Protection against Plasmodium falciparum challenge induced in Aotus monkeys by liver-stage antigen-3-derived long synthetic peptides†
Abbreviations
LSA3liver stage antigen-3
LSPlong synthetic peptide
NRPnon-repeat peptide
pLDHparasite lactate dehydrogenase
RPrepeat peptide
Abstract
The vaccine potential of Plasmodium falciparum liver stage antigen-3 (LSA3) was investigated in Aotus monkeys using two long synthetic peptides corresponding respectively to an N-terminal non-repeat peptide (NRP) and repeat 2 (R2) region of the LSA3, adjuvanted by ASO2. Both 100–222 (NRP) and 501–596 repeat peptides induced effector B- and T-cell responses in terms of antigen-driven antibodies and/or specific IFN-γ secretion. Animals challenged with P. falciparum sporozoites were protected following immunization with either the NRP region alone or the NRP combined with the R2 repeat region, as compared with controls receiving the adjuvant alone. These results indicate that the NRP may be sufficient to induce full, sterile protection and confirm the vaccine potential of LSA3 previously demonstrated in chimpanzees and in Aotus.
Introduction
A solid and justified hope for an effective pre-erythrocytic malaria vaccine has emerged from the evidence that sterile immunity can be obtained by immunization with irradiated sporozoites both in humans and in animal models 1. Others and we have brought evidences that the so-called antisporozoite immunity is in fact liver-stage dependent. There is now convergent data indicating that this protective immunity is induced by the arrested liver trophozoites that persist in the liver and target-infected hepatocytes 2. This has been the primary driving force behind our work on the liver stages and has led to identification of new antigens expressed during the pre-erythrocytic phase of the parasite life cycle. The selection of the vaccine candidates relied on experimentally induced protection in humans immunized by irradiated sporozoites. By analyzing the differential immune responses observed between protected and non-protected volunteers upon a viable sporozoite challenge, we selected within a subset of Plasmodium falciparum pre-erythrocytic antigens a protein that we named liver stage antigen-3 (LSA3), which was preferentially recognized by sera from protected humans 2, 3. This antigen is highly conserved, is expressed in sporozoite and liver stages, and its protective potential against challenge with P. falciparum sporozoites has been demonstrated in non-human primates using a large set of antigen-presentation systems 3, 4.
The protective efficacy shown by this antigen prompted us to further analyze its antigenicity and immunogenicity using 17 long synthetic peptides (LSP) ranging between 44 and 186 amino acids in length, spanning the whole P. falciparum LSA3 protein. Most of the peptides were shown to be highly immunogenic in mice and antigenic in humans exposed to malaria in a hyperendemic area of Africa 5. The chemical synthesis of LSP provides several practical advantages and has contributed to the evaluation of the antigenicity and immunogenicity of both P. falciparum and P. vivax proteins in animals and humans 6. We selected two LSA3-LSP from the N-terminal non-repeated (100–222) and the repeat region R2 (501–596), which cover the sequence of the LSA3-729 subunit protein used in previous protective experiments 3, 4 and which is a target of Th1 protective responses in primates. In order to determine the region of greatest importance for the induction of protective mechanisms, we immunized Aotus monkeys with either the non-repeat region peptide alone, or mixed with the repeat region peptide and evaluated the efficacy of the immune responses induced in this manner against challenge by P. falciparum sporozoites.
