Enhanced B-cell activation mediated by TLR4 and BCR crosstalk†
Abbreviations
IRAKIL-1R-associated kinase
NIP3-nitro-4-hydroxy-5-iodo-phenylacetate
RLPSrough-form LPS
SLPSsmooth-form LPS
TIRToll/IL-1R
TRAFTNFR-associated factor
TRAMTRIF-related adaptor molecule
TRIFTIR domain-containing adaptor protein inducing IFN-β
Abstract
Despite the important role of B lymphocytes as a bridge between the innate and the adaptive immune system, little is known regarding lipopolysaccharide (LPS) recognition, activation of signalling networks or conceivable cooperation between LPS and the B-cell antigen receptor (BCR). Here, we show that primary B cells can efficiently discriminate between different LPS chemotypes, responding with at least 100-fold higher sensitivity to rough-form LPS compared with smooth-form LPS. Using genetically modified mice, we demonstrate that B lymphocytes recognize all LPS chemotypes via Toll-like receptor 4 (TLR4). In addition, we dissect the signalling pathways that lead to CD69 upregulation upon TLR4 and BCR activation in primary B cells. Our data suggest that TLR4 and BCR induce CD69 transcription via two distinct sets of signalling molecules, exerting quantitative and qualitative differences in B-cell activation. Finally, we show that simultaneous stimulation of TLR4 and BCR additively elevates B-cell activation. In contrast, co-engagement of TLR4 and BCR by antigen-coupled LPS synergistically enhances activation of B cells, pointing out attractive targets for signalling crosstalk in B lymphocytes.
Introduction
Considerable evidence supports an important role of B lymphocytes in immune responses against gram-negative bacteria. Lipopolysaccharide (LPS) is the main structural component of the cell wall of these pathogens and is a primary target for recognition by the immune system 1, 2. Stimulation of B cells by LPS induces B-cell proliferation, antibody secretion, and promotes B cells to function as antigen-presenting cells by enhancing expression of MHC class II and co-stimulatory molecules 3, 4. Thus, B cells are an important bridge between the innate and the adaptive immune system, due to their ability to be activated by pathogen-associated molecules like LPS and to generate antigen-specific antibodies.
Toll-like receptors (TLR) are a family of transmembrane proteins responsible for recognition of pathogen-associated patterns and initiation of the respective responses 5. Genetic experiments have shown that TLR4 is the signal-transducing receptor for LPS 6-8. TLR4 requires interaction with the glycoprotein MD-2 for LPS recognition, as has been demonstrated using gene-disrupted mice 9. TLR4 is a leucine-rich repeat protein with an intracellular signal-transducing Toll/IL-1R (TIR) homology domain 10. In addition to TLR4, another TLR family receptor, RP105 (CD180), plays a role in LPS recognition by B lymphocytes 11-13. Surface expression of RP105 is dependent on co-expression of the MD-2 homolog MD-1 14. RP105 also contains a leucine-rich extracellular domain, but lacks the signal-transducing TIR domain, having instead a short tail with no homology to other known proteins 11. To date, the molecular mechanism by which TLR4/MD-2 and RP105/MD-1 co-operate in LPS responses remains controversial 12, 13, 15.
The current model for LPS recognition and initiation of signalling is guided by the interaction between LPS and the LPS-binding protein. LPS-binding protein delivers LPS to the membrane-bound or the soluble form of the glycoprotein CD14 16. However, CD14 is devoid of a cytoplasmic domain, and recent studies showing that certain LPS chemotypes are able to induce cell responses in the absence of CD14 have questioned the central role of CD14 in TLR4 signalling 17, 18. Nevertheless, binding of LPS to the TLR4/MD-2 complex leads to the recruitment of the cytosolic adaptor proteins MyD88 and Mal, initiating a signalling cascade that culminates in nuclear translocation of the transcription factors NF-κB and AP-1 19, 20.
In detail, recruitment of IL-1R-associated kinase (IRAK)-4 by MyD88 facilitates phosphorylation of IRAK-1, which dissociates from the receptor complex to interact with the TNFR-associated factor 6 (TRAF6). The IRAK-1/TRAF6 complex mediates subsequent signalling steps leading to the activation of the IκB kinase complex, which in turn phosphorylates IκB and targets it for degradation. NF-κB then translocates to the nucleus initiating gene transcription. The IRAK-1/TRAF6 complex can lead to further activation of the Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways and the transcription factor AP-1. Engagement of TLR4 can also induce activation of a MyD88-independent signalling pathway involving the adaptor proteins TIR domain-containing adaptor protein inducing IFN-β (TRIF) and TRIF-related adaptor molecule (TRAM). This signalling pathway results in the activation of the IFN-responsive factor 3, which mediates transcription of IFN-inducible genes and activates a delayed wave of NF-κB transcription 21. This consensus model of the TLR4 signalling network has emerged mainly from studies with macrophages and transfected cell lines. How valid this information is for other cell types, e.g. B lymphocytes, still has to be elucidated.
