 1). After day 19, however, LHVS treatment did not affect the clinical severity of EAE in this model (Fig. 1B). When LHVS treatment was initiated only after the clinical onset of EAE in these animals became apparent, we similarly observed a significantly lower clinical score from day 3 after disease onset (0.5±0.3 versus 1.9±0.1, p<0.01) until day 18 (2.5±0 versus 3.5±0.3, p<0.05; Fig. 1C and Supporting Information Table 1), indicating that LHVS treatment was effective in preventing disease progression, albeit only for a limited period of time.
To investigate whether LHVS is affecting EAE by the inhibition of Ii proteolysis and/or by impairment of the autoantigen degradation, we furthermore induced the disease with the full extracellular domain of MOG (MOG1–125) instead of the immunogenic peptide. Because LHVS treatment had a similar effect on disease induced by either MOG1–125 or the respective immunogenic peptide, we conclude that the therapeutic effect of LHVS is unlikely to be mediated by alteration of MOG-processing (Supporting Information Fig. 1).
To determine whether LHVS treatment in MOG-immunized mice had affected T-cell reactivity, the recall responses to MOG35–55 were evaluated day 12 post immunization (p.i.) measuring MOG35–55-specific IFN-γ secretion and T-cell proliferation. We observed a significant reduction in the number of IFN-γ producing cells with splenocytes of mice that had been treated with LHVS versus vehicle-treated controls (39.2±2.2 versus 141.5±8.8, p<0.001; Fig. 1D). Similarly, LHVS treatment suppressed the proliferative responses of T cells against the MOG peptide (counts per minute: LHVS, 10019.6±2869.6; vehicle-control, 37578.4±6119.0; p<0.01) consistent with the later onset and clinical reduction in the severity of EAE observed in LHVS-treated animals. We therefore conclude that the inhibition of cysteine proteases, in particular CatS, delays both the clinical onset of de novo EAE and progression of the established disease. This is accompanied by a reduced antigen-specific activation of CD4+ T cells in the periphery.
Proteasome inhibition ameliorates EAE
We immunized C57BL/6 with MOG1–125 emulsified in complete Freund's adjuvant (CFA). The animals then received 0.5 mg/Kg of bortezomib or vehicle-control, intravenously (i.v.) twice weekly, starting at the day of immunization over a 4-week period. The treatment resulted in a significant reduction in the clinical EAE score in the bortezomib treated group, compared with the vehicle-control-treated group (average score, 0.7±0.2 treated versus 1.8±0.3 untreated; p<0.01; Fig. 2A and Supporting Information Table 2); interestingly, discontinuation of bortezomib treatment by week 4 resulted in an increase in the clinical signs of EAE in this group of animals (Fig. 2A). When bortezomib treatment was prolonged continuously for an additional week (5-week period), the clinical score of these animals was further stabilized (Fig. 2B).
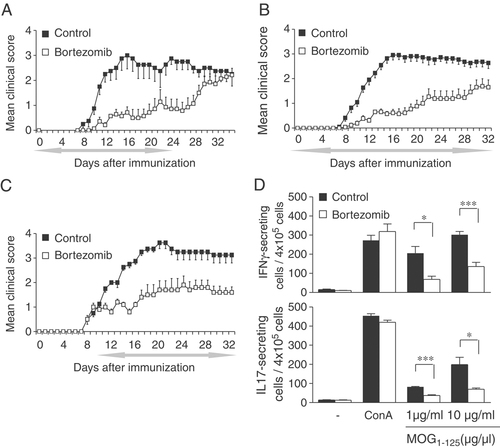
Effect of prophylactic and therapeutic administration of bortezomib. (A–C) C57BL/6 mice were injected with MOG1–125 emulsified in CFA, 100 μL of bortezomib (0.5 mg/Kg) or vehicle-control (NaCl 0.9%) were administered i.v. twice weekly, starting on day 0 and for a period of 4 weeks (A), 5 weeks (B), or administered therapeutically, starting 2 days after disease onset until day 32 (C). The period of treatment is indicated by the grey left-right arrow. The mean daily score for each group (n=6) is shown. Data are representative of 3 experiments. (D) Spleens from mice immunized with MOG1–125 after twice weekly injections of bortezomib or vehicle-control were isolated 12 days p.i., cultured with MOG1–125 peptide, and IL17 and IFN-γ cytokines measured by ELISPOT assays (mean±SD from four individual mice in each group). Data are representative of two experiments, *p<0.05, ***p<0.001, as compared with vehicle-control-treated group.