Results and discussion
General remarks
The present study was performed in view of the protective effect induced previously by immunization with different formulations of LSA3 (Fig. 1) against a P. falciparum sporozoite challenge in chimpanzees and in Aotus monkeys 3, 4. Four formulations have been found able to induce protection: the LSA3-729 recombinant protein either adsorbed to microparticles without adjuvant or adjuvanted by ASO2, lipopeptides formulations of the NRI and NRII peptides with the non-lipid-tailed R peptide 3, 4, and genetic immunization using the nearly full-length gene 7 (Daubersies et al., in preparation). Here, we perform a preliminary evaluation of the immunogenicity and protective efficacy in A. l. griseimembra of two LSP covering either the non-repeat (100–222) or the repeat (501–596) sequences derived from the P. falciparum LSA3-729 protein (Fig. 1). In view of the strong Th1 responses obtained against the NRI and NRII peptides in previous studies relying on lipopeptide or protein-adjuvanted formulations, the importance of the non-repeated region for protection was evaluated by immunizing with the non-repeat peptide (NRP) (including NRI and NRII sequences) either alone or combined with the RP.
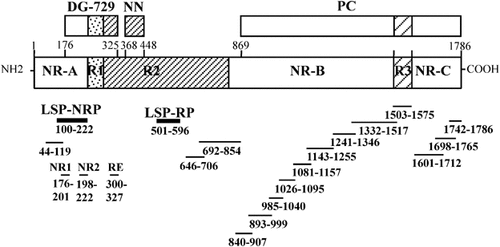
Schematic representation of P. falciparum LSA3 antigen. Recombinant proteins DG729, NN and PC are shown in the upper part and synthetic peptides in the lower part. The latter were used for antigenicity studies 5. Immunogenicity and protective efficacy were evaluated previously in chimpanzee and Aotus monkeys using either DG 729 or peptides derived from the latter or a mix of 729, NN and PC 3, 4. Here, we employed in Aotus the LSP-NRP (100–222) and LSP-RP (501–596) regions.
The LSP formulations were safe, since neither local nor generalized reactions were detected and hematological parameters, liver and kidney function remained within normal values in Aotus following subcutaneous immunizations with the LSA3-LSP in ASO2 adjuvant.
LSA3-LSP induce high IFN-γ response
At the T-cell level, the production of IFN–γ is of particular interest, since this cytokine is known to efficiently stimulate the elimination of P. falciparum liver forms even at low concentrations 8. Its role in protection has been observed in vitro in P. falciparum-infected human hepatocytes, at concentrations ranging from 1 to 10 I U 8 as well as in vivo in mice immunized by irradiated sporozoites 9 and is supported by studies in chimpanzees immunized either with irradiated sporozoites or with LSA3-derived antigens 2, 10, or in Aotus immunized with particulate formulations 4. Because peptides NRP and RP contain epitopes, which are main targets of Th1 responses and the protection is associated with strong Th1 responses 4, analysis of T-cell responses in immunized Aotus was limited to the study of IFN-γ production by specific T cells. PBMC from immunized monkeys produced substantial amounts of IFN-γ as determined by ELISPOT upon stimulation with at least one of the short or long synthetic peptides, or in one animal in culture supernatants. The frequency of specific IFN-γ-producing cells detected by the ex vivo ELISPOT assay using cells from monkeys immunized with either NRP or the mixture NRP+RP was highest in response to in vitro stimulation with the NRP (Fig. 2A). Lymphocytes from Aotus V114 and M88 from NRP and NRP+RP groups, respectively, also showed high IFN-γ response (determined either by ELISPOT or by ELISA) when challenged in vitro by the shorter peptides NRI and NRII contained in the NRP sequence. Responses were actually higher than those recorded previously by immunization with the DG 729 recombinant protein 4. The specificity of the response was demonstrated by negative results obtained using lymphocytes from either pre-immunization samples or control monkeys. The high and specific IFN-γ secretion against the LSP-NRP and LSA3-729 shorter peptides is in agreement with previous results obtained in Aotus, mice, and chimpanzee immunized with LSA3-729-derived lipopeptides without adjuvant 10, 11 and recombinant proteins 4. It indicates that the immunization with LSP-NRP is an efficient means of induction of strong T-cell responses.
