Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1α†
Abbreviations
HIF-1hypoxia-inducible factor-1
siRNAshort-interfering RNA
Abstract
Recent data suggest that hypoxia and its principal molecular signature HIF-1 (hypoxia-inducing factor-1) may tune down inflammation by dictating anti-inflammatory programs. We tested the effects of hypoxia and HIF-1α on the homeostasis of naturally occurring regulatory T cells (Treg) and their transcriptional activator Foxp3. Hypoxia induced a time-dependent increase in HIF-1α in mouse and human T cells. Hypoxia upregulated the expression of Foxp3 in Jurkat T cells, human and murine mononuclear cells. The effects of hypoxia on Foxp3 expression were HIF-1α-dependent as they were abolished upon transfection with short-interfering RNAs for HIF-1α and promoted by HIF-1α overexpression. Hypoxia increased the potency of Treg, as hypoxic CD4+CD25+ lymphocytes were more effective than normoxic cells in suppressing the proliferation of CD4+CD25− effectors. In vivo expression of HIF-1α achieved by hydrodynamic injection of the respective naked DNA similarly induced an increase in Foxp3 expression and an increase in the number of functionally active Foxp3+CD4+CD25+ Treg. Thus, hypoxia dictates an anti-inflammatory program by driving expression of HIF-1α that acts to increase the number and suppressive properties of naturally occurring CD4+CD25+ Treg.
Introduction
Under hypoxia, hypoxia-inducible factor-1 (HIF-1) plays a key role in controlling glycolysis, angiogenesis, erythropoiesis and cell survival 1. HIF-1 constitutes an oxygen-dependent α-subunit and a constitutively expressed β-subunit that interact through mutual basic helix-loop-helix domains 2. HIF-1α oxygen-dependent domain undergoes site-specific proline hydroxylation by prolyl hydroxylases, which allows subsequent ubiquitination mediated by the von Hippel-Lindau protein 3-5. In the absence of molecular oxygen, HIF-α hydroxylation is abrogated, leading to its cellular accumulation. HIF subunits heterodimerize, and activate a wide range of target genes by binding to hypoxia response elements.
T-lymphocytes that comprise a principal effector component of the cellular immune response are exposed to hypoxia in target microenvironments in which they are assumed to dictate inflammatory programs. Target sites include tumors and healthy organs that confront T cells with gradients of low oxygen tension. Recent literature suggests that blunted HIF-1α expression results in a net proinflammatory program evident by increased production inflammatory cytokines upon TCR engagement 6. Similar results were obtained upon knocking out HIF-1 selectively in T cells 7. Additionally, HIF-1α was reported to play an inhibitory role in the regulation of T-cell receptor signal transduction by controlling intracellular calcium balance 8. Collectively, these observations suggest that HIF-1 drives a Th2 favored response by downregulated Th1 programs 9-11. Naturally occurring CD4+CD25+ regulatory T cells (Treg) comprise a relatively newly characterized population that has evolved to tune down cellular immune responses 12. The transcriptional factor governing Treg has been identified as Foxp3 and their mechanism of action is via cell-to-cell contact resulting in suppression of effector T-cell proliferation.
Herein, we tested the hypothesis that HIF could exert an immunomodulatory role by influencing the Treg population and their transcriptional activator Foxp3.
Results and discussion
The effect of hypoxia and HIF-1α overexpression on Foxp3 expression in Jurkat T cells
We first determined the effect of hypoxic conditions (1% O2) and HIF-1α stabilization on the expression of Foxp3 in human Jurkat T cells. Incubation of Jurkat cells under hypoxia for 16 h resulted in a marked increase in HIF-1α activity (eight fold; p<0.005) as determined by ELISA (Fig. 1A). Hypoxia-induced HIF-1α increased activity was abrogated by retroviral infection with short-interfering RNAs (siRNA) directed against HIF-1α but not with control siRNA. Flow cytometry analysis revealed a significant increase in Foxp3 expression in hypoxic cultures, compared with normoxic controls (Fig. 1B). This effect was markedly reduced in the presence of siHIF-1α, clearly pointing at a HIF-1α-dependent effect on Foxp3 expression. We then assessed Foxp3 expression in Jurkat cells following retrovirus-mediated overexpression of HIF-1α. To circumvent von Hippel-Lindau-mediated ubiquitination of HIF-1α, we employed a mutant form of HIF-1α, HIF-1αP564A, in which mutation of proline 564 to alanine leads to enhanced HIF-1α stability 13. Jurkat cells were infected with different dilutions (1:0, 1:1, 1:2, 1:4) of pMX-HIF-1αP564A virus (∼6×106 IU/mL). A dose-dependent increase in mRNA level of HIF-1α and Foxp3 was evident by semi-quantitative RT-PCR (Fig. 1C). The increase in Foxp3 mRNA following HIF-1α overexpression was further validated by quantitative real-time PCR (Fig. 1D). At the protein level, enhanced HIF-1α activity (7.94-fold; p<0.005) was validated by ELISA (Fig. 1E). Finally, FACS analysis confirmed a direct dose-dependent association between HIF-1α and Foxp3 expression (Fig. 1F). Altogether, these findings pointed at clear causal relationship between HIF-1α and Foxp3 expression.
