Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies†
Abbreviation
TAAtumour-associated antigens
Abstract
Immunostimulatory mAb as vaccine adjuvants for the treatment of cancer hold considerable potential for boosting weak responses when used against immunogenic tumours, or in combination with various other vaccines. We now show that when administered with OVA, the combination of anti-4-1BB mAb with anti-CD40, anti-OX40 or anti-CD25 resulted in a fourfold enhancement in the antigen-specific T-cell response compared with anti-4-1BB mAb alone, with a similar enhancement in memory responses following rechallenge with OVA. Although the number of antigen-specific T-cells generated after treatment with each of the combinations was similar, marked functional differences were detected. In particular, anti-4-1BB/anti-CD25 resulted in excellent expansion of specific CD8+ T cells but produced fewer IFN-γ-secreting effector cells than the other combinations. Anti-4-1BB/anti-OX40 proved to be the most potent, inducing the most effective T-cell responses in the RIPmOVA diabetes model with adoptively transferred OVA-specific T cells, and, when given with a peptide vaccine, protecting mice against the poorly immunogenic B16-F10 tumour. Overall the results suggest that although these combinations of mAb look promising in terms of their therapeutic potential, further functional assays are needed to compare their effects.
Introduction
One of the most exciting and potentially important recent developments in cancer therapy has been the identification of immunostimulatory mAb. Unlike conventional anti-cancer mAb that bind directly to the tumour cell targets, these agonistic and antagonistic mAb operate by promoting anti-tumour immunity, either by triggering or blocking co-receptors in the immune system, or by deleting regulatory T cells. The target receptors are key accessory molecules required during the generation of functional effector T cells, including members of the TNF receptor and the immunoglobulin superfamilies. Importantly, in a range of models this strategy has provided anti-tumour responses and long-term protection, without the need to identify individual tumour-associated antigens (TAA).
Three classes of immunostimulatory mAb acting at different levels of the immune system have been identified 1. First, anti-CD40 mAb that mimic the action of CD40L on helper T cells and activate or ‘licence’ APC to generate CTL effectors. CD40 is also expressed by certain tumours, allowing for direct targeting as a secondary mechanism 2, 3. Second, a number of mAb such as agonistic anti-4-1BB, anti-CD27 and anti-OX40, which bind directly to T cells to promote CTL effectors. Third, a group that modulates or blocks immune regulation of CTL responses by binding to inhibitory receptors such as CTLA-4 or PD-1 4 on effector cells, or by deleting CD4+CD25+ natural Treg cells.
To date, two of these targets, CD40 and CTLA-4, have been investigated in patients. In the first anti-CD40 mAb trial 5, 6 23 patients with multiple myeloma and 12 with NHL were treated weekly with a humanised mAb, SGN-40. Two partial responses were reported without serious adverse events, and four myeloma patients showed a reduction in M-protein. More recently, a phase I trial using a fully human IgG2 agonistic mAb in various tumours has shown four partial responses in melanoma patients, but importantly with dose-limiting toxicity at only 0.3 mg/kg due to systemic inflammatory syndromes 7. In the case of CTLA-4, two blocking mAb are in phase I–III trials, following encouraging early testing in melanoma and ovarian carcinoma 8. They are also being used in combination with tumour vaccines (peptides) 9, 10 or various forms of chemotherapy (http://clinicaltrials.gov/). Importantly, tumour responses often develop over an extended period of weeks or months and tend to correlate with autoimmune manifestations and systemic inflammation, seen in at least a third of patients 11, 12.
Of the other immunostimulatory mAb in development, anti-4-1BB is particularly interesting and attractive since it is expressed mainly on activated T cells, has activity in developing anti-tumour immunity, yet paradoxically has been shown to prevent autoimmunity in a number of situations. It also shows inducible expression on Treg, NK and NKT cells, monocytes, mast cells, granulocytes and DC. Mice deficient in 4-1BB or its ligand are less able to cope with viruses such as influenza, and point to 4-1BB playing a critical role late in the development of a T-cell response, most likely in the formation of memory. Early results with anti-4-1BB mAb reinforced this and suggested that triggering 4-1BB on responding CD8 T cells extended their survival and boosted tumour or viral immunity 13. Recently it has been reported that 4-1BB can also deliver a unique antigen-independent growth signal for memory T cells, dependent on a direct effect of 4-1BB on the T cells themselves, implying the expression of 4-1BB on memory T cells 14.
However, recently apparently contradictory evidence has shown that anti-4-1BB mAb can also suppress humoral immunity, airway allergic responses and numerous autoimmune responses 15, thus perhaps triggering 4-1BB skews responses, even when fully established, away from Th2 and towards Th1 16, 17. Such a dichotomy of functions has been suggested to make it ideal for tumour vaccines, particularly for combining with agents that are known to promote autoimmunity as a side-effect of tumour therapy 18.
We now report the use of anti-4-1BB mAb alone and in combination with other immunostimulatory mAb, which might be complementary by operating at different levels within the immune system. Our results underline that one of the important features of such reagents is their ability to extend the survival of effector T cells and prevent antigen-driven AICD. They also point to anti-4-1BB mAb combined with either anti-CD40 or anti-OX40 as being particularly effective, especially when used with an appropriate tumour peptide vaccine.