To examine whether bortezomib treatment would also be effective when initiated after the clinical onset of EAE, we administered bortezomib or vehicle-control in individual mice only after the first clinical signs of EAE had occurred. This treatment likewise led to a significant clinical improvement compared with the control group treated with vehicle-control only (average score, 1.1±0.2 in treated versus 2.1±0.1 in untreated mice; p<0.01; Fig. 2C and Supporting Information Table 2). Strikingly, we observed a similar degree of protection against EAE progression, irrespective whether treatment was initiated in the early induction phase of the disease or after the disease had become clinically evident.
To assess the T-cell responses against MOG1–125 after bortezomib treatment, we used ELISPOT assays for IFN-γ and interleukin (IL)-17. We immunized C57BL/6 mice with MOG1–125, treated with either bortezomib or vehicle-control twice weekly until day 12 p.i. and analyzed splenocytes. In two independent experiments (four samples each), there was a significantly lower number of MOG1–125-IFN-γ producing cells in spleens of mice that had been treated with bortezomib versus vehicle-control (68.5±9.4 versus 205.8±19.1, p<0.05) after stimulation with 1 μg/mL MOG1–125 and (135.8±13.8 versus 302.3±11.6, p<0.001) with 10 μg/mL MOG1–125, respectively (Fig. 2D). A similar picture was observed when the number of IL-17 producing cells was assessed in the same manner (36.9±3.3 versus 83.9±5.4, p<0.001, with 1 μg/mL MOG1–125 and 69.8±4.4 versus 198.6±20.6, p<0.05 with 10 μg/mL MOG1–125, Fig. 2D).
Proteasomal activation in LEW.1N rats with MOG-EAE
Genetic heterogeneity is a critical feature in MS, which might also influence treatment efficacy of certain compounds. This influence can be partially investigated by testing the therapeutic effect of a compound in parallel in different models of EAE. To this end, we examined the effect of bortezomib in a rat model of EAE. The disease was induced in female LEW.1N rats by intradermal injection of 50 μg of MOG1–125 emulsified in CFA at the base of the tail. Rats were clinically inspected daily for signs of EAE and weight was measured from day 12 p.i. until sacrifice. Treatment with 0.25 mg/kg bortezomib twice weekly for 4 weeks starting on day of immunization improved neurological outcome, reducing the cumulative clinical score from 78.8±3.9 in vehicle-control treated rats to 32.3±13.2 in bortezomib-treated rats (p<0.05; Fig. 3A). Furthermore, bortezomib treatment also prevented the decrease in body weight in EAE rats during the course of EAE (65.87±4.93 g in vehicle-control treated rats compared with 17.74±0.47 g in bortezomib-treated rats; p<0.05; data not shown).
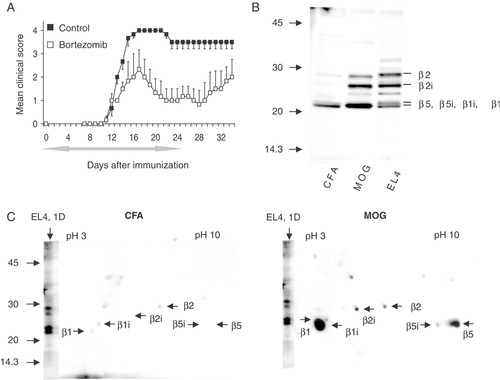
Increased activity of proteasomal subunits in macroglia and neurons from MOG immunized rats. (A) LEW.1N rats were injected with MOG1–125 emulsified in CFA, starting on day 0 rats were treated with 100 μL of bortezomib (0.25 mg/Kg) or vehicle-control (NaCl 0.9%) for 4 wk. The period of treatments is indicated by the grey left-right arrow. The mean daily score for each group (n=3) is shown. (B) For analysis of proteasomal activity, macroglia cells derived from MOG or control CFA-immunized LEW.1N rats were labeled with DALVS immediately after purification and the DALVS-modified active proteasome polypeptides were visualized by SDS-PAGE and Western blot. Murine EL-4 cells served as controls. (C) Resolution of active proteasomal subunits in macroglia cells by two-dimensional gel. Macroglia cells from MOG- or CFA-immunized rats were labeled with DALVS and analyzed by two-dimensional gel electrophoresis (pH range 3–10). Labeled active species were visualized by Western blot.