The term LPS does not describe a monomorphic molecule. Most wild-type (WT) gram-negative bacteria produce a highly heterogeneous mixture of LPS molecules (smooth-form LPS, SLPS) with different degrees of complexity of the polysaccharide component 18. LPS consists of three regions, a lipid A backbone containing up to seven fatty acid chains, a core oligosaccharide and an O-polysaccharide (O-antigen) comprising up to 80 repeating oligosaccharide units (Fig. 1A). Bacteria, either naturally or due to genetic alterations, synthesize LPS lacking the O-antigen and this LPS is known as rough-form LPS (RLPS) 22. In addition, several bacterial mutations are known to affect the length of the core oligosaccharide itself, generating a whole collection of distinct RLPS, named RaLPS to ReLPS (Fig. 1A). The physicochemical properties of LPS, as well as its biological characteristics, change with the length of the sugar part. Comparative studies using different LPS chemotypes showed that RLPS are more efficient activators than SLPS in human granulocytes 23, neutrophils 24 and mouse bone marrow-derived mast cells and splenocytes 18. The type of LPS (smooth or rough) also influenced the efficiency of gut colonization by Salmonella 25 and the resistance of gram-negative bacteria to complement-mediated killing 26.
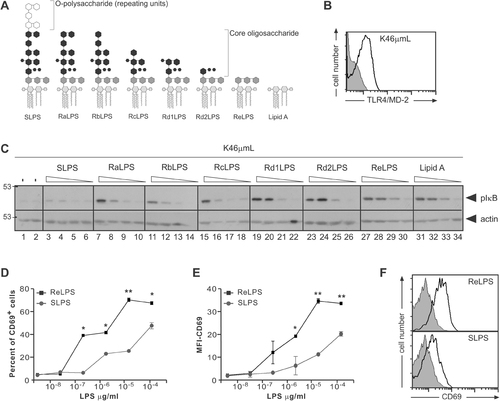
Stimulation potential of different LPS chemotypes. (A) Schematic representation of the different LPS chemotypes used in this study. (B) TLR4/MD-2 expression on the B-cell line K46µmL was analyzed by flow cytometry. The gray-filled histogram shows autofluorescence and the black histogram shows TLR4/MD-2-specific signal. (C) K46µmL cells were stimulated with the indicated LPS chemotypes at four concentrations each: 250, 25, 2.5 and 0.25 µg/mL (from left to right) for 5 min. Cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho-IκB and, as a loading control, with anti-actin antibodies. (D, E) K46µmL B cells were stimulated with the indicated concentrations of SLPS or ReLPS. After 18 h CD69 upregulation was assayed by flow cytometry, and the percent of CD69+ cells (D) or the MFI depicted (E). (F) Representative histograms of CD69 upregulation upon ReLPS and SLPS stimulation are shown. **p<0.01, *p<0.05.
In the present report, we systematically compared the activation of B lymphocytes in response to highly purified LPS chemotypes. We show that B cells can efficiently discriminate between the different LPS chemotypes. Furthermore, dissection of the signalling pathways that lead to CD69 upregulation in B cells upon TLR4 and B-cell antigen receptor (BCR) activation suggests that TLR4 and BCR induce CD69 transcription via two distinct subsets of signalling molecules and point out possible targets for signalling crosstalk in B lymphocytes.
Results
Activation potential of different LPS chemotypes
SLPS, RLPS (Ra to Re) and lipid A were highly purified as described 27, 28 and used to study their capacity to activate TLR4 signalling in murine B lymphocytes. Since the amount of TLR4/MD-2 expression in B cells is hardly detectable 29, 30, and because the amount of TLR4 expression determines the degree of LPS responsiveness 8, we screened different B-cell lines in order to identify those constitutively expressing high amounts of this receptor. The murine mature B-cell line K46µmL 31 was chosen due to its high expression of TLR4/MD-2 (Fig. 1B). In B cells, the NF-κB pathway can be activated in response to LPS, resulting in the phosphorylation of IκBα at conserved serine residues and its subsequent degradation by the proteasome 32.
Stimulation of K46µmL cells with the different LPS chemotypes caused IκBα phosphorylation (Fig. 1C). An inverse correlation between the length of the LPS core oligosaccharide and its activation potential was observed. RdLPS, ReLPS and lipid A were the most potent inducers of IκBα phosphorylation. RLPS with increasing length of the core (Ra to Rc) displayed intermediate activity, and SLPS was inactive (Fig. 1C). This pattern was also observed using the plasmacytoma B-cell line J558Lδδm/mb-1 33 (Supporting Information Fig. 1). Furthermore, the degree of IκBα degradation upon stimulation also correlated with the complexity of the core of LPS, ReLPS and lipid A being the strongest stimuli (data not shown). Analyzing a kinetically more downstream event, ReLPS was also more potent than SLPS when upregulation of the early activation marker CD69 was assessed using the K46µmL B-cell line (Fig. 1D–F). Therefore, the different LPS chemotypes have different activation potencies in B cells.