Results
Immunostimulatory mAb provide synergistic activity for expansion of OT-I cells in vivo
The potency of immunostimulatory mAb was investigated in vivo by transfer of OVA-specific OT-I T cells followed by immunisation with OVA and one or more immunostimulatory mAb. The optimum concentration for each mAb was established in preliminary experiments (not shown). Compared with control IgG, agonistic anti-4-1BB mAb resulted in a 14-fold increase in the percentage of OT-I cells in the circulation (4% versus 0.3% on day 6) (Table 1). Anti-CD40 mAb increased OT-I cells to a similar level. In contrast, three other mAb, anti-CTLA-4, anti-CD25 and anti-OX40, which have all shown therapeutic activity in tumour settings 19-21, boosted OT-I cells only modestly (Table 1), with anti-CTLA-4 mAb being the most active with around 2% OT-I cells at the peak response. It is important to note that the anti-CD25 mAb (PC61) was shown to deplete CD25+ cells when assessed with a second non-blocking anti-CD25 mAb.
| Immunisation regimen 0.5 mg OVA+mAb | Peak% of circulating OT-I cellsb) | Peak fold-increase relative to OVA+control IgGc) | Peak fold-increase relative to OVA+anti-4-1BBc) |
|---|---|---|---|
| control IgG | 0.30±0.07 | – | – |
| anti-4-1BB | 4.18±0.83 | 13.9* | – |
| anti-CD40 | 5.08±0.88 | 16.9* | – |
| anti-CD25 | 0.55±0.05 | 1.8*** | – |
| anti-OX40 | 0.59±0.21 | 1.9 (NS) | – |
| anti-CTLA-4 | 1.99±1.1 | 6.6 (NS) | – |
| anti-4-1BB/anti-CD40 | 17.27±3.44 | 57.6* | 4.1** |
| anti-4-1BB/anti-CD25 | 18.41±1.16 | 61.4* | 4.4* |
| anti-4-1BB/anti-OX40 | 17.71±3.22 | 59.0* | 4.2** |
| anti-4-1BB/anti-CTLA-4 | 9.90±2.98 | 33.0* | 2.4 (NS) |
- a) a) Experimental set-up was as described in Fig. 1.
- b) b) OT-I cells, mean% of total lymphocytes in peripheral blood at the peak of response with the SE indicated. n=5 mice per group.
- c) Ratio of maximum percentage of OT-I cells after treatment with immunostimulatory mAb relative control IgG, or anti-4-1BB mAb, p values calculated using the unpaired t-test. *p<0.002; **p<0.01; ***p<0.05; NS-not significant.
Next we tried to augment the activity of the anti-4-1BB mAb by combining it with each of the other immunostimulatory mAb. Figure 1 shows that anti-CD25, anti-CD40 and anti-OX40 given with anti-4-1BB mAb boosted the OT-I response to a similar extent, by about four-fold compared with anti-4-1BB mAb alone (Table 1). However, the advantage of including anti-CTLA-4 was relatively small and it was dropped from further investigation.
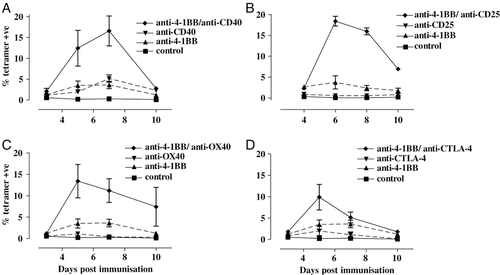
Primary in vivo expansion of OT-I cells in response to OVA and immunostimulatory mAb. Mice received 5×105 OT-I cells i.v. (5 mice/treatment), followed by i.p. injection of 0.5 mg OVA and either anti-4-1BB alone or in combination with (A) anti-CD40, (B) anti-CD25, (C) anti-OX40 or (D) anti-CTLA-4 or IgG (control) on day 0. Blood samples were taken to measure circulating SIINFEKL tetramer + CD8 cells over the course of the response, and levels are expressed as a % (mean±SEM) of total lymphocytes. Data are from two separate experiments. Data are summarised in Table 1, which also gives p values for comparisons.
The expansion of OT-I cells was followed as expected by contraction to leave a small population of cells that persisted for at least 4 wk. Boosting these mice with OVA 28 days after the primary immunisation resulted in the expected secondary expansion of OT-I cells, which was generally proportional to the primary response (Table 2). Thus, anti-4-1BB/anti-OX40 and anti-4-1BB/anti-CD25 had the most impressive effect with secondary responses almost ten times those seen with anti-4-1BB or any of the other mAb alone. Although the potency of the anti-CD40/anti-4-1BB mAb combination was less pronounced, the secondary response was still four times that following a primary immunisation with OVA and anti-4-1BB mAb alone. These results underline the benefit of certain combinations of immunostimulatory mAb on both primary and secondary responses.
| Primary immunisation regimen 0.5 mg OVA+mAb | % OT-I on day 28a) | Peak % OT-I after secondary challenge | Peak fold increase relative to control mAbb) | Peak fold increase relative to 0VA+anti-4-1BBb) |
|---|---|---|---|---|
| control mAb | 0.08±0.03 | 0.12±0.03 | – | |
| anti-4-1BB | 0.25±0.03 | 0.52±0.12 | 4.3* | – |
| anti-CD25 | 0.21±005 | 0.60±0.1 | 5.0* | 1.1 (NS) |
| anti- OX40 | 0.45±0.15 | 0.27±0.1 | 2.2 (NS) | 0.52 (NS) |
| anti-CD40 | 0.75±0.01 | 0.75±0.01 | 6.2* | 1.4 (NS) |
| anti-4-1BB/anti-OX40 | 0.52±0.2 | 5.00±1.6 | 41.7* | 9.8* |
| anti-4-1BB/anti-CD25 | 0.77±0.008 | 4.64±0.72 | 38.6* | 9.0* |
| anti-4-1BB/anti-CD40 | 0.67±0.02 | 2.08±0.15 | 17.3* | 4.0* |
- a) a) OT-I cells, mean percentage of total lymphocytes in peripheral blood on day 28 after the primary immunisation and at the peak of the secondary response are shown.
- b) b) Ratios of the peak % of OT-I cells after treatment with immunostimulatory mAb relative to control mAb and anti-4-1BB mAb alone, p values calculated using the unpaired t-test. *p<0.01; NS-not significant. Combined results from two separate experiments using at least 3 mice per group.
Combinations of immunostimulatory mAb enhance the survival of antigen-activated OT-I cells
We next investigated whether immunostimulatory mAb influenced T-cell acquisition by controlling their rate of proliferation or by prolonging their survival. First, OT-I proliferation as assessed by CFSE dilution was determined in lymph nodes and spleen on days 2 and 3 after immunisation. The results showed that, regardless of the mAb treatment, OT-I cells in the lymph nodes (Fig. 2) and spleen (data not shown) divided at a similar rate, with the majority of the OT-I cells having undergone at least six rounds of division by day 3. Similar results were obtained on day 2 (not shown).