The specific involvement and activity pattern of proteasome catalytic subunits in EAE have not been characterized to date. To visualize proteasome activity and to assess the activity profile of proteasome subunits in intact glia cells and neurons, we used the cell permeable, activity-based probe Dansyl-Ahx3L3VS (DALVS), which irreversibly binds to all active subunits of the constitutive and the immunoproteasome with comparable affinity. The dansyl moiety allows the detection of the labeled polypeptides in a semiquantitative manner after resolution by SDS-PAGE and anti-dansyl blot. Owing to the activity-based and irreversible nature of DALVS labeling, the polypeptides visualized by this method directly correspond to the amount and pattern of active proteasomal species in the intact cell.
LEW.1N rats were vaccinated with MOG1–-125-CFA while control animals received only CFA. As expected, only the MOG-immunized animals developed clinical signs of EAE by day 12. Brainstems were dissected at day 13 p.i. and cells were prepared as described in Materials and methods. Macroglia cells resulting from immunized rats and murine EL-4 cells (a murine thymoma line with constitutive activity of the constitutive proteasome and the immunoproteasome) were labeled with DALVS immediately after purification and the DALVS-modified active proteasome polypeptides were visualized by SDS-PAGE and Western blot. Labeled proteasome polypeptides were identified based on published data and by direct comparison to the established labeling pattern of EL-4 cells, which constitutively express all active species 12, in microglia of MOG immunized rats we detected a 30 kDa band previously identified as the proteasomal β2 subunit while the signal at slightly lower molecular weight represented the β2i subunit. The 20 kDa signal combined polypeptides derived from the β1, β5, β5i and β1i subunits at poor resolution. The β1-derived polypeptides were predominant in the higher molecular weight portion of the band, the β5- and β5i-derived polypeptides were predominant in its central part and the β1i-derived signals were predominantly retrieved from the lower part (Fig. 3B). We observed a striking increase in the amount of active proteasome polypeptides, thus visualized in macroglia cells from MOG-immunized rats, compared with CFA-treated controls. This was evident for β2-type as well as β1/β5-type of proteasomal activity. Although the β1 and β5-type of active species and the respective immunoproteasome subunits cannot be unequivocally differentiated by one-dimensional SDS-PAGE, the robust induction of active β2i species in macroglia cells from the MOG-immunized rats was striking.
To improve the level of resolution for the analysis of active proteasome subunits in macroglia cells and to differentiate between the β1 and β5 subunits, MOG samples and control cells were resolved by two-dimensional SDS-PAGE after labeling with DALVS (Fig. 3C). The identity of the labeled species was deferred from identical experiments with EL-4 cells in our laboratory, in which the polypeptides had been identified by tryptic digest and mass spectrometry as well as by comparison to the published mobility patterns of proteasomal subunits in comparable 2D SDS-PAGE 12, 27. We found a higher amount of all active proteasome polypeptides species in the MOG-immunized rats. In addition to the increased amount of active β2i subunit, a strong upregulation of active β1i and β5 species compared with the CFA-treated rats was in particular observed, confirming our observations made by one-dimensional resolution. A qualitatively very similar two-dimensional activity profile was observed with primary microglia cells, although in this case the β2i subunit was found to be more prominent in the MOG cells than in CFA cells (Supporting Information Fig. 2). The dominant upregulation of active β5, β5i and β1-species in EAE-affected animals corresponds well with the inhibitory profile of bortezomib, which preferably blocks these species, which again matches the therapeutic effect of bortezomib observed in this study.