LPS chemotypes activate B cells via TLR4
Next, we were interested whether primary B-cell responses are also activated by the different LPS chemotypes despite their low expression of TLR4/MD-2. Since it is known that LPS induces CD69 upregulation in splenic B cells 34, we used this read-out of B-cell activation. Stimulation of red blood cell (RBC)-depleted splenocytes with both ReLPS and SLPS induced a dose-dependent increase in CD69 (Fig. 2A and B) and CD86 (Fig. 2C) expression. As observed before, ReLPS had a stronger activation potential than SLPS. A rough estimation shows that 100-fold more SLPS than ReLPS is needed to reach optimal B-cell activation as measured by CD69 upregulation (Fig. 2B). This inverse correlation between the length of the LPS core oligosaccharide and its activation potential was also observed when cell growth, cell proliferation, survival or differentiation in plasma cells were evaluated (Supporting Information Fig. 2). In addition, marginal zone and follicular B cells exhibited the same selectivity to the different LPS chemotypes regarding CD69 upregulation (Supporting Information Fig. 2). Splenic B cells from mice deficient in TLR4 expression did not respond to any LPS chemotype tested (Fig. 2B and C and data not shown), indicating that in B cells all LPS chemotypes are recognized and signal via TLR4.
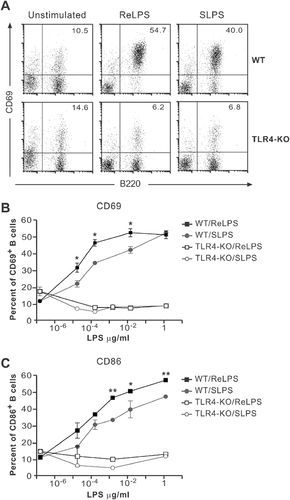
LPS chemotypes induce upregulation of CD69 in splenic B cells via TLR4. (A) Splenocytes from WT and TLR4-deficient mice were stimulated with 2.5×10−2 µg/mL ReLPS, 2.5×10−2 µg/mL SLPS or left untreated. CD69 upregulation was analyzed by flow cytometry after 18 h. Numbers indicate the percentage of B cells (B220+) expressing CD69. (B) Upon stimulation with the indicated concentrations of SLPS or ReLPS, CD69 upregulation was assayed as in (A) and the percent of CD69+ cells depicted. (C) CD86 upregulation was detected using specific antibodies and flow cytometry. Each point shows the mean of triplicate±SD. **p<0.01, *p<0.05.
Combined stimulation of B cells via TLR4 and BCR enhances B-cell activation
We and others have recently shown that co-stimulation of mast cells with antigen/antibody complexes and LPS leads to synergistic cell activation 18, 35. Likewise, stimulation via TLR9 augments BCR-induced B-cell activation by dual engagement of both receptors 36. In addition, a previous publication suggested collaboration between TLR and the BCR in T-dependent responses 37. To directly address the existence of a functional collaboration between TLR4 and the BCR, splenocytes from B1-8 transgenic mice expressing a BCR specific for the hapten 3-nitro-4-hydroxy-5-iodo-phenylacetate (NIP) were stimulated via TLR4 using different forms of LPS, via the BCR with NIP-coupled bovine serum albumin (NIP–BSA), or with a combination of these stimuli. For this purpose, sub-optimal amounts of the individual stimuli were used.
Both ReLPS and SLPS could enhance NIP–BSA-induced B-cell activation as measured by CD69 upregulation (Fig. 3A and B). Owing to the lower potency of SLPS (Figs. 1, 2), 100 times more SLPS than ReLPS had to be used (Fig. 3A and B). The same results were obtained when a highly heterogeneous mixture of LPS chemotypes from WT bacteria (“commercial LPS”) was assayed (see below and data not shown). We included this LPS in our study because it is the preparation most commonly used in the literature. However, it has been shown to contain lipoproteins that can modulate the LPS response, most probably by binding to other members of the TLR family 38. Using this experimental approach, the TLR4 and the BCR are stimulated simultaneously, but most probably co-engagement of the receptors is not taking place.
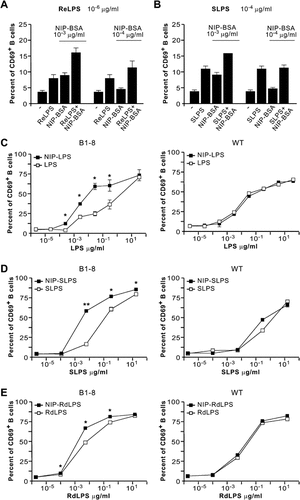
Enhanced B-cell activation by combined stimulation with different LPS chemotypes and antigen. (A, B) Splenocytes from B1-8 mice were treated with the indicated stimuli and CD69 expression was assayed by flow cytometry after 18 h. Each bar represents the mean of triplicate±SD. (C–E) Splenocytes from B1-8 mice and C57BL/6 mice (WT) were stimulated with (C) “commercial LPS” coupled (NIP–LPS) or not to NIP, (D) SLPS or SLPS coupled to NIP (NIP–SLPS), or (E) RdLPS or RdLPS coupled to NIP (NIP–RdLPS). CD69 expression was analyzed by flow cytometry. Each point represents the mean of duplicate±SD. **p<0.01, *p<0.05.