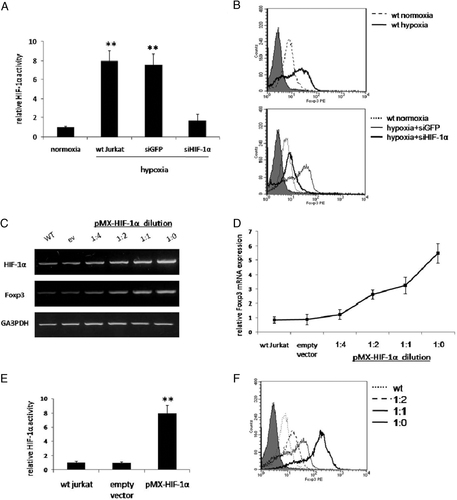
The effects of hypoxia and HIF-1α overexpression on Foxp3 expression in Jurkat T cells. Jurkat cells were incubated for 16 h in normoxia (20% O2) or hypoxia (1% O2). In parallel, hypoxic cells were infected with retroviral siHIF-1α or siGFP, as control, and HIF-1α binding activity was determined by ELISA as described in Materials and methods (A). Cell groups were analyzed for Foxp3 expression by flow cytometry; wild-type normoxic (broken line) and hypoxic (bold line) cells, compared with isotype control (gray) (B; upper panel); hypoxic siHIF-1α (bold line) and siGFP (fine line) compared with normoxic (dotted line) and isotype control (gray) (B; lower panel). Jurkat cells were then transduced with pMX-eGFP or with decreasing dilutions (1:0, 1:1, 1:2, 1:4) of pMX-HIF-1αP564A virus; HIF-1α as well as Foxp3 expression was then assessed by semi-quantitative RT-PCR (C; upper panel). Foxp3 mRNA following HIF-1α overexpression was quantified by real-time PCR (D). HIF-1α activity in Jurkat cells, infected with undiluted retrovirus, evaluated by ELISA is shown (E). Jurkat cells, infected with decreasing dilutions of pMX-HIF-1αP564A virus, were analyzed by flow cytometry for Foxp3 expression; no dilution (bold line), 1:1 dilution (fine line), 1:2 dilution (broken line) compared with wild-type (dotted line) and isotype control (gray) (F). For all experiments triplicate were performed; mean value±SD of a representative of three independent assays is shown (**p<0.005).
The effect of hypoxia on the number and function of primary mouse and human Treg
To confirm the validity of the above findings in primary cells we tested the effects of hypoxia in human peripheral blood mononuclear cells (PBMC) and mouse splenocytes. Human PBMC as well as mouse splenocytes were subjected to different periods of hypoxia and analyzed by ELISA for HIF-1α DNA binding activity (Fig. 2A). Annexin V-PE/7-AAD staining showed no significant differences in apoptosis between normoxic and hypoxic cell groups (data not shown). A time-dependent increase in HIF-1α activity was demonstrated, showing a peak elevation after 16 h (6.41- and 5.32-fold in PBMC and splenocytes, respectively, p<0.005). Thus, human PBMC and mouse splenocytes were exposed for 16 h hypoxia, allowed to recover for 24 h, and analyzed by flow cytometry for CD4+CD25+ expression (Fig. 2B). The Foxp3+ population among CD4+CD25+ suppressor cells was found to increase from 30.56±6.3 to 48.90±4.9% in human cells (p<0.05) (Fig. 2C; upper panels). Likewise, in mouse splenocytes hypoxia treatment increased Foxp3+CD4+CD25+ T cells from 57.95±6.2 to 70.05±5.1% (p<0.05) (Fig. 2C; lower panels).