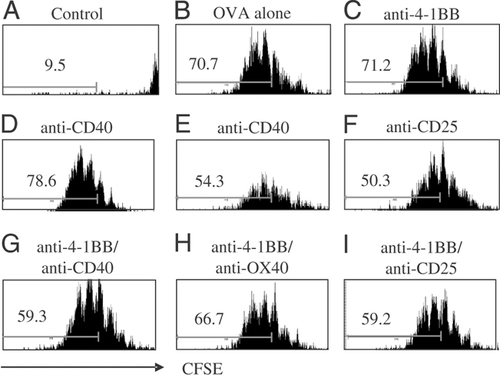
Early in vivo proliferation of OT-I cells in response to OVA and mAb. Mice received 1×106 CFSE labelled OT-I cells i.v., followed by of 0.5 mg OVA and mAb i.p. on day 0 as described in Fig. 1. Mesenteric lymph nodes and spleens were removed on days 2 and 3 and the level of CFSE labelling in OT-I cells analysed by flow cytometry. The histograms show the CFSE fluorescence (FL1) of gated Kb OVA257-264 tetramer+ CD8 cells in the lymph nodes on day 3, and the % of OT-I cells that have undergone more than six rounds of cell division are indicated. The histograms are representative plots from one of two experiments, each with 2 mice per group.
We also investigated proliferation later in the response by assessing the incorporation of BrdU into dividing OT-I cells 5–7 days after immunisation. While there were the expected differences in the total numbers of OT-I T cells following treatment with the immunostimulatory mAb (see Fig. 1), the actual proportion of dividing cells remained relatively constant (Supporting Information Figure 1). Thus, although with OVA alone very few cells survive to day 7, those surviving continue to proliferate at a similar rate to those in mice that have received potent combinations of immunomodulating mAb, such as anti-4-1BB/anti-OX40. These results suggest that the increased accumulation of OT-I cells after treatment with immunostimulatory mAb is not the result of a direct effect on cell proliferation.
If the mAb do not act by increasing the proliferation of the Ag-activated T cells, then, in order for more OT-I cells to accumulate, they must prolong their survival. Annexin V was used to determine the number of apoptotic OT-I cells in the spleen after treatment with the various regimes. Representative histograms are shown in Fig. 3A–D, and the results are summarised in Fig. 3E. By day 6 after OVA alone, more than 75% of OT-I cells are annexin V+ (Fig. 3A). Importantly, this level was reduced to approximately 30% with anti-4-1BB and anti-CD40 mAb to 50% with anti-OX40 mAb and to less than 15% with anti-4-1BB/anti-CD40 anti-4-1BB/anti-OX40 and anti-4-1BB/anti-CD25 mAb combinations. Similar results were obtained on days 4, 5 and 8 (data not shown). These results indicate that the predominant effect of immunostimulatory mAb, either alone or in combination, is to promote the survival of antigen-activated OT-I cells.
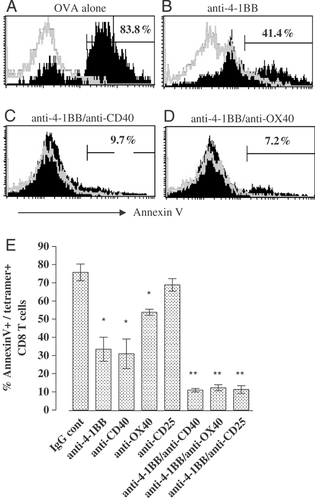
Survival of OT-I cells after vaccination with OVA and mAb. Mice received OT-I cells, mAb and OVA on day 0 as in Fig. 1. On day 6, spleens were harvested and labelled with FITC-anti-Annexin V, APC-anti-CD8α and PE-H-2Kb OVA257-264 tetramer and analysed by flow cytometry. (A)–(D) are representative histograms of annexin V staining of Kb OVA257-264 tetramer positive (solid) and negative (open) CD8+ T cells. Numbers show the % of annexin V positive/Kb OVA257-264 tetramer+/CD8+ cells; (E) summarises the results obtained for each mAb, alone and in combination. Bars show mean (±SEM) values obtained from three experiments with a total of five mice per group. p Values calculated using unpaired t-test: anti-4-1BB, anti-CD40 and anti-OX40 compared with IgG control, all p≤0.002 (*); anti-4-1BB/anti-CD40, anti-4-1BB/anti-OX40 and anti-4-1BB/anti-CD25 compared with anti-4-1BB alone, all p≤0.015 (**).
Immunostimulatory mAb generate OT-I effector cells
Clonal expansion of antigen-specific T cells without acquisition of effector function has been reported in a number of models 22. To ascertain whether the OT-I cells generated with the mAb regimes were functional, splenocytes taken seven days after immunisation were assessed for IFN-γ production after in vitro stimulation with SIINFEKL peptide (Fig. 4). OVA alone resulted in the generation of very few (∼0.3%) IFN-γ-secreting CD8 T cells (Fig. 4A). This increased approximately fivefold in mice receiving anti-4-1BB, anti-CD40 and to a slightly lesser extent anti-OX40 mAb. Anti-CD25 mAb did not promote IFN-γ production. With anti-4-1BB/anti-CD40 and anti-4-1BB/anti-OX40 mAb, IFN-γ-secreting cells increased markedly, with, respectively, 10–15 times more positive cells than with OVA alone (Fig. 4A). IFN-γ production was specific, only occurring after re-stimulation in vitro with SIINFEKL peptide. Interestingly, anti-4-1BB/anti-CD25 mAb, although being one of the most potent combinations for driving OT-I accumulation (Fig. 1), did not increase the number of IFN-γ-secreting cells over that achieved with anti-CD40 alone. The results are shown in Fig. 4B.