To co-cluster the BCR with TLR4, we used commercially available NIP–LPS where NIP was covalently bound to “commercial LPS”. On average, 0.7 molecules of NIP were coupled per LPS molecule, minimizing BCR clustering. Both primary B cells from B1-8 transgenic mice and the K46µmL B-cell line were more potently stimulated with NIP–LPS than with LPS alone (Fig. 3C, left panel and data not shown). Moreover, stimulation of WT primary B cells, which do not express a NIP-specific BCR, showed no difference in the effect between LPS or NIP–LPS (Fig. 3C, right panel).
To rule out that the observed phenomenon was due to potential impurities contained in the “commercial LPS”, we coupled NIP to the highly purified Rd1LPS and SLPS. Both preparations stimulated B1-8 primary B lymphocytes more potently than their uncoupled counterparts (Fig. 3D and E, left panels). As control, NIP-coupled or uncoupled LPS forms exhibited indistinguishable stimulation of WT lymphocytes (Fig. 3D and E, right panels). In addition, B1-8 primary B lymphocytes proliferated more potently upon stimulation with NIP–SLPS than with SLPS (Supporting Information Fig. 3). These data confirm that BCR and TLR4 are indeed the receptors involved in the augmented stimulation observed by the NIP–LPS preparations.
CD69 upregulation via TLR4 or BCR follows different kinetics
Both TLR4 and BCR engagement lead to CD69 upregulation (Figs. 2, 3). However, whether these receptors activate the same B-cell signalling pathways downstream of the receptor to trigger CD69 upregulation is unknown. As a first approximation, we studied the kinetics of CD69 expression in response to ReLPS or NIP–BSA. ReLPS induced rapid CD69 upregulation in 95% of the B cells. CD69 expression was maintained at high level up to at least 2 days (Fig. 4A–C). Engagement of the BCR also led to rapid upregulation of CD69, but in contrast to ReLPS, CD69 expression was transient and decayed in most B cells after 10 h (Fig. 4A–C). The observed differences were not due to divergences in cell survival (Fig. 4D). We also observed differences in receptor internalization upon antigen versus LPS stimulation (Fig. 4E). Since internalization is a phenomenon controlled via signal transduction, these results suggest the involvement of different signalling intermediates after BCR or TLR4 stimulation.
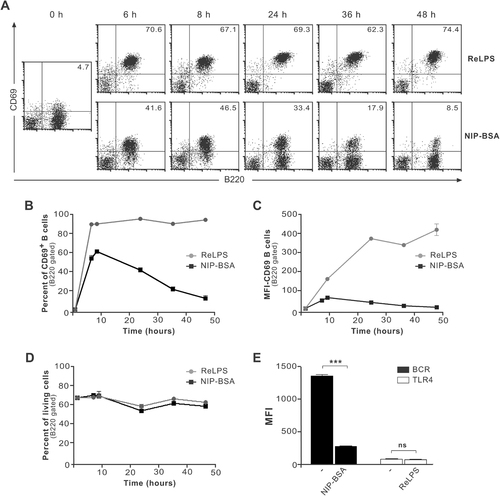
CD69 upregulation induced by LPS or antigen follows different kinetics. (A) Splenocytes from B1-8 mice were stimulated with 10–2 µg/mL ReLPS or 10−2 µg/mL NIP–BSA at 37°C. At the indicated time points, cells were harvested, stained, and analyzed by flow cytometry. Representative dot plots are displayed using a lymphocyte gate. The percentage of CD69+B220+ cells is indicated. (B–D) B cells were gated based on B220 expression and the data presented in (A) were quantitatively analyzed for the percent of CD69+ cells (B), the MFI of CD69 (C) or the percent of living cells upon stimulation (D). (E) Cells were stimulated for 24 h as in (A) and the MFI of the indicated receptors was measured. Each point shows the mean of triplicate±SD; ***p<0.001, ns: non-significant.
TLR4 and BCR induce CD69 upregulation via differential signalling pathways
To address in more detail the signalling pathways involved in TLR4- or BCR-mediated CD69 upregulation, we pre-incubated primary B cells from B1-8 mice with inhibitors of specific signalling proteins, prior to stimulation with either ReLPS or antigen. Inhibition of ERK activation by the MEK inhibitor UO126 abolished BCR-mediated CD69 upregulation as previously described (Fig. 5A, left panel and 39). Surprisingly, we found that ERK inhibition had no effect on TLR4-mediated CD69 upregulation (Fig. 5A, right panel), despite efficient inhibition of LPS-induced ERK phosphorylation (Fig. 5B). CD69 promoter activation is dependent on NF-κB in different experimental systems 40, 41. Since signalling from both TLR4 and BCR leads to induction of NF-κB activity, we blocked NF-κB activation using BAY11-7082, which irreversibly inhibits IκB phosphorylation and subsequent degradation 42. Our results show that induction of CD69 expression by NIP–BSA or ReLPS is highly dependent on NF-κB activity, since inhibition of IκB phosphorylation blocked CD69 upregulation in response to both stimuli (Fig. 5C). Experiments titrating BAY11-7082 indicated, however, that BCR-induced CD69 upregulation was less sensitive to NF-κB blockage than TLR4-induced CD69 upregulation (Supporting Information Fig. 4).