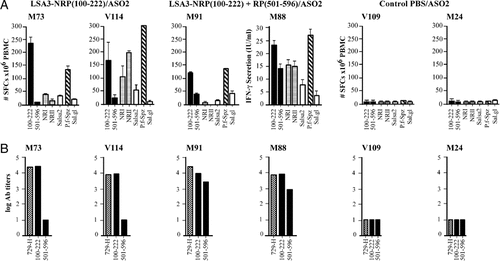
T- and B-cell responses in immunized and control Aotus. Two groups of Aotus monkeys were immunized with either NRP (100–222) or with the mix of NRP+RP (501–596) peptides adjuvanted in ASO2 as described in Materials and methods. A control group was immunized with PBS mixed with the same adjuvant. (A) IFN-γ production was evaluated on PBMC 15 days after the last immunization. Cell suspensions were mixed with the synthetic peptides LSA3-NRP, -RP, -NRI, -NRII, and with P. falciparum sporozoites as a native protein. Peptide SALSA-2 and salivary glands from non-infected An. albimanus were used as negative controls. Results are expressed as the mean+SD of the triplicate determination of IFN-γ spot forming cells per 106 PBMC. For Aotus M88, IFN-γ was titrated in culture supernatants by ELISA and expressed in IU/mL. (B) Specific antibody responses were assessed by ELISA against both peptides NRP and RP, and the LSA3-729 recombinant protein. Results are expressed as log titers.
Responses are relevant to the parasite protein
The relevance to the native LSA3 protein of T-cell responses induced by immunization with LSP was demonstrated by high specific responses to P. falciparum sporozoite. All immunized Aotus showed significant amounts of IFN-γ detected specifically by ELISPOT, or in culture supernatants, in response to in vitro exposure of lymphocytes by a P. falciparum sporozoite extract, at levels similar to those produced using LSP. Results indirectly confirm the antigenicity of the molecule and indicate that substantial amounts of LSA3 are expressed at the sporozoite stage. In contrast, no IFN-γ response was detected in control animals or in lymphocytes from immunized animals in response to non-infected Anopheles salivary glands, used as controls. Based on previous studies 2, 3, 10, strong IFN-γ responses to the native protein are considered to be of good predictive value for protection against pre-erythrocytic stages of P. falciparum.
Antibody responses target the NRP region
Antibodies determined by ELISA showed titers ranging between 900 and 27 000 (Fig. 2B). Monkeys immunized with either NRP or the mixture NRP+RP produced high levels of antibodies to both NRP and the 729 recombinant proteins, indicating that B-cell epitopes contained in the NRP alone are strongly immunogenic in Aotus. Antibody titers were lower in the RP region, in contrast to results obtained in exposed populations, and non-significant in the group immunized with NRP alone, as expected. These results are similar to those observed in immunized mice 5 and chimpanzees 10, confirming that the sequences from the N-terminal region of the protein contain potent B-cell epitopes.
LSA3-LSP induce protection against P. falciparum sporozoite challenge
The challenge was performed 60 days after the last immunization in order to avoid a possible nonspecific effect upon liver stages development of pro-inflammatory mediators, such as IFN-α, TNF-α, free oxygen and nitrogen radicals, induced by the adjuvant 2. As shown in Fig. 3, the three challenge control monkeys, two immunized with PBS in ASO2 (M24, V109) and one naive animal (V111) developed a P. falciparum infection detected by the parasite antigen lactate dehydrogenase (pLDH) assay from day 14 to 28. Although there were some variations from one animal to the other, results are in agreement with previous data showing that Aotus monkeys can be successfully infected with sporozoites derived from the P. falciparum Santa Lucia strain in a reproducible manner 4, 12. In contrast, pLDH was negative in all LSA3-immunized animals in all of the 120 samples collected during the 60 days of follow-up, indicating the induction of a sterile protection.