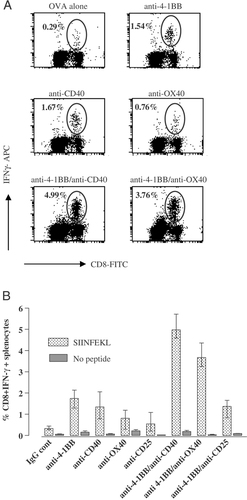
In vitro IFN-γ secretion by OT-I cells after immunisation with OVA and mAb. Mice received OT-I cells, mAb and OVA on day 0 as described in Fig. 1. Spleens were harvested on day 7 and splenocytes stained for intracellular IFN-γ after re-stimulation in vitro for 5 h with 0.1 μM SIINFEKL peptide. (A) Representative flow cytometry profiles with gated regions indicating CD8+ IFN-γ+ cells as a % of total lymphocytes. (B) Summary of data showing percentage CD8+ IFN-γ+ splenocytes with or without SIINFEKL peptide restimulation. Bars show mean and individual values obtained from two mice.
We next investigated whether the immunostimulatory mAb regimes would also stimulate the generation of functional effector cells during an endogenous anti-OVA T-cell response using the more sensitive ELISPOT assay that detects as few as 1 in 50 000 antigen-specific T cells. This is a more rigorous test of in vivo immunity and avoids OT-I cells that are acutely sensitive to TCR triggering. Spleens were taken at the peak of the response (approximately day 7 after immunisation) for assessment of IFN-γ-secreting SIINFEKL-specific T cells by ELISPOT. The results in Fig. 5 show a barely detectable (<10 spots/4×105 splenocytes) SIINFEKL-specific response after immunisation with OVA alone. However, a clear response was detectable with anti-4-1BB mAb in lymph nodes and spleen (∼60 spots/4×105 splenocytes). This was further increased by four- to fivefold with anti-4-1BB/anti-CD40 and anti-4-1BB/anti-OX40 mAb combinations.
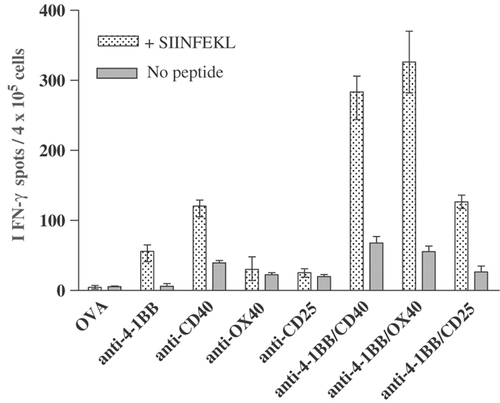
Endogenous SIINFEKL-specific T-cell responses to immunisation with OVA and mAb. Mice received OT-I cells, mAb and OVA on day 0 as in Fig. 1. Splenocytes were harvested on day 7 and assessed for IFN-γ secretion by ELISPOT. For each mouse, triplicate wells, each containing 4×105 splenocytes, were incubated for 24 h with and without 0.1 μM SIINFEKL peptide. The histogram shows the mean and range of the number of IFN-γ spots per 4×105 splenocytes. Data are from one of two experiments, each with 2 mice per group.
Immunostimulatory mAb can promote OT-I effectors to promote diabetes in RIPmOVA mice
An important aspect of generating effective tumour immunity is overcoming tolerance to tumour antigens, which may be very similar to self-antigens and expressed at low levels. The ability of different combinations of mAb to overcome tolerance was examined using RIPmOVA transgenic mice. These mice express membrane-bound OVA in the pancreatic islets of the pancreas, the kidney and the thymus 23, and the thymic expression of OVA has been reported to result in the development of complete central tolerance to OVA antigens, with deletion of OVA-reactive T cells 24. The adoptive transfer of OT-I cells into RIPmOVA mice is a useful model in which to study functional OVA-specific T-cell responses, the induction of peripheral tolerance and ways in which this tolerance may be overcome. After transfer, OT-I cells home into the pancreatic lymph nodes where cross-presentation of OVA by DC occurs, and they undergo limited proliferation and are then rapidly depleted 25. Destruction of OVA-expressing pancreatic islet cells (and hence diabetes) only occurs if a large number (>5×106) of OT-I cells are transferred 26 or if the induction of tolerance can be overcome by transferring the cells with an immune adjuvant, such as poly(I:C) or LPS 27. Thus, in this model the development of diabetes acts as a readout for the overcoming of tolerance. We examined the ability of different combinations of mAb to prevent the development of tolerance and thus promote the development of diabetes in these mice.
The minimum number of OT-I cells that needed to be transferred into RIPmOVA mice to induce diabetes with each mAb therapy was established (Table 3). Anti-4-1BB mAb reduced the number of OT-I cells needed to produce diabetes by at least tenfold, to less than 5×105 cells per mouse (Table 3); anti-CD40 alone was somewhat less effective, requiring between 5×105 and 1×106; anti-OX40 and anti-CD25 alone were ineffective. However, with anti-4-1BB/anti-CD40 and anti-4-1BB/anti-OX40 mAb, this was reduced even further (Table 3 and Fig. 6), the latter being most effective, with some mice becoming diabetic after receiving just 2.5×104 OT-I cells, almost 20 times fewer than with anti-4-1BB mAb and 200 times fewer than without mAb.