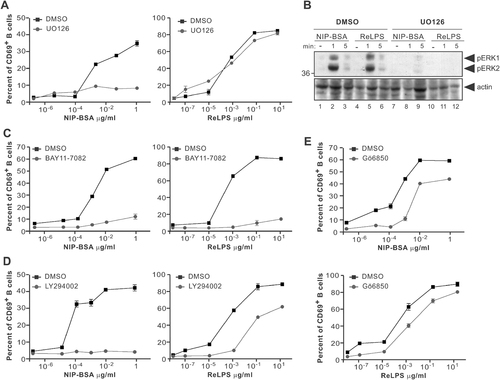
LPS and antigen induce CD69 upregulation via different signalling pathways. (A) Splenocytes from B1–8 mice were pre-treated with DMSO (carrier) or the MEK inhibitor UO126 (10 µM) for 30 min prior to stimulation with NIP–BSA or ReLPS. CD69 upregulation was assayed by flow cytometry after 8 h stimulation. Each point shows the mean of triplicate±SD. (B) Splenocytes from B1-8 mice were pre-treated as in (A) prior to stimulation. Cell lysates were separated by SDS-PAGE and Western blotting performed using anti-phospho-ERK and anti-actin antibodies. (C–E) Splenocytes from B1-8 mice were pre-treated with DMSO or BAY11-7082 (1.25 µM; IκB kinase inhibitor) (C), LY294002 (25 µM; PI3K inhibitor) (D) or Gö6850 (2 µM; PKC inhibitor) (E), stimulated with NIP–BSA or ReLPS, and processed as in (A).
NF-κB activation is also dependent on phosphatidylinositol 3-kinase (PI3K) activity upon both BCR and TLR4 engagement in primary B cells 43. Using LY294002, an inhibitor shown to specifically block PI3K, we observed that BCR-induced CD69 upregulation required PI3K activity (Fig. 5D, left panel). In contrast, PI3K is only partially involved in ReLPS-mediated CD69 expression (Fig. 5D, right panel). Previous reports have suggested that LPS and antigen lead to NF-κB activation via PI3K using two distinct pathways: a protein kinase C (PKC)-dependent pathway for antigen and a PKC-independent one for LPS 43. To study the role of PKC in CD69 expression, we used the inhibitor bisindolylmaleimide-I (Gö6850), which can inhibit both conventional and novel PKC isoforms. As shown in Fig. 5E, PKC have only a limited role in CD69 induction in primary B cells. Together, these data suggest that BCR and TLR4 induce CD69 expression in B cells by distinct signalling pathways.
Differential signalling pathways are employed upon co-ligation
We also used these inhibitors to gain more insight into the signalling pathways that are activated during combined stimulation of B cells via TLR4 and BCR. As shown in Fig. 6A, enhanced CD69 upregulation, which was observed when both stimuli were added, was due to an additive effect of the different pathways. As expected, inhibition of ERK blocked NIP–BSA-induced CD69 upregulation and had no effect on ReLPS-mediated CD69 expression. When NIP–BSA and ReLPS were added simultaneously in the presence of ERK inhibition, the additional effect of NIP–BSA on LPS-induced CD69 upregulation was reduced to the level induced by ReLPS alone. Similarly, combined stimulation with NIP–BSA and ReLPS under conditions of IκB inhibition still evoked NF-κB-independent CD69 expression equivalent to that mediated by NIP–BSA. Here a lower concentration of the IκB inhibitor BAY11-7082 was used, compared with Fig. 5C, to better appreciate the NF-κB-independent CD69 expression mediated by NIP–BSA (Supporting Information Fig. 4). This additive effect was further observed when PI3K (LY294002, Fig. 6A), PKC, Src kinases or Syk were inhibited (data not shown). In addition, a similar summative effect was observed when “commercial LPS” was used in combination with antigen (Fig. 6B).