Bortezomib inhibits activation of NF-κB in vivo
Activation of NF-κB has been demonstrated as a crucial molecular event for the initiation of the inflammatory reaction in the pathogenesis of EAE 28, 29. Inhibition of transcription factor NF-κB in the CNS ameliorates EAE in rats 29. Since proteasome inhibition induced by bortezomib has been shown to inhibit NF-κB activity in malignant cells 30-32, we investigated whether the proteasome inhibition by bortezomib would likewise interfere with the NF-κB pathway in the CNS, providing a molecular basis for the observed therapeutic effect of bortezomib. To this end, we examined the DNA-binding activity of NF-κB by electrophoretic mobility shift assay (EMSA). Mice were immunized with MOG1–125 and treated i.v. with 0.5 mg/Kg of bortezomib twice weekly. On the 13th day of immunization, mice were sacrificed and EMSA was carried out in nuclear extracts isolated from spinal cords and spleens. As illustrated in Fig. 4, bortezomib treatment diminished the DNA-binding activity of NF-κB in nuclear extracts in bortezomib-treated mice not only in splenocytes but also within the CNS, when compared with untreated controls.
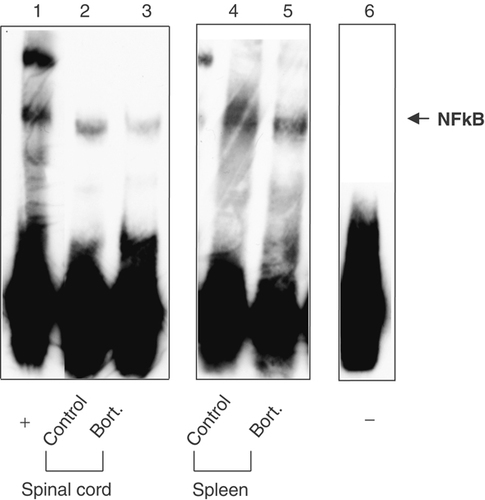
NF-κB activation is impaired after bortezomib treatment. MOG-immunized C57BL/6 mice were treated for 2 wk either with bortezomib or vehicle-control, spinal cord and spleen were removed 13 days p.i. and the nuclear extracts were prepared as described in Materials and methods. Nuclear proteins (10 μg) were incubated with the NF-κB probe followed by EMSA. Free probe is shown in lane 6. The blots represent single representative experiments.
Effect of combined bortezomib and LHVS treatment in EAE
As demonstrated above, the treatment of EAE with either an inhibitor of lysosomal cysteine proteases (LHVS) or with a proteasome inhibitor (bortezomib) had a therapeutic effect on the course of the disease. Since LHVS and bortezomib appeared to impact differentially on EAE, the combination of both inhibitors was tested in vivo.
Four groups of mice (n=6) were immunized with MOG1–125 and treated during the induction and effector phases (from day 0 to day 32) of EAE with vehicle-control, LHVS, bortezomib and a combination of both inhibitors (same dose and regimen used in the previous experiments). Consistent with the results shown in Fig. 1B and Supporting Information Table 1, LHVS treatment significantly prolonged the time to EAE manifestations and reduced their severity when applied during the induction and early effector phase (mean cumulative score day 0–day 18, 2.3±0.8 versus 23.0±1.6; p<0.001; Fig. 5A and Supporting Information Table 3). Similarly as shown in Fig. 2B, the treatment with bortezomib significantly reduced the overall disease compared with the vehicle-control-treated group (mean cumulative score day 0–day 32, 27.0±6.2 versus 64.8±4.4, p<0.01). Mice treated with a combination of LHVS and bortezomib showed significant reduction in clinical signs, compared with vehicle-control-treated mice (average score 0.3±0.1 versus 2.0±0.1; p<0.001, Fig. 5B and Supporting Information Table 3) as well as a delay in the disease onset (17.5±1.3 versus 8.5±0.3 days; p<0.001; Fig. 5A and Supporting Information Table 3). When we compared the effect of the combined therapy with either monotherapy, we found that bortezomib in combination with LHVS significantly reduced the overall clinical severity score compared with LHVS or bortezomib alone (mean cumulative score, bortezomib and LHVS: 9.3±3.8 versus bortezomib 27.0±6.2; p<0.05 or LHVS: 39.6±3.8; p<0.01; Fig. 5A and Supporting Information Table 3).