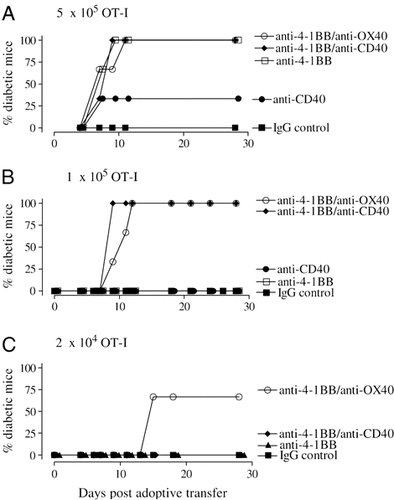
Induction of diabetes in RIPmOVA using combinations of mAb. (A) 5×105, (B) 1×105 and (C) 2×104 OT-I T cells were transferred into RIPmOVA on day 0 along with mAb as in Fig. 1. The number of OT-I cells transferred to each mouse were as shown in (A)–(C). Mice were monitored daily for the development of diabetes. Graphs show cumulative incidence of diabetes in each group, 3 mice per group. The results are summarised in Table 3.
| Number of OT-I cells | Control | anti-41BB | anti-CD40 | anti-OX40 | anti-CD25 | anti-4-1BB/antiCD40 | anti-4-1BB/anti-OX40 | anti-4-1BB/anti-CD25 |
|---|---|---|---|---|---|---|---|---|
| 5×106 | 2/3 | |||||||
| 1×106 | 1/6 | 3/3 | 3/3 | 0/3 | 0/3 | 3/3 | 3/3 | 2/3 |
| 5×105 | 0/3 | 3/3 | 1/3 | 0/3 | 0/3 | 3/3 | 3/3 | 2/3 |
| 2.5×105 | 0/3 | 1/3 | 0/3 | 2/3 | 3/3 | 1/3 | ||
| 1×105 | 0/3 | 0/3 | 0/3 | 3/3 | 3/3 | 0/3 | ||
| 5×104 | 0/3 | 0/3 | 1/3 (3/3)b) | 3/3 | ||||
| 2.0×104 | 0/3 | 0/3 | 0/3 | 2/3 |
- a) a) Table shows incidence of diabetes in mice receiving different immunostimulatory mAb. OT-I T cells were adoptively transferred into RIPmOVA Tg mice at the numbers indicated, together with a single or a combination of mAb: anti-4-1BB, 0.5 mg; anti-CD40, 0.5 mg; anti-OX40, 0.2 mg; and anti-CD25, 0.5 mg. Mice were monitored on alternate days for the development of diabetes by urine dip-sticking for glucose.
- b) b) Two mice with glucose in urine for <48 h.
With each of these combinations, the onset of diabetes was delayed with fewer OT-I cells (Fig. 6), presumably reflecting the time needed to acquire sufficient effector cells to mediate disease. Thus, treatment with anti-4-1BB/anti-OX40 with transfer of just 2.5×104 OT-I cells did not result in diabetes until day 14, 9 days later than with 5×105 cells. The enhanced OT-I response with this treatment may be due to an increased initial expansion of the effector cells or because deletional tolerance is being prevented. However, the former seems unlikely in view of the constant level of proliferation regardless of treatment (Fig. 3).
Interestingly, the anti-4-1BB/anti-CD25 mAb combination was less effective at provoking diabetes than anti-4-1BB mAb alone. This is consistent with our previous findings demonstrating that although this combination is highly effective at enhancing the accumulation of OT-I cells (Fig. 1), the number of IFN-γ secreting cells was not significantly increased (Fig. 4).
Despite extensive investigation, we could find little evidence of an endogenous OVA-specific T-cell response in RIPmOVA mice following immunisation with OVA and mAb, either by ELISPOT or SIINFEKL tetramer staining (not shown), even after 6 days in vitro culture with SIINFEKL to allow expansion of antigen-specific T cells. Importantly, with splenocytes from wild-type C57/BL/6 mice under the same conditions, 20–30% of the CD8+ lymphocytes were SIINFEKL tetramers +ve at this time.
The anti-4-1BB/anti-OX40 mAb combination therefore appeared to be the most potent for generating effective anti-OVA responses in RIPmOVA model, but showed similar efficacy to the other combinations in wild-type mice immunised with 0.5 mg OVA. Since RIPmOVA mice express only very low levels of OVA (∼2.2 ng/μg pancreatic protein) 23, we reasoned that this difference may be related to the level of antigen priming the response, with the T-cell response being more dependent on co-stimulation when the amount of antigen is very limited. The more potent effects of anti-4-1BB/anti-OX40 may therefore only be exposed by the very low levels of antigen in the RIPmOVA model. To test this hypothesis, the OT-I transfer experiments in wild-type mice were repeated with lower (50 μg/mouse) doses of OVA. It can be seen in Fig. 7 that at this dose the anti-4-1BB/anti-OX40 combination was significantly (p<0.0001) more effective at enhancing OT-I expansion than the others, generating a peak almost 12-fold greater than anti-4-1BB mAb alone. In contrast, the other combinations achieved responses only three- to fourfold greater than anti-4-1BB alone. It therefore seems that when the antigen dose is limiting, as is often the case with TAA, the anti-4-1BB/anti-OX40 mAb combination provides the most effective immunostimulatory signals.
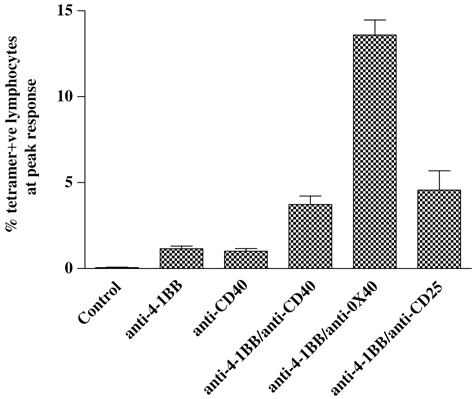
Primary in vivo expansion of OT-I cells in response to a low dose of OVA and immunostimulatory mAb. The procedure was as described in Fig. 1 except mice (3/group) received 50 μg OVA instead of 500 μg. Levels of tetramer +ve CD8 cells are expressed as a % (mean±SEM) of total lymphocytes.