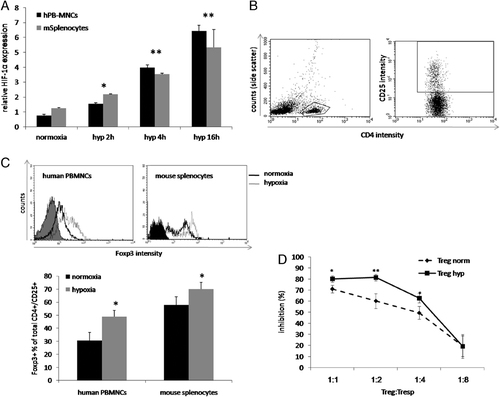
Number and function of primary Treg following hypoxia preconditioning. Human PBMC (hPB-MNCs, black) and mouse splenocytes (gray) were incubated under hypoxia for 0 (normoxia), 2, 4 or 16 h. Cells were then analyzed for HIF-1α activity (A). After 24 h of recovery, cells were analyzed by flow cytometry for Foxp3 expression in CD4+CD25+ gated cells. CD4 and CD25 gating steps are shown in B. Human (C; left histogram) and mouse (C; right histogram) cells were then assessed for Foxp3 expression – the mean values+SD (representative of three independent assays) are presented in the chart. For the suppression assay, mouse splenocytes were submitted to normoxic or hypoxic conditions. CD4+CD25+ suppressor and CD4+CD25− responder T cells were sorted by flow cytometry. Normoxic responders activated by anti-CD3 and anti-CD28 monoclonal antibodies were co-cultured with normoxic/hypoxic suppressors at different ratios (1:1, 1:1/2, 1:1/4 and 1:1/8, respectively). After 72 h, [3H]thymidine proliferation assay was performed (D) (percent inhibition of proliferation was determined from the following formula: 1−(median [3H]thymidine uptake of 1:1 CD4+CD25+:CD4+CD25− coculture/median [3H]thymidine uptake of CD4+CD25− cells). For all experiments, each group was performed in triplicate/quadruplicate; mean value±SD of a representative of three independent assays is shown (*p<0.05, **p<0.005).
On the functional aspect, hypoxia-treated Treg more potently suppressed the proliferation of CD4+CD25− responders as compared with normoxic CD4+CD25+ cells, in a dose-dependent manner (Fig. 2D).
The effect of in vivo overexpression of HIF-1α in mouse splenocytes on the levels of Foxp3+CD4+CD25+ Treg
We next explored the effect of HIF-1α on Treg in vivo. For this purpose, we employed the hydrodynamic naked DNA injection in order to induce HIF-1α overexpression in mouse splenocytes 14. A noticeable expression of human HIF-1α mRNA (Fig. 3A) and enhanced HIF-1α activity (Fig. 3B) were evident in the splenocytes 24 h after injection. Foxp3 mRNA levels were elevated in the HIF-1α-injected group 48 h after DNA administration (Fig. 3A). FACS analysis of spleen-derived Treg 72 h after injections showed significantly elevated levels of Foxp3+CD4+CD25+ cells in HIF-1α-injected mice (80.16±2.26%) compared with controls (68.98±2.25%) (p<0.005) (Fig. 3C). These overall findings support the assumption that HIF-1α activation contributes to an increase in the expression of Foxp3 as well as to an increase in the number of functionally active Foxp3-Treg.
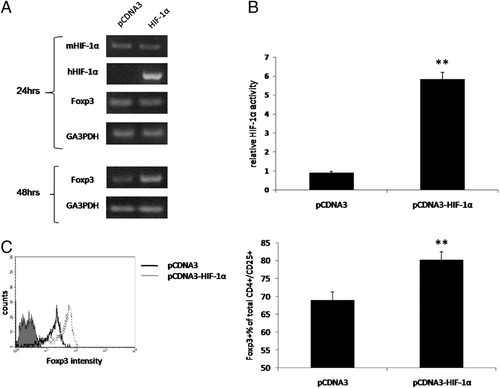
The effect of HIF-1α overexpression in lymphocytes on the levels of CD4+/CD25+/Foxp3+ Treg. Hydrodynamic naked DNA systemic injection of pCDNA3 empty vector or pCDNA3-HIF-1αP564A was performed. C57BL/6J mice (n=8 per group) were injected i.v. for 5 s with 12 μg of plasmid DNA. Murine (m) and human (h) HIF-1α, Foxp3 and GA3PDH mRNA expression were analyzed after 24 h and (Foxp3 and GA3PDH) 48 h by RT-PCR (A). HIF-1α activity was evaluated after 24 h (B). Flow cytometric analysis of spleen-derived CD4+CD25+Foxp3+ Treg was performed 72 h after injection (C); mean value+SD is shown (**p<0.005).