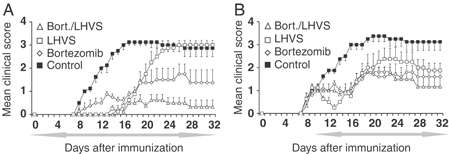
Combined administration of LHVS and bortezomib reduces EAE. (A, B) C57BL/6 mice were injected with MOG1–125 emulsified in CFA and mice were given daily intraperitoneal injections of 25 mg/Kg LHVS (open squares), twice weekly intravenous injections of 0.5 mg/Kg bortezomib (diamonds) and combination of both treatments as described before (triangles) and vehicle-control (filled squares) in a prophylactic (A) or therapeutic setting (B). The period of treatment was indicated by the left-right arrow. The mean daily score for each group (n=6) is shown.
We next assessed the effect of the combination treatment with LHVS and bortezomib on the course of the disease when treatment was initiated after there was already clinical evidence for EAE. To this end, EAE was again induced with MOG and treatment with vehicle-control, LHVS, bortezomib or both inhibitors combined, respectively, was initiated after the first clinical signs of EAE became apparent and continued until the end of the experiment (from day 10 to day 32). As shown in Fig. 1C, therapeutic application of LHVS significantly reduced the mean clinical score compared with the vehicle-control-treated group, albeit only for a short period of time (from day 12 to day 18, p<0.05; Fig. 5B and Supporting Information Table 3). By contrast, treatment with bortezomib resulted in significant amelioration of the clinical score compared with the control group (average score, 1.1±0.2 versus 2.0±0.2, p<0.01; Fig. 5B). Furthermore, the group of mice treated with LHVS and bortezomib showed a significant reduction in the clinical signs (average score, 1.0±0.1 versus 2.0±0.2, p<0.01), compared with the control group.
Discussion
The two major intracellular proteolytic systems, the proteasome and lysosomal proteases, not only play a key role in cellular homeostasis but also provide peptide ligands for MHC I and II mediated immunity. In the present study, we have evaluated intracellular proteases as therapeutic targets in EAE. We demonstrate that (i) pharmacological inhibition of the key lysosomal protease CatS by LHVS reduces the severity of MOG-induced EAE and reduces associated proinflammatory immune responses; (ii) glia cells and neurons from MOG-immunized rats have an increased activity of proteasome polypeptides compared to CFA-immunized rats; (iii) administration of the proteasome inhibitor bortezomib improved the clinical signs of EAE in mice and rats. Bortezomib application was further associated with a suppression of the secretion of proinflammatory cytokines by MOG-stimulated T cells and decreased NF-κB activity in CNS resident cells and (iv) combining the inhibition of lysosomal cysteine proteases with the inhibition of the proteasome reduced EAE severity compared with either monotherapy or controls.
CatS is essential for the MHC II-mediated presentation of antigenic peptides to CD4+ T cells. It is involved in both the intracellular breakdown of li 9 and proteolytic processing of different antigens including myelin basic protein, a potential autoantigen implicated in the pathogenesis of MS 33. CatS activity is required for induction of the MHC II-mediated collagen-induced arthritis 34. Likewise, pharmacological inhibition of CatS suppresses autoimmunity in thyroiditis and pulmonal hypersensitivity 26. We here demonstrate that inhibition of CatS by LHVS attenuates the clinical signs of EAE- and MOG-specific T-cell activation. This may either be the result of impaired antigen processing, Ii processing, or both. To address this issue, we induced EAE in mice either with the antigenic peptide MOG35–55 or with the extracellular MOG domain, MOG1–125. Because LHVS had a comparable effect under both conditions, we argue that the therapeutic effect of LHVS is unlikely to be mediated by the alteration of MOG processing. Indeed, LHVS treatment in vitro and in vivo has been shown to impair Ii proteolysis, which in turn affects the activation of CD4+ T cells 6-10. On the other hand, LHVS may affect the pathogenesis of EAE independently from MHC II antigen presentation, e.g. by decreasing the activity of extracellular cysteine proteases such as CatS, CatL or CatK, which may be involved in local inflammation and tissue destruction. However, it is unlikely that this represents the major mechanism of action of LHVS in our model, because LHVS is not detected in the brain 3 h after i.v. administration, in contrast to the plasma, suggesting that LHVS acts mainly in the periphery and not in the CNS 35. Therefore, it is conceivable to assume that LHVS is most efficient during the induction and early effector phase of EAE, where it interferes with the activation of T cells in peripheral lymphoid organs.