Combinations of immunostimulatory mAb can immunise against melanoma
The ability of a regime to enhance CD8 T-cell responses does not always translate into improved anti-tumour immunity. Therefore, having established that anti-4-1BB and anti-OX40 appeared to be the most potent of the combinations screened, this was evaluated for its ability to overcome tolerance and generate protective immunity in the poorly immunogenic B16-F10 melanoma model. Although there are limitations to the usefulness of syngeneic murine tumour models in predicting clinical efficacy of immunotherapies, B16-F10 is considered a robust and challenging model with features representing many of the obstacles to achieving tumour immunity in patients. The tumour antigens expressed by this tumour line closely resemble those expressed by human melanoma cells. In addition, similar mechanisms of peripheral tolerance, including anergy, ignorance and immunoregulation, have been described in both patients with melanoma and mice carrying B16-F10 28. Treatment with anti-4-1BB mAb alone is not therapeutic in animals with established systemic B16-F10. However, anti-4-1BB mAb in conjunction with TRP-2 peptide vaccination has been reported to prolong survival and result in a small number of long-term survivors 29. Mice received 1×105 B16-F10 cells i.v. followed three days later by s.c. TRP-2 peptide in incomplete Freund's adjuvant together with either anti-4-1BB mAb, anti-4-1BB/anti-OX40 or an irrelevant mAb. Control mice received an irrelevant peptide. A second dose of mAb was given three days later. It can be seen in Fig. 8 that anti-4-1BB/anti-OX40 mAb, given in conjunction with TRP-2, provided effective therapy and significantly prolonged survival compared with anti-4-1BB mAb alone, with approximately 40% of the mice remaining without detectable tumour beyond 150 days. Treatment without TRP-2 peptide was not protective. Interestingly, the majority of mice immunised with anti-4-1BB/anti-OX40 mAb and TRP-2 peptide, but not single mAb, developed loss of fur pigmentation at the vaccination site, which persisted for the duration of the experiment. Histochemistry revealed no other signs of autoimmunity. Therefore, this combination of mAb, together with peptide, is able to overcome ignorance about the TRP-2-petide and generate effective anti-tumour immunity.
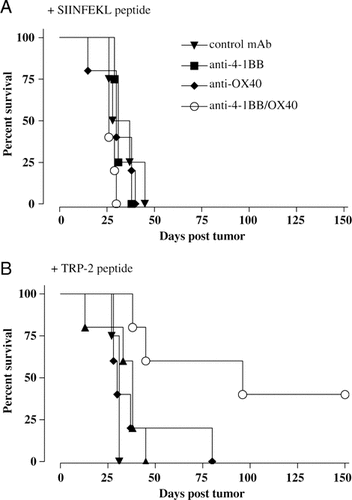
Treatment of B16-F10 with combinations of mAb and peptide vaccine. Mice received 1×105 B16-F10 tumour cells i.v. on day 0 and then on day 3 i.d. injections of SIINFEKL (A) or TRP-2 (B) peptide (50 μg in incomplete Freund's adjuvant) and mAb either anti-4-1BB (500 μg), anti-OX40 (200 μg) or both, with a second dose of mAb i.p.on day 6. The combination of anti-4-1BB and anti-OX40 mAb significantly prolonged survival compared with either mAb alone (p=0.02). The survival curves show data from one of two experiments with similar results.
Discussion
Anti-4-1BB mAb have proven highly effective in promoting effector T-cell proliferation and survival 16, 30, and delivered impressive anti-tumour effects in a number of murine models 31-33, in many cases clearing widespread and metastatic disease. However, in a number of less immunogenic models therapy fails and it is generally felt that, against poorly immunogenic spontaneous tumors, anti-4-1BB mAb alone is unlikely to generate effective responses 34. This means that the level of tolerance that must be overcome in order to provoke clinically effective anti-tumour immunity is likely to be greater than in most animal models. One way in which this might be achieved is by combining anti-4-1BB mAb with other immunostimulatory mAb. As well as potentially providing extra direct co-stimulation to CD8+ T cells, such an approach may further potentiate responses by enhancing antigen presentation, augmenting CD4+ T-cell help or counteracting immunoregulation. In this paper we have examined how anti-4-1BB mAb can be combined with other mAb to generate the most potent T-cell responses.
We have shown that the expansion of OT-I cells in response to OVA was enhanced almost 60-fold by treating with anti-4-1BB mAb in conjunction with anti-CD40, anti-OX40 or anti-CD25 mAb and that this appeared to be largely attributable to increased survival of the antigen-activated T cells. Although similar numbers of OT-I cells were generated with each of these combinations, anti-4-1BB/anti-OX40 and anti-4-1BB/anti-CD40 mAb resulted in the most functionally active, IFN-γ-secreting cells, both after the adoptive transfer of OT-I cells and when measuring endogenous anti-OVA responses. We have also demonstrated that treatment with anti-4-1BB mAb, either alone or in combination, reduced the number of OT-I cells needed to precipitate diabetes in the RIPmOVA model, with anti-4-1BB/anti-OX40 mAb being the most effective in preventing deletional tolerance and generating productive T-cell responses. With this combination, as few as 2.5×104 OT-I cells were sufficient to provoke diabetes, 20-fold fewer than when using 4-1BB mAb alone and 200-fold fewer than with a control mAb. The magnitude of this difference suggests a potentially useful therapeutic benefit that might be achieved by using the mAb in combination. In RIPmOVA mice, endogenous anti-OVA T-cell responses could not be generated even when using the most potent combination in conjunction with large doses of OVA and incomplete Freund's adjuvant. However, while a therapeutic effect in patients will rely on the stimulation of an endogenous response, the level of deletional tolerance to tumour antigens may be less complete, and they may retain the ability to generate an effective response from a small number of low-affinity endogenous T cells. Alternatively, such mAb therapies may be useful in augmenting existing adoptive transfer therapies.
Interestingly, despite causing impressive accumulation of OT-I cells in all models, treatment with anti-4-1BB together with anti-CD25 mAb consistently appeared to result in fewer functional anti-OVA effector cells. One explanation is that the anti-CD25 mAb deletes CD25-expressing activated OT-I cells, skewing the expanded OT-I cell population towards a less activated phenotype. However, if this was the case it might have been anticipated that the secondary response to antigen would have been diminished, and it is perhaps surprising that in these animals it was robust. Many groups have shown that depleting anti-CD25 mAb, such as PC61, can enhance the generation of functional effector T cells 20, 35-37. We can only surmise that the outcome of anti-CD25 mAb therapy is model dependent and that the timing of administration of anti-CD25 mAb relative to immunisation with antigen is critical. Further studies are needed to explore this issue, but the use of anti-CD25 mAb does raise the age-old concern that any benefit gained by eliminating regulatory T cells could be counteracted by depleting activated effector cells. The combination of anti-OX40 and anti-4-1BB mAb together with TRP-2 peptide immunisation provided useful therapy in the poorly immunogenic B16-F10 melanoma model and, importantly, this was achieved without generating widespread autoimmunity, despite the expression of TRP-2 on normal melanocytes. This suggests that this combination of mAb may be potent enough to overcome tolerance to tumour antigens that are similar, or identical, to self-antigens, without necessarily incurring unacceptable toxicity. Using anti-OX40 and anti-4-1BB mAb in combination with peptide immunisation may be useful in overcoming immunological ignorance when the levels of antigen expressed by tumour cells are low.