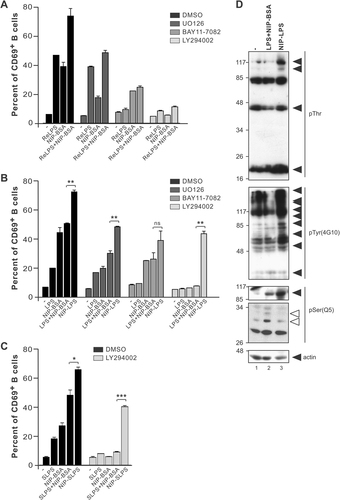
B-cell activation by NIP–LPS is distinct from that elicited by combined stimulation using NIP–BSA and LPS. (A) Splenocytes from B1-8 mice were pre-treated with DMSO or the indicated inhibitors for 30 min. Stimulation with 10−3 µg/mL NIP–BSA, 10−4 µg/mL ReLPS or both was done at 37°C for 8 h. Cells were stained with anti-CD69 antibodies for subsequent analysis by flow cytometry. (B) Cells were pre-incubated with the indicated inhibitors and stimulated with 1.25×10−3 µg/mL LPS or NIP–LPS and 10−3 µg/mL NIP–BSA as described in (A). (C) Cells were pre-incubated with the PI3K inhibitor LY294002 (25 µM) and stimulated with 1.25×10−3 µg/mL SLPS or NIP–SLPS and 10−3 µg/mL NIP–BSA as described in (A). (D) K46µmL cells were left untreated or stimulated with 30 µg/mL of LPS and 10 µg/mL of NIP–BSA or 30 µg/mL of NIP–LPS for 10 min. Cell lysates were separated by SDS-PAGE, and Western blotting performed using the indicated antibodies. Black arrows indicate proteins mainly phosphorylated upon NIP–LPS stimulation. White arrows point to proteins mainly phosphorylated upon NIP–BSA and LPS simultaneous stimulation. ***p<0.001, **p<0.01, *p<0.05, ns: non-significant.
Finally, we asked whether a different signalling response was elicited when TLR4 and BCR were co-engaged by NIP-coupled LPS. NIP–LPS is thought to bring TLR4 and BCR in close proximity, which could allow physical interaction of the receptors and their signalling intermediates or promote receptor relocalization into the same membrane microdomain. Therefore, new signalling pathways could be stimulated. NIP–LPS-induced CD69 expression was only partially blocked by the inhibitors used, even when they efficiently inhibited CD69 expression mediated by the individual stimuli or the combination of them (Fig. 6B). The same results were obtained when highly purified SLPS was used (Fig. 6C), indicating that the synergistic effect observed was not due to the potential impurities contained in the “commercial LPS” preparations. These results indicate that co-engagement of TLR4 and BCR enabled cellular activation under conditions where either of the individual stimuli or a combination of them is unable to elicit stimulation.
Lastly, we stimulated K46µmL cells with either a combination of LPS and NIP–BSA, or by co-clustering the receptors with NIP–LPS. To broadly analyze the signalling intermediates activated under these experimental conditions, we performed immunoblotting with antibodies that recognized phosphorylated tyrosine, threonine or serine residues. We observed striking differences in the pattern of protein phosphorylation (Fig. 6D). NIP–BSA was used at suboptimal conditions, minimizing BCR clustering and BCR-derived signalling. We found that several effector proteins were phosphorylated to a higher extent under co-ligation conditions (black arrows), whereas others were predominantly phosphorylated under simultaneous stimulations (white arrows). This experiment demonstrates that co-ligation of BCR and TLR4 activates signalling cascades qualitatively different from those activated by the stimulation of the individual receptors. Therefore, we describe for the first time evidences of functional crosstalk between TLR4 and BCR under co-engagement conditions.
Discussion
Despite the important role of B cells as a bridge between the innate and the adaptive immune systems, little is known regarding LPS recognition by B cells, respective signalling cascades, or conceivable crosstalk between LPS and antigen receptor. In the present study, we identified mouse B-cell lines expressing high amounts of TLR4/MD-2, as a useful tool to gain insights into TLR signalling in B lymphocytes. We showed that these B-cell lines and primary B lymphocytes can discriminate between different LPS chemotypes, leading to quantitative differences in B-cell activation (with ReLPS being a superior activator of B cells compared with SLPS). In contrast to macrophages, to which CD14 confers the ability to respond to SLPS 17, 18, B lymphocytes lack CD14 expression, and soluble LPS-binding protein cannot enhance B-cell activation by either form of LPS 18. Therefore, one interpretation for the activation by SLPS is that B cells are indeed responding to the RLPS present in all SLPS preparations.
RLPS is a metabolic precursor in the SLPS synthesis pathway. Therefore, LPS synthesized from WT gram-negative bacteria is a highly heterogeneous mixture of SLPS molecules with variable lengths of the O-polysaccharide, together with varying amounts of RLPS 18. The fact that 100 times more SLPS than ReLPS is needed to reach maximal B-cell activation (Fig. 2B) could provide support to this interpretation. However, different RLPS (Ra to Re) have different activation potencies, suggesting that the TLR4/MD-2 complex can discriminate between highly related ligands. Therefore, the TLR4/MD-2 complex itself could have some discriminatory capability that confers specificity to the LPS recognition event. In addition, the physicochemical properties of the LPS molecules differ based on the length of the oligosaccharide chain and this might also contribute to the observed differential stimulation potential.