Although proteasome inhibitors have been shown to mediate anti-inflammatory and immunomodulatory effects, their effect on the pathogenesis of autoimmune diseases and in inflammation-induced neurodegeneration is unexplored. Bortezomib, licensed for treating human myeloma, reversibly ablates both β1 and β5 activity and reduces β2 proteasomal activity in peripheral blood cells 27. Based on the increased activities of β1i, β2i and β5 subunits in glia cells and neurons of rats with MOG-EAE shown in this report, we hypothesized that bortezomib might be ideally suited to block proteasome activity in the CNS in the course of EAE. Consistent with this assumption, bortezomib treatment ameliorated the course of the disease.
To our knowledge this is the first report showing a differential activation of proteasome subunits in resident cells of the CNS in the course of EAE a model of inflammation-induced neurodegeneration. We not only observed a general upregulation of proteasome activity in glia cells of MOG-immunized rats but also an induction of immunoproteasome activity. A higher proteasomal activity may lead to (i) an increased supply in antigenic peptides to be presented on MHC 1 to CD8+T cells or/and (ii) regulation of the inflammatory responses via the upregulation of several pro-inflammatory molecules promoted by NF-κB activation or/and (iii) changes in histone de-ubiquitination with general effects on transcription. Our study suggests that the presentation of antigenic MOG peptides on MHC I molecules might not be the key for the development of EAE, because we observed no difference in the course of EAE, irrespective whether treatment was initiated together with the antigenic stimulus or after clinical manifestation of the disease.
DNA-binding activity of NF-κB is induced in the spinal cord of LEW rats during EAE and induction of NF-κB activation plays an important role in the pathogenesis of EAE. Consistent with this, inhibition of activation of NF-κB by pyrrolidine dithiocarbamate in vivo attenuates clinical symptoms of EAE 29. It is well established that inhibition of the NF-κB pathway leads to effects on a variety of cell types with reduced expression of pro-inflammatory factors such as cytokines and chemokines as well as adhesion molecules on lymphoid cells. Furthermore NF-κB inhibition has antiproliferative and pro-apoptotic effects on lymphoid cells. The effects of selective absence of NF-κB in CNS resident cells in EAE has been recently investigated by inducible knockdown of IKK2 36. Consistent with the inhibitory effect of bortezomib on the activation of NF-κB in different tumor cells 20, 23, we here demonstrate that the therapeutic effect of bortezomib in EAE is accompanied by inhibition of NF-κB activation in spleen and spinal cords in vivo. Thus, both the proteasome and NF-κB activity contribute to the pathogenesis of EAE. One possible scenario is that the increased proteasomal activity leads to a higher rate of degradation of IκB, thus resulting in less efficient inhibition of NF-κB, which then mediates the inflammatory response. Likewise, the upregulation of the proteasome observed may be the consequence of NF-κB activation, leading to increased protein expression. Interestingly, proteosomal activity affects ubiquitinylation that in turn affects transcription by histone remodeling 37.
Our data suggest that the proteasome activation in CNS resident cells is strongly involved in the development of EAE. This in turn points toward the new observation that CNS resident cells are primary regulators of EAE development and possibly influence development of T-cell-induced CNS autoimmunity by mechanisms not completely elucidated so far.
In conclusion, the present work demonstrates that increased proteasomal activity in glia cells and neurons is a hallmark of EAE. Inhibition of the proteasome with bortezomib can significantly ameliorate disease in mice and rats by attenuation of NF-κB-promoted inflammatory events. Therefore, the proteasome is an important target for therapeutic interventions in inflammatory CNS diseases. In addition, we show that selective inhibition of CatS has the potential to modulate autoantigen driven immune responses in class II-restricted autoimmune diseases. Combined targeting of the proteasome and lysosomal proteases therefore appears as a promising therapeutic option in EAE and possibly MS, because it controls activation of autoreactive T cells in the periphery and modulates specific cellular responses that lead to tissue damage in the target organ, the CNS.