Concluding remarks
In this study, we demonstrate, for the first time, an association between hypoxia and the homeostasis of Treg, mediated by upregulating HIF-1α. We have shown enhancing effects of hypoxia both on the number as well as on the activity of Treg, potentially driving anti-inflammatory programs. These findings are in line with previous observation suggesting that the targeted lymphocyte knockdown of HIF-1α results in a Th1-skewing of the immune response. It may be hypothesized that hypoxic sites (tumoral, ischemic, inflammatory) may downregulate local early inflammatory response by inducing expression of HIF-1α within local lymphocytes with consequent upregulation of the Treg pool.
Materials and methods
Cell culture
Experiments using animals were done with the permission of the local Ethical Committee. Local Ethical Committee approval was received also for human studies and informed consent of all participating subjects was obtained. Male C57BL/6J mice were obtained from Harlan Laboratories. Human peripheral blood mononuclear cells PBMC were isolated from healthy subjects by Ficoll density-gradient centrifugation (Sigma-Aldrich) of 20 mL blood samples. Mouse spleens were explanted, mechanically minced and red cells were lysed using ammonium chloride solution. Jurkat T cells, human PBMC and mouse RBC-depleted splenocytes were cultured in complete RPMI medium supplemented with 10% FBS, 5% CO2 at 37°C. For hypoxic preconditioning, cells were placed in a hypoxic chamber (Billups-Rothenberg) and a gas mixture of 94% N2, 1% O2 and 5% CO2 was infused for 4 min at a rate of 2 psi. The chamber was placed in an incubator maintained for the indicated time courses at 37°C.
Retroviral transduction
pMX-eGFP constructs were kindly provided by Dr. I. Spiegel. pCDNA3-hHIF-1αP564A was a gift from Dr. M. Safran. The HIF-1α mutant form was inserted into the BamH1/NotI sites of pMX-eGFP, excluding eGFP, to yield pMX-hHIF-1αP564A. pMX-eGFP served as control vector. siRNA that correspond to the HIF-1α and GFP genes, cloned into pSuperRetro vector (OligoEngine, WA, USA), were kindly provided by Prof. R. Ratan 15. All plasmids were confirmed by DNA sequencing (Hylabs).
Retroviral plasmids were transfected into Phoenix packaging cells (ATCC) using standard Ca2PO4 protocol. Medium was changed after 24 h. Cells were grown for additional 24 h and virus collected and centrifuged 5 min/1500 rpm/4°C. Viral titer was determined as >6×106 IU/mL using NIH 3T3 cells. Jurkat cells were incubated with viral supernatant for 6 h in the presence of 4 μg/mL polybrene (Sigma-Aldrich). Infection was repeated after 24 h.
Active HIF-α ELISA
Human/Mouse Active HIF-1α DuoSet® Intracellular ELISA (R&D) was used according to manufacturer's instructions. Briefly, a biotinylated oligonucleotide containing the consensus hypoxia response element HIF-1α binding site was incubated with nuclear extracts. HIF-1α-ds oligonucleotide complexes were captured by immobilized anti-HIF-1α antibody. Unlabeled oligonucleotide was used to test assay's specificity. Detection was performed utilizing Streptavidin-HRP and ELISA reader.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from cultured 3−4×106 cells by phenol/chloroform/isoamyl alcohol (Biological Industries). RNA was treated with DNase I (Ambion). RNA (2 μg/reaction) was transcribed using AMV-RT (Promega). Amplification reactions were carried out using REDTaq ReadyMix (Sigma-Aldrich). Reaction conditions were calibrated for each gene and primers. The following primers were used: hHIF-1α fw′-CCACCTATGACCTGCTTGGT, rev′-GGCCAGCAAAGTTAAAGCAT; mHIF-1α fw′-TGCTCATCAGTTGCCACTTC, rev′-CTTCCACGTTGCTGACTTGA; hFoxp3 fw′-CTCTTCTTCCTTGAACCCCA, rev′-CACTTGCAGACACCATTTGC; mFoxp3 fw′-GGCCCTTCTCCAGGACAGA, rev′-GCTGATCATGGCTGGGTTGT; h/mGA3PDH fw′-TCCACCACCCTGTTGCTGTA, rev′-ACCACAGTCCATGCCATCAC.
Real-time polymerase chain reaction
RNA was transcribed as described above. Foxp3 mRNA expression was quantified using ABsolute SYBER Green ROX mix (Thermo) according to manufacturer's instruction and using default cycling parameters on an ABI Prism 7200 Sequence Detector. The gene-expression results were expressed as arbitrary units relative to the expression of the gene encoding GA3PDH, as the mean±SD. Sequences for Foxp3 primers were the same used for semi-quantitative RT-PCR.