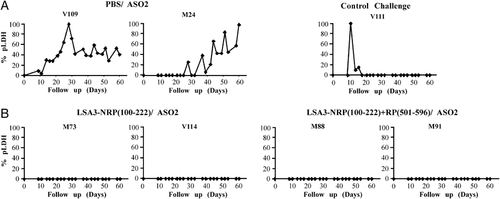
Parasitemia follow-up after challenge with P. falciparum sporozoites. Immunized and control Aotus as described in Fig. 2 were challenged 2 months after the third immunization by intravenous inoculation of 105 sporozoites from P. falciparum Santa Lucia strain. (A) The negative controls are two monkeys (V109, M24) that received the adjuvant ASO2 alone with PBS and one naive monkey (V111). (B) Aotus immunized with either NRP (100–222) or with the mix of NRP+RP (501–596) peptides. Parasitemia was evaluated by measuring circulating parasite pLDH in 30 blood samples from each animal collected every 2 days during 60 days. The results are expressed as percentages of maximal OD value observed in challenged control Aotus, as described in the Materials and methods.
Although the number of animals was limited in this preliminary study, the fact that the Aotus immunized with the NRP alone or the mixture NRP+RP were fully protected suggests that epitopes contained within the NRP region alone are sufficient to induce protection against P. falciparum-sporozoites challenge, i.e. induce critical mechanisms. Therefore, further analysis of responses elicited by NRP in models and in humans may contribute to elucidation of these mechanisms. Although, the number of monkeys does not allow to reach statistical significance, these results are consistent with previous data obtained with P. falciparum LSA3-DG729 region 3, 4, particularly with the protection induced by lipopeptide formulations of the peptides NRI and NRII contained in the NRP employed in the present work 3.
It is noteworthy that the vaccination with LSA3-LSP induced immune responses that protected against a challenge with a strain heterologous to that used to design the vaccine. This is in agreement both with previous challenge data also performed using heterologous P. falciparum NF54 parasites 3 and with the high degree of LSA3-sequence conservation 3.
Concluding remarks
The present study confirms the high immunogenicity of LSA3 and particularly the induction of IFN-γ-secreting T cells; it suggests that responses elicited by the NRP region of the molecule are of particular significance, as NRP defines a minimal region associated with protection against P. falciparum heterologous challenge. It lends support to the LSP strategy as means to readily investigate the interest of diverse regions from large molecules and provides yet another vaccine formulation to the list of four LSA3-derived antigen presentations that successfully induced protection against P. falciparum challenge in a strain-independent manner. It also supports the use of Aotus monkey as a valuable alternative to chimpanzees for the discovery and pre-clinical development of P. falciparum pre-erythrocytic vaccines.
However, data obtained in pre-clinical models can only be taken as indicative, even when they are convergent and promising. Results indicate that immunization by full-length LSA3 may not be required. This and earlier studies rather suggest that formulations based on the DG 729 protein and/or the NRP, which can be easily produced under GMP conditions, should now be evaluated for safety and protective efficacy in clinical trials.
Materials and methods
Long synthetic peptides
Two peptides covering the DG729 protein employed in previous studies 3, 4 were synthesized and selected from a panel of 17 LSP based on previous studies, which showed strong antigenicity in human donors and strong immunogenicity in mice 5. Peptide 100–222 (NRP) represents most of non-repeat region A from the N-terminal part of the protein. Peptide 501–596 (RP) is representative of the repeat region 2 (R2).
Immunization of monkeys
Two groups of two adult, naive, male and female Aotus lemurinus griseimembra monkeys, originating from the northern forests of Colombia, were immunized three times subcutaneously at 3-wk intervals with the two peptides mixed with the adjuvant ASO2. Aotus of group I received 50 μg of NRP alone, whereas Aotus of group II received a mix of 50 μg of NRP and 50 μg of RP. A control group of two monkeys was immunized with PBS mixed with the ASO2 adjuvant. In order to check the safety of the vaccine candidate, animals were inspected daily by a veterinary doctor performing physical examinations throughout the experimental period. Hematological parameters including Hemoglobin and Hematocrit, and liver and kidney biochemical parameters (BUN, Creatinine ASAT, ALAT) were evaluated before and during the immunization and challenge process. The experimental protocol was reviewed and approved by the animal ethical committee of Universidad del Valle and it was conducted following the US National Institutes of Health guidelines for the care and use of laboratory animals.