An emerging theme is whether crosstalk between co-stimulated receptors can determine the outcome of an immune response. Previous reports suggested that sequential engagement of the BCR and intracellularly expressed TLR9 36 and TLR7 44 can augment B-cell activation. Although B lymphocytes can be activated by LPS, secrete LPS-specific antibodies, and undergo class switching upon TLR4 activation, little is known about eventual crosstalk between TLR4 and the BCR. It has been shown that a persistent signal via the BCR can abolish LPS-induced plasma cell differentiation, in which the ERK pathway was the molecular switch 45. In addition, B cells of mice deficient in molecules such as Lyn, Btk, PI3K, SLP65 or PLCγ that are directly implicated in the BCR signalosome exhibit impaired proliferative responses to LPS 46-50.
Here, we stimulated primary B cells from transgenic mice expressing a NIP-specific BCR with antigen only (NIP–BSA), LPS only, NIP–BSA together with LPS, and NIP-coupled LPS (NIP–LPS). Combined stimulation with antigen and LPS led to enhanced B-cell activation, independent of the LPS chemotype used. Furthermore, when primary B cells were stimulated with NIP–LPS, B-cell activation was strongly enhanced. Using B cells of WT mice, we showed that this enhancement was dependent on recognition by the BCR. To dissect the contributions of BCR- and TLR4-mediated signalling pathways to this enhanced activation, we used well-characterized inhibitors against signalling elements involved in the initiation of CD69 transcription (Supporting Information Fig. 5). BCR-mediated CD69 expression is tightly regulated by the activation of several signalling pathways: ERK, NF-κB and PI3K (39 and Fig. 5). In contrast, we showed that TLR4-mediated CD69 upregulation was mostly dependent on NF-κB pathway activation and only partially affected by PI3K or PKC inhibition. Strikingly, it was completely independent of ERK signalling. Although it was previously suggested that IκB phosphorylation and subsequent NF-κB activation were dependent on PI3K activity upon LPS stimulation 43, we observed an NF-κB-dependent, PI3K-independent pathway that can promote considerable CD69 expression.
Next, we applied these inhibitors to the simultaneous stimulation with NIP–BSA and LPS. In the presence of ERK inhibition, CD69 upregulation was equivalent to that obtained only with LPS. Similarly, blockage of NF-κB led to CD69 upregulation to the level reached by only NIP–BSA stimulation under NF-κB-blocking conditions. These data indicate that simultaneous stimulation via BCR and TLR4 leads to an additive effect on B-cell activation, in the absence of any observable crosstalk between the signalling networks activated by each receptor. In contrast, when primary B cells were activated with NIP–LPS, a situation that induces co-clustering of TLR4 and BCR, the resulting synergistic CD69 upregulation was hardly blocked by any of the inhibitors tested. Even under conditions that completely abolished CD69 upregulation by the individual stimuli, or combined stimulation with LPS and NIP–BSA, a strong CD69 expression was detected after NIP–LPS stimulation.
In contrast to simultaneous stimulation, NIP–LPS can bring TLR4 and BCR in close proximity. This might promote physical interaction of the receptors including their associated proteins leading to activation of a distinct set of signalling molecules. We considered PKCε, Btk and the MAPK JNK and p38 as plausible candidates. (1) Mice lacking PKCε display defective responses when stimulated with LPS 51. In addition, PKCε phosphorylates the N terminus of TRAM, contributing to its signalling potency 52. It is thus possible that BCR-mediated PKCε activation 53 could increase TRAM activation and TRIF-dependent transcription by NF-κB and IFN-responsive factor 3. We found that the synergistic activation by NIP–LPS was barely blocked by the PKC inhibitor Gö6850 (Fig. 5E and Supporting Information Fig. 4), which also inhibits novel PKC, such as PKCε. Thus, it is unlikely that PKCε is the molecule behind the described phenomenon.
(2) Btk activity is increased upon BCR cross-linking 54 and its deficiency results in reduced proliferative responses of B cells to LPS 46. Moreover, Btk interaction with MyD88, Mal and IRAK-1 and an important role for Btk in LPS-mediated NF-κB activation have been reported 55. Treatment of primary B cells with the Btk-specific inhibitor LFM-A13, however, leads to a moderate augmentation of LPS-mediated CD69 upregulation (Supporting Information Fig. 4), fitting with the recently described negative regulatory role of Btk in TLR4 signalling 56. Moreover, treatment with LFM-A13 has no effect on the synergistic response induced by NIP–LPS (Supporting Information Fig. 4). Thus, Btk seems not to be involved in the synergy described.
(3) Sequential stimulation of BCR and TLR9 induced enhanced JNK and p38 phosphorylation and therefore augmented AP-1 activity 57. Furthermore, there are two AP-1-binding sites within the CD69 promoter 41. However, BCR-mediated p38 activation is PKC-dependent 57 and treatment with the inhibitor Gö6850 did not significantly inhibit the NIP–LPS-induced synergism in CD69 upregulation (Supporting Information Fig. 4). Since JNK activation upon BCR engagement seems to be Syk-dependent 58, we are currently performing experiments with a novel Syk-specific inhibitor (R406) and specific JNK inhibitors. Preliminary data indicate that this inhibitor can reduce the NIP–LPS-triggered CD69 upregulation by 60%, which is the strongest block so far observed.