Materials and methods
Animals
Female mice and rats 10–14 wk of age were used in all experiments. C57BL/6 mice were purchased from Charles Rivers Laboratories, Germany. LEW.1N rats were obtained from H. Hedrich (Central Animal Laboratory, Hannover Medical School, Hannover, Germany). Animals were bred and kept under specific pathogen-free conditions at Hertie-Institute, University of Tübingen, Tübingen, Germany. All experiments were approved by the regional boards in Tübingen, Germany.
Immunogens and antigens
MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) was obtained from EMC microcollections GmbH, Tübingen, Germany. The N-terminal sequence of rat MOG (amino acids 1–125) was expressed in Escherichia coli and purified to homogeneity by chelate chromatography 38.
Induction and evaluation of EAE
For induction of EAE, C57BL/6 mice were immunized subcutaneously with either 50 μg of MOG35–55 or rrMOG1–125 dissolved in PBS and emulsified with an equivalent volume of CFA supplemented with 2 mg/mL of heat-inactivated Mycobacterium tuberculosis H37RA (Difco Laboratories, Michigan, USA). On day 0 and 2, each mouse was administered 150 ng of pertussis toxin (Calbiochem, Germany). EAE was induced in LEW.1N rats by intradermal injection at the base of the tail with a total volume of 200 μL of inoculum containing 50 μg of MOG1–125 in saline mixed (1:1) with CFA, with 200 μg of Mycobacterium tuberculosis. Animals were clinically scored and weighed on a daily basis up to 40 days p.i. Severity of clinical disease was assessed as following: score 0=no paralysis, 1=limp tail, 2=limp tail and weak gait, 3=hind limb paralysis, 4=fore limb paralysis and 5=death or moribund animal.
Treatment regimen
Mice received 25 mg/Kg of LHVS 5 i.p. daily and/or 0.5 mg/Kg of bortezomib (Ortho Biotech, Neuss, Germany) i.v. twice weekly. Rats were treated with 0.25 mg/Kg of bortezomib i.p. Control animals received an identical volume of PBS. Treatments started on the day of immunization (prophylaxis) or after disease onset (treatment) for 4–5 wk periods.
Proliferation assay
Spleen cells from mice immunized with MOG35–55 and treated daily with LHVS were isolated at day 12 after immunization. Proliferative experiments were performed in triplicates in 96-well round-bottom microtiter plates (Nunc, USA). A total of 4×105 splenocytes/well were cultivated in 200 μL of complete medium containing RPMI (Invitrogen, Germany) supplemented with 5% FCS (FCS gold, PAA), 1% penicillin/streptomycin, 1% glutamine and 50 μM 2-ME (all from Invitrogen), in the presence or absence of 10 μg/mL MOG35–55 peptide. After 40 h, cells were pulsed with 1 μCi/mL [3H]-TdR (Hartmann Analytic, Braunschweig, Germany) per well for an additional 16 h. DNA was collected on glass fiber filters (Wallac, Turku, Finland) and [3H]-TdR incorporation was measured in a scintillation counter (1450 Microbeta Plus, Wallac).
ELISPOT analysis
Ninety-six well nitrocellulose plates (Millipore, Schwalbach, Germany) were coated with 10 μg/mL capture antibodies either for IFN-γ (Mabtech, Stockholm, Sweden) or IL-17 (natutec-ebioscience, Germany) overnight at 4°C. After washing, the membrane was blocked with RPMI containing 10% FCS for 1 h. A total of 4×105 splenocytes/well were cultured for 40 h in 37°C, 5% CO2. For each Ag or mitogen, triplicates were used. Background levels of cytokine production (<15 spots) were measured by plating the cells in the absence of peptide. ConA was used as a positive control. After 40 h, the cells were discarded and the membranes were thoroughly washed by immersing the plates six times in PBS. To visualize areas of the membranes that had bound secreted IFN-γ and IL-17, the respective biotinylated detector antibodies (1 μg/mL) were added for 2 h and staining performed with streptavidin peroxidase complex and chromogen solution containing carbazole (Sigma-Aldrich). Areas of the membrane where a specific color reaction had occurred appeared as dark brown-red spots and were counted by an automated ELISPOT counter (Autoimmun-Diagnostika, Albstadt, Germany) and manually cross-checked. The average numbers of spots in triplicates secreted after exposure with Ag or mitogen were expressed as numbers of IFN-γ- or IL-17-secreting cells per 4×105 cells added initially to the wells.