B- and T-cell responses
Immune responses in immunized Aotus were assessed to the two NRP and RP used as immunogens, to the recombinant protein LSA3-729 overlapping part of both regions, and the peptides LSA3-NRI, LSA3-NRII covering the short non-repeated sequence found in the NRP and in the LSA3-729 recombinant protein.
Serum samples collected from immunized and control monkeys before and after immunization were used for evaluation of specific antibody responses to the peptides by ELISA as described elsewhere 4.
T-cell assays were performed using Aotus PBMC from blood taken by femoral venipuncture at day 0 (pre-immunization) and 15 days after the third immunization. Cell suspensions were mixed with the corresponding LSA3 synthetic peptides described above or with a sonicated P. falciparum sporozoites. An irrelevant peptide from the SALSA protein 13 and salivary glands obtained from non-infected Anopheles albimanus mosquitoes were used as negative controls. IFN-γ production was evaluated here because high levels of this cytokine were shown to be associated with protection against sporozoite challenge and were found to-date the best surrogate marker of protection in chimpanzees and Aotus 3, 4, 10. The frequency of PBMC producing IFN-γ ex vivo was evaluated using a commercial kit for human IFN-γ ELISPOT (MABTECH, Stockholm, Sweden) as described elsewhere 4. Results are expressed as the mean number of IFN-γ spot forming cells per 106 PBMC. Since numbers of PBMC recovered from one of the studied Aotus were insufficient, in this case we performed a more classical determination of IFN-γ in supernatants collected on day 5 from in vitro antigenic stimulation of lymphocyte cultures. IFN-γ was titrated by a two-site capture ELISA as described previously 10. Negative and positive controls (unstimulated cells and cells stimulated with PHA) were included in each assay. The specificity was verified by the absence of signal in control supernatants.
Challenge and parasitemia follow-up
P. falciparum Santa Lucia strain previously adapted by serial passages in Aotus monkeys and able to produce infective gametocytes 14 was used to infect laboratory reared An. albimanus mosquitoes by artificial membrane feeding. Mature infective sporozoites collected on day 15 post-feeding were used to challenge monkeys as described previously 4. The challenge was performed 2 months after the last immunization. Blood from all challenged animals was sampled every 2 days during 60 days. Parasitemia was monitored by the detection of the pLDH using a double-site enzyme-linked lactacte dehydrogenase immunodetection assay 15. The OD values recorded with this double-site antigen-capture assay have been found to be a direct function of parasite densities both for P. falciparum and for P. vivax 15, 16. The assay proved to be more sensitive for the detection of P. falciparum and P. vivax than thick blood smears and was able to detect a parasitemia as low as 1 infected RBC in 108 RBC 15, 16. The method was further validated by assessment of pLDH in samples from previous challenge studies in test and control animals 4. The baseline absorbance value, above which a sample was considered positive, was the mean value plus 3 SD of the OD values obtained using blood samples collected before challenge. Positive parasitemia were expressed as the percentage of the highest OD value obtained from each of the three control Aotus. Negative parasitemia were expressed as 0%. At the end of the follow-up period, the animals were treated with the combination Sulfadoxine–Pyrimethamine, as in previous studies 4.
Acknowledgements
Thanks to Dr. Jean Louis Perignon for critical reading of the manuscript. We also thank A. Rincon and N. Puchot for technical assistance and V. M Salazar and L. A. Ruiz for care and handling the Aotus monkey colony. The present work received financial support from the European Commission (INCO-DC contracts No. QLRT-2000-0186) and from the Colombian Research Council (Colciencias-contract No. 118-2000).
Conflict of interest: The authors declare no financial or commercial conflict of interest.