Recently, Zhang et al. 38 have reported studies in lymphocytic choriomeningitis virus-infected mice, which show that, depending on the timing of treatment, anti-4-1BB mAb can either cause activation-induced cell death or enhance anti-viral immunity. When given early post-infection, anti-4-1BB was immunosuppressive, with depletion of virus-specific CD8+ T cells. In contrast, at later time points, the CD8 response was augmented. This contrasts with the results in our OVA models, and in our immunotherapy, in which anti-4-1BB was given at the same time as antigen exposure. However, these models may be seen to represent the extremes in terms of endogenous CD8 responses, and whereas the lymphocytic choriomeningitis virus model involves the development of a strong endogenous response that clears viral infection and generates long-term immunity, in the OVA model, as with many TAA, a very weak endogenous response is generated and in the absence of stimulatory mAb, tolerance develops. It is perhaps not surprising that the effects of anti-4-1BB are different in the two models. However, this disparity does underline the importance of gaining a clear understanding of the effects triggered by anti-4-1BB, and other immunostimulatory mAb, in order to achieve their most successful application in immunotherapy.
Several mechanisms may contribute to the synergy between 4-1BB and OX40 and it is likely that their relative importance varies in different models. The wide cellular distribution of these two receptors only increases the potential targets and the possibility that agonistic anti-4-1BB and –OX40 mAb act on one or more types of cell. Since most of the current work has used OT-1 cells, it seems likely that CD8 cells are a common target for this combination of reagents. This suggestion is consistent with previously reported synergy between anti-OX40 and anti-4-1BB, which was not abrogated by CD4+ T-cell depletion 39. In addition, in RIPmOVA mice used in this study, central tolerance to CD4+ T-cell epitopes is reported to be complete 40, suggesting that the CD8+ T-cell responses we have seen are not dependent on CD4+ T-cell help. However, such observations do not exclude other mechanisms, including the involvement of other cell types, such as DC or Treg (below).
Although 4-1BB is most strongly associated with CD8+ T-cell co-stimulation and OX40 most closely with CD4+ T-cell function, there is considerable overlap in their reported expression, function and signalling mechanisms. Importantly, both receptors are expressed on CD8+ T cells following TCR signalling and appear to have similar roles in activation 31 with agonistic mAb directly enhancing CD8+ T-cell responses, largely through improved survival of antigen-activated T cells 30, 41. Both 4-1BB and OX40 interact with common TRAF proteins, activate NFκB pathways and induce expression of anti-apoptotic Bcl-2 family molecules 42-44. Such overlap might suggest some redundancy in function, with consequently less potential for synergy, although deficiencies in either 4-1BB or OX40 on CD8+ T cells result in impaired T-cell responses 15, indicating that signalling through both is necessary for optimal function. However, there are differences between the in vivo expression patterns of the two molecules, with expression of OX40 on CD8+ T cells occurring slightly later than that of 4-1BB, and being more transient 31, suggesting that their signalling might be important at different time points during the T-cell response. In addition, studies directly comparing the function of 4-1BB- and OX40-deficient CD8+ T cells indicate distinct differences between the roles of the two molecules 15. In particular, OX40 appears to be important in the acquisition of effector function, with activated OX40-deficient CD8+ T cells demonstrating reduced expression of granzyme B, TNF and IFN-γ. Dual co-stimulation with anti-OX40 and anti-4-1BB could therefore enhance effector function as well as T-cell survival. There is also evidence that the signalling mechanisms for the two proteins are distinct. Although both interact predominantly with TRAF 2, the cytoplasmic tail of 4-1BB has two potential binding sites whereas OX40 only has one 45. In addition, 4-1BB is reported to interact with TRAF 1 whereas OX40 also binds to TRAF 3 45. These differences in binding between 4-1BB and OX40 and the TRAF proteins may result in differing downstream signalling events 45. It is therefore possible that signalling through 4-1BB and OX40 molecules on the same CD8+ T cell may have different effects, which could be additive. These effects could become synergistic if ligation of OX40 on the T cell somehow enhanced 4-1BB function. This could potentially be achieved by an increased expression of 4-1BB, a redistribution of the 4-1BB molecules into a more favourable distribution for binding ligand (e.g. by clustering) or a change in the expression or orientation of the TRAF molecules.
An alternative to a direct effect on CD8+ T cells is that the anti-OX40 mAb is exerting its effects by enhancing CD4+ T cell help. Although, given the role of OX40 in CD4+ T-cell function, this would seem a likely possibility, previously reported synergy between anti-OX40 and anti-4-1BB has not been abrogated by CD4+ T-cell depletion 39. In addition, in RIPmOVA mice, central tolerance to CD4+ T-cell epitopes is reported to be complete 40, suggesting that the CD8+ T-cell responses seen in this model were not dependent on CD4+ T-cell help.
Augmenting DC function is another potential mechanism by which anti-4-1BB/anti-OX40 may enhance CD8 T-cell function. Both 4-1BB and OX40 are expressed by DC 46, 47 and ligation of 4-1BB on DC has been shown to enhance cytokine secretion, promote B7-1 and B7-2 expression and enhance their ability to stimulate T-cell proliferation in vitro 46. We are currently assessing whether the anti-4-1BB + anti-OX40 combination endows DC with the ability to activate CD8 cells more efficiently.