FACS analysis
After Ficoll (Sigma-Aldrich) separation, human PBMC were stained with combinations of the following monoclonal antibodies and corresponding isotype controls (eBioscience): APC- anti-human CD4, FITC-anti-human CD25. Intra-cytoplasmic Foxp3 staining was performed using the PE-anti-human Foxp3 staining kit according to the manufacturer's instructions. Mouse splenocytes were stained using the following antibodies (eBioscience): anti-mouse FITC-CD4, anti-mouse PE CD25, anti-mouse APC-Foxp3 staining kit and corresponding isotype controls.
In both cases, 8×104 cells were acquired by flow cytometry (FACSCalibur, Becton Dickinson). To calculate Foxp3 percentage in CD4+CD25+ cells, the analysis was performed as follows (Fig. 2B): CD4+ PBMC or splenocytes were gated on CD25 marker. Then, CD4+CD25+ gated cells were presented as Side Scatter versus Foxp3 (Fig. 2C human, Fig. 2D mouse). Gating on Foxp3 was done with respect to isotype control. Jurkat T cells were stained with anti-human PE-Foxp3 staining kit and 1×104 cells were acquired for flow cytometry.
Apoptosis in the different cell groups was assessed using SouthernBiotech ApoScreen Annexin V Apoptosis detection kit (Annexin V-PE, 7-AAD solution and Annexin V binding buffer).
Cell sorting
The CD4+CD25 (responders) and CD4+CD25+ (suppressors) populations were isolated from 108 RBC-depleted splenocytes by FACS (FACSAria, Becton Dickenson) after FITC-anti-CD4 and PE-anti-CD25 staining for 30 min.
Functional suppression assay
Ninety-six well plates (CoStar, Corning) were coated with 1 μg/mL anti-CD3 monoclonal antibody (17A2, eBioscience) overnight at 4°C and washed. CD4+CD25− and CD4+CD25+ T cells (104 cells/well) were cultured in RPMI 10% FBS medium at different responder–suppressor ratios (1:1, 1: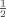 , 1:
, 1: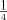 and 1:
and 1: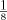 ). All cells were cultured in a final volume of 200 μL and 2.5 μg/mL anti-CD28 monoclonal antibody (eBioscience). After 72 h, [3H]thymidine (1 μCi/well) was added for 16 h before proliferation was assayed by scintillation counting (beta counter). Percent inhibition of proliferation was determined from the following formula: 1−(median [3H]thymidine uptake of 1:1 CD4+CD25+:CD4+CD25− coculture/median [3H]thymidine uptake of CD4+CD25− cells).
). All cells were cultured in a final volume of 200 μL and 2.5 μg/mL anti-CD28 monoclonal antibody (eBioscience). After 72 h, [3H]thymidine (1 μCi/well) was added for 16 h before proliferation was assayed by scintillation counting (beta counter). Percent inhibition of proliferation was determined from the following formula: 1−(median [3H]thymidine uptake of 1:1 CD4+CD25+:CD4+CD25− coculture/median [3H]thymidine uptake of CD4+CD25− cells).
Hydrodynamic naked DNA systemic injections and gene expression analysis
Male C57BL/6J mice (18–20gr) were injected via tail vein up to 5 s with 12 μg plasmid of pCDNA3 empty vector or pCDNA3-HIF-1αP564A in a volume of 1.6 mL of normal saline, as previously described 14. Animals were sacrificed at different time points (24, 48, 72 h; n=4 per group at each point) and spleens were harvested using standard surgical protocol. Splenocytes were then isolated and gene expression was assessed by RT-PCR. HIF-1α activity in splenocytes was evaluated by ELISA, as well as CD4, CD25 and Foxp3 expression was assessed by flow cytometry as described in section “FACS analysis”.
Statistical analysis
All comparisons were performed using a one-way ANOVA test. p<0.05 was accepted as statistically significant.
Acknowledgements
This work was performed as a part of the thesis of Jeremy Ben-Shoshan. The work was supported by a grant from the Israel Science Foundation (J.G.; 832/06). We thank Dr. Ivo Spiegel (Molecular Cell Biology Department, Weizmann Institute of Science) for his assistance with retroviruses. We are grateful to Dr. Michal Safran (Pediatric Hematology Department, Sheba Medical Center) for providing the HIF-1α mutant.
Conflict of interest: The authors declare no financial or commercial conflict of interest.