Taken together, our results show that antigen and LPS activate primary B cells to upregulate CD69 expression by distinct signalling pathways. In contrast to simultaneous TLR4 and BCR activation, co-engagement of these receptors induces a synergistic B-cell response. This synergy might be the result of the physical proximity of TLR4 and BCR promoted only by receptor co-engagement. As a consequence, new signalling pathways could be activated that account for the observed synergistic activation. These data might have important implications for vaccine design, since recent vaccine strategies have been based on the use of TLR ligands as adjuvants to increase immune responses 37. Our results suggest that relatively small amounts of antigen coupled to LPS could augment B-cell activation and therefore provide efficient immune protection upon vaccination.
Materials and methods
Cell lines and mice
K46µmL 31 and J558Lδδm/mb-1 cells were grown in RPMI 1640 complete medium containing 5% FCS, 50 U/mL penicillin, 50 mg/mL streptomycin, 2 mM L-glutamine and 50 mM 2-mercaptoethanol. RBC-depleted splenic B cells from B1-8 59, TLR4-deficient C57BL/10SnCr and C57BL/6 mice were stimulated in RPMI 1640 containing 1% FCS, 50 U/mL penicillin, 50 mg/mL streptomycin, 2 mM L-glutamine and 50 mM 2-mercaptoethanol.
Reagents
SLPS from Salmonella abortus equi and RLPS from Salmonella minnesota mutants R595 (Re), R3 (Rd1), R7 (Rd2), R5 (Rc), R345 (Rb) and R60 (Ra) were extracted and purified as described 27, 28. Lipid A from S. minnesota R595 was prepared as indicated 60. LPS (“commercial LPS”) was purchased from Sigma. NIP–BSA (15 NIP per BSA molecule) and NIP–LPS (0.7 NIP groups per LPS molecule) were obtained from Biosearch Techologies (Novato, CA). NIP–succinimide ester (NIP–OSu; Biosearch Techologies) was used to couple NIP to highly purified RdLPS and SLPS according to the manufacturer's instructions. UO126 was purchased from Promega (Madison, WI), BAY11-7082, LY294002, and Gö6850 from Calbiochem (Germany).
Antibodies
Antibodies against phosphorylated IκB (S32) and phospho-threonine were purchased from CST (Germany). Phosphorylated ERK1/2 were detected with anti-activated-MAPK (12D4; Nanotools, Germany). Anti-phospho-serine (Q5) antibody was purchased from Quiagen and anti-phospho-tyrosin (4G10) from UBI. Anti-actin (I-9) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Other antibodies used in this study include: anti-B220-FITC, anti-CD23-PE, anti-CD21-allophycocyanin, anti-CD69-biotin, anti-CD86-biotin, anti-CD138-biotin, anti-IgM-FITC (Pharmingen, Canada) and anti-TLR4/MD-2-Alexa 647 (Alexis/MobiTec). Streptavidin-PE was purchased from Molecular Probes.
Stimulation and Western blot analysis
Cells were resuspended in medium without serum, and incubated for 1 h at 37°C prior to stimulation with the indicated stimulus at 37°C. All lyses were done in lysis buffer containing 20 mM Tris-HCl (pH 8), 137 mM NaCl, 2 mM EDTA, 10% glycerol, 10 µg/mL leupeptin, 10 µg/mL aprotinin, 1 mM PMSF, 500 µM sodium orthovanadate, 1 mM NaF and 0.5% Brij96. Postnuclear supernatants were boiled with sample buffer and subjected to SDS-PAGE separation. Immunoblotting was performed by conventional methods.
Upregulation of surface markers CD69, CD86
RBC-depleted splenocytes and K46µmL cells were stimulated with the indicated stimuli at 37°C for the displayed time. Cells were harvested and stained with the indicated antibodies for analysis by flow cytometry as previously described 61. B cells were gated based on B220 expression.
Statistical analysis
Statistical analysis was done using the GraphPad Prim 4.0 program (GraphPad Software, San Diego, CA). Data were compared by the Student's t-test; ***p<0.001, **p<0.01, *p<0.05 and ns: non-significant.
Acknowledgements
We would like to thank Nadja Goos for expert technical assistance, and P. Nielsen for critical reading of the manuscript. This work was supported by an Emmy Noether Fellowship to W.W.S. from the DFG (SCHA 976/1), the European Union-funded grant EPI-PEP-VAC to S.M. and M.R., grant SFB620 from the DFG to W.W.S., M.H. and M.R., and grant SPP1110 from the DFG to M.A.F.
Conflict of interest: The authors declare no financial or commercial conflict of interest.