Finally, OX40 expression is also reported on Treg cells, and agonistic anti-OX40 mAb have been reported to abrogate Treg-mediated immunosuppression 48, 49. Such an inhibition of Treg cells may be particularly important in the context of anti-4-1BB mAb therapy, given that the latter has been reported to enhance Treg function 48, 50. Counteracting anti-4-1BB-mediated Treg immunosuppression may expose more of the co-stimulatory effects of anti-4-1BB mAb. The contribution that OX40 signalling on Treg cells makes to the synergistic effects of anti-OX40 mAb in the models used here is difficult to assess, and a detailed analysis of Treg cell numbers in these models would be interesting although anti-OX40 mAb-mediated inhibition of Treg function is reported not to be due to Treg depletion 49; hence, Treg numbers may be unchanged.
Regardless of the mechanism, the combination of anti-OX40 and anti-4-1BB mAb appears to be the most promising to take forward to clinical trial as we have demonstrated that it converts a very weak, or tolerant, T-cell response into a potent therapeutic response. The current work suggests that ideally such treatment would be performed in the setting of peptide immunisation or perhaps adoptive transfer of effector cells. Clearly, such combinations will be logistically difficult to organise, particularly the selection of appropriate mAb, and intensifying the costimulation with combination treatments would increase the likelihood of serious autoimmunity, especially where the peptide vaccine introduced a strong autoantigen. However, our current results suggest that the rewards in terms of tumour immunity are likely to justify the effort required.
Materials and methods
Animals and cells
C57BL/6 and OT-I mice (originally supplied by Charles River Laboratories) were bred and maintained in local facilities. RIPmOVA mice were originally generated by Professor W. Heath (Friedrich–Wilhelms-Universität, Bonn) 25 and provided for this study by Professor Chris Kurts (Friedrich–Wilhelm's University, Bonn) and were bred heterozygously and screened by PCR for OVA expression. The B16-F10 melanoma line was obtained from the ATCC. Animal experiments were conducted under licence according to the UK Home Office licence welfare guidelines and approved by the University of Southampton Ethical Committee.
Antibodies
LOB12.3 (anti-4-1BB) 31 and Mc106A5 (anti-BCL1 idiotype, irrelevant control) 51, in house; 3/23 (anti-CD40)(originally provided by G. Klaus, NIMR, London) 52; UC10 4F10-11 (anti-CTLA-4) (ATCC), PC 61 5.3 (anti-CD25) (ATCC); OX86 (anti-OX40) (ECACC).
Adoptive transfer OT-I cells
A single cell suspension of lymph node (inguinal, brachial and mesenteric) and spleen cells was prepared from OT-I mice and stained using PE-H2-Kb OVA 257-264 tetramer (Beckman Coulter) and APC- anti-CD8α (BD Biosciences) to determine SIINFEKL-positive OT-1 CD8 T cells (typically 15–20%). A total of 5×105 OT-I cells were transferred by i.v. injection into C57BL/6 recipients, sex-matched with the OT-I donors and 4 h later, T cells were primed in vivo by i.p. administration of OVA (50 or 500 μg) and mAb, either singly or in combination: anti-4-1BB, 0.5 mg; anti-CD25, 0.5 mg; anti-CD40, 1 mg; anti-OX40, 0.2 mg; anti-CTLA-4, 1 mg. Secondary stimulation was by i.p. injection of OVA (0.5 mg).
For CFSE dilution analysis, the splenocyte/lymph node suspension was labelled with CFSE (Molecular Probes, Leiden, The Netherlands) (5 μM) and the labelled cells (1×106 OT-I cells) transferred by i.v. injection into C57BL/6 mice. The mice were immunised with OVA and mAb as above.
For transfer of OT-I cells into RIPmOVA mice, the lymph node/splenic suspension was enriched for OT-I T cells using CD8 immunocolumns (Cedarlane Laboratories Limited, Ontario, Canada), according to the manufacturer's instructions. Enriched OT-I cells (2×104–5×105) were transferred by i.v. injection into RIPmOVA recipients, sex-matched with the donors and 4 h later mice received mAb as described above. Mice were monitored daily for the development of diabetes by urine dip-sticking for glucose.
Flow cytometry
For tracking OVA-specific T cells, blood samples or lymph node/spleen cells were stained with PE- H-2Kb OVA257-264 tetramers and APC- anti-CD8α. Dead and dying cells by were detected using 1 μg/ml FITC–annexin V (BD Biosciences).
Intracellular IFN-γ
Splenocytes were incubated for 6 h at 37°C with 0.1 μg/mL SIINFEKL, media alone, or 50 ng/mL PMA and 1 μM ionomycin, and 5 μg/mL brefeldin A. Cells were then stained with FITC- anti-CD8α with 10 μg/mL anti-FcR mAb (2.4G2) (to block binding through FcR) and 5 μg/mL brefeldin A, fixed with 1% formaldehyde and then permeablised in PBS/BSA/0.5% saponin and intracellular IFN-γ labelled with APC-anti-IFNγ (BD Biosciences).
Measurement of cell proliferation using BrdU
Proliferation status was determined using a FITC-BrdU Flow kit (BD Biosciences). Mice received BrdU (1 mg, i.p.), sacrificed 1 h later, splenocytes labelled with PE- H-2Kb OVA257-264 tetramer and APC- anti-CD8α mAb, and intracellular staining with an anti-BrdU antibody performed according to the manufacturer's instructions
IFN-γ ELISPOT
To determine endogenous OVA-specific T-cell responses, mice were immunised with OVA (5 mg) and mAb. Spleens were harvested on day 7, and splenocytes incubated for 24 h with or without 0.1 μM SIINFEKL. Cells secreting IFN-γ were then detected using a BD™ ELISPOT kit (BD Biosciences) according to the manufacturer's instructions.
Acknowledgements
This work was supported by Tenovus of Cardiff and Cancer Research, UK. We thank Professor Chris Kurts for the RIPOVA mice and members of the Tenovus Laboratory who provided expert technical support and valuable discussion.
Conflict of interest: The authors declare no financial or commercial conflict of interest.