Affinity labeling of active cysteine proteases
DCG-0N, a derivative of JPM-565 with identical labeling characteristics, was synthesized and purified as previously described 39. Spleen lysates were prepared from 5×105 cells in 2×lysis buffer (100 mM citrate/phosphate, 2 mM EDTA, 1% NP40, pH 5) and were lysed for 30 min at 4°C, followed by removal of membrane fragments by centrifugation. Spleen cell lysate protein (12 μg) was incubated with 10 μM of DCG-0N and 50 mM DTT at ambient temperature for 30 min Reactions were terminated by addition of 6× SDS reducing sample buffer and immediate boiling at 95°C for 10 min. Samples were resolved by 12.5% SDS-PAGE gel, and then blotted on a PVDF membrane (Amersham Biosciences). After blocking with PBS-0.2% Tween 20–10% Roti®-Block (Roth, Karlsruhe, Germany) and extensive washing with PBS-0.2% Tween 20, the membrane was probed with Vectastatin® (Vector Laboratories, Burlingame, CA, USA) in PBS-0.2% Tween 20 for 60 min, followed by washes with PBS-0.2% Tween 20. The ECL-Detection kit (Amersham Biosciences) was used for visualization. E-64 was obtained from Sigma.
Affinity labeling of active proteasomes
The proteasome-specific affinity probe DALVS was synthesized as described 40. Both the constitutive [β1, β2, β5)] and the immunoproteasome subunits (β1i, β2i, β5i) were labeled by DALVS in intact cells, which were incubated with 1 μM DALVS in complete medium at 37°C for 1 h. Afterwards, cells were washed with 0.9% NaCl and lysed in 1% Triton X-100 on ice for 30 min followed by the removal of debris and membrane fractions by centrifugation. Total protein content in cell lysates was determined using the Bradford method. For the two-dimensional gels, samples were adjusted for equal amounts of protein with IEF-buffer (9 M urea, 4% CHAPS, 0.5% Pharmalyte (all GE Healthcare, Germany), 0.4% DTT). IPG-strips pH 3–10NL (Invitrogen) were rehydrated with the samples overnight, followed by isoelectric focusing using the Ettan IPG-multiphor device (GE Healthcare). Consecutively, strips were reduced in DTT, alkylated with iodoacetamide and resolved by SDS-PAGE on NuPage 4–12% precast gels (Invitrogen). Visualization of the labeled species was performed by Western blot, using a rabbit polyclonal dansyl-sulfonamidohexanoyl-specific antibody (Molecular Probes) and a HRP-coupled secondary reagent, followed by enhanced chemoluminescence as described 27.
Isolation of cells from rat brain
After dissecting the rat brainstems, cells were squeezed through a mesh in collagenase and DNase containing RPMI medium. Tissue was incubated while shaking at 37°C until homogenized. Isolated cells were separated from the myelin on a percoll gradient. After removing the myelin, cells were collected, washed and plated in a culture flask overnight. Non-adherent cells (macroglia) were removed from the adherent cells (microglia).
Electrophoretic mobility shift assay
Nuclear protein extracts were obtained following the protocol of Andrews and Faller 41. Protein concentrations in the nuclear extracts were determined using the Bio-Rad/Bradford assay. EMSA was conducted according to the manufacturer's instructions (Panomics, CA, USA). Consensus biotinylated NF-κB oligonucleotides were provided by the manufacturer (Panomics). For the supershift assays, 10 μg of the nuclear protein extracts was incubated with the biotinylated probes. The reaction products were separated on a 6% non-denaturing polyacrylamide gel and visualized by autoradiography.
Statistical analysis
Data shown in figures and tables are reported as the mean±SD of at least three experiments. Differences between normally distributed groups were compared using the Student's t test and in non-normally distributed groups with the Mann–Whitney U test.
Acknowledgements
This study was supported by the German Research Foundation (DFG We 1947/4-1/2 and DFG We 1947/5-1).
Conflict of interest: The authors declare no financial or commercial conflict of interest.




