IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells
Abstract
Epstein-Barr virus-induced gene 3 (EBI3) and the p35 subunit of IL-12 have been reported to form a heterodimeric hematopoietin in human and mouse. We have constructed a heterodimeric protein covalently linking EBI3 and p35, to form a novel cytokine which we now call IL-35. The Fc fusion protein of IL-35 induced proliferation of murine CD4+CD25+ and CD4+CD25– T cells when stimulated with immobilized anti-CD3 and anti-CD28 antibodies in vitro. The IL-35-expanded CD4+CD25+ T cell population expressed Foxp3 and produced elevated levels of IL-10, whereas the IL-35-induced CD4+CD25– T cells produced IFN-γ but not IL-4. The in vitro expanded CD4+CD25+ T cells retained their suppressive functions against CD4+CD25– effector cells. Furthermore, when cultured with soluble anti-CD3 antibody and antigen-presenting cells, IL-35 suppressed the proliferation of CD4+CD25– effector cells. Moreover, IL-35 inhibited the differentiation of Th17 cells in vitro. In vivo, IL-35 effectively attenuated established collagen-induced arthritis in mice, with concomitant suppression of IL-17 production but enhanced IFN-γ synthesis. Thus, IL-35 is a novel anti-inflammatory cytokine suppressing the immune response through the expansion of regulatory T cells and suppression of Th17 cell development.
Abbreviations:
-
- CIA:
-
collagen-induced arthritis
-
- CII:
-
type II collagen
-
- EBI3:
-
Epstein-Barr virus-induced gene 3
Introduction
Epstein-Barr virus-induced gene 3 (EBI3) is a homologue to IL-12 p40 and to the ciliary neurotrophic factor receptor, whose expression is induced in B lymphoblastoid cell lines by EBV infection 1. EBI3 encodes a 34-kDa glycoprotein with 27% amino acid identity to the IL-12 p40 subunit. Both EBI3 and p40 are encoded by mRNA with a 3′ untranslated Alu repeat sequence, lacking a membrane anchoring motif, and are predicted to be secreted 1. IL-12 was identified and purified from culture supernatants of EBV-transformed B cell lines and would be expected to be closely associated with EBI3. IL-12 p35 is ubiquitously expressed whereas IL-12 p40 expression is inducible. The dissociation between p35 and p40 gene regulation suggests that either subunit may associate with other partners 2–4. p40 associates with p19 to form IL-23 5, and EBI3 associates with a p28 protein to form IL-27 6. EBI3 has been reported to associate with the p35 to form a heterodimeric hematopoietin in vivo 7. Thus, the EBI3-p35 heterodimer may represent a novel cytokine of the IL-6 family, which includes IL-12, IL-23 and IL-27. We now demonstrate that EBI3-p35 indeed has a profound cytokine activity. We propose to call the novel cytokine IL-35.
EBI3 is expressed at high levels in human B lymphoblast cells, tonsils and spleen 1. Importantly, a large fraction of p35 in extracts of the trophoblast component of a human full-term normal placenta specifically co-immunoprecipitated with EBI3, suggesting that IL-35 may be important in immune regulation. We show here that IL-35 could expand CD4+CD25+ Treg cells and also directly suppressed the proliferation of CD4+CD25– effector cells and inhibited Th17 cell polarization. Furthermore, mice treated with IL-35 developed markedly less severe collagen-induced arthritis (CIA), indicating that IL-35 is immune suppressive and may be a novel reagent against inflammatory disease.
Results
Construction and purification of IL-35
We have constructed IL-35 by covalently linking EBI3 with the p35-Fc fusion protein, as described for the construction of IL-23 5 and IL-27 6 (Fig. 1A). The human Fc fusion protein was introduced for ease of purification and also for a longer half life of the protein in vivo. We constructed murine as well as human IL-35, according to published sequences for EBI3 and IL-12 p35 1, 2. The proteins were purified by agarose columns, and the purity was checked by Coomassie blue staining (Fig. 1B) and was found to be >98%. Because of the lack of good antibodies against mouse EBI3 and p35, the protein was also checked by Western blot against human Fc (Fig. 1C). A single band at MW 78 kDa was detected, consistent with the predicted MW of the IL-35 fusion protein. The human IL-35 was tested for purity by silver staining and Western blot with anti-human EBI3 and anti-human p35 antibodies. In all cases, a single band at 78 kDa was detected (Fig. 1D). In addition, we also constructed the EBI3-Fc fusion protein as a control. We also used the human Fc fragment as an additional control.
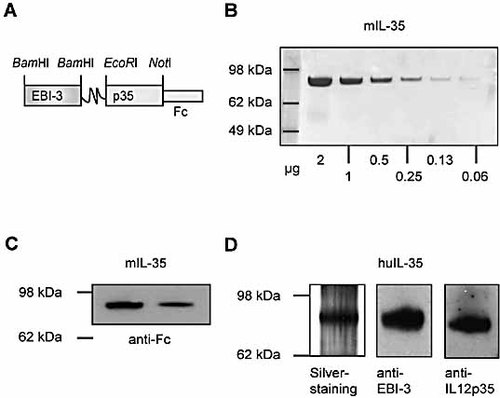
Construction and characterization of IL-35. (A) The structure of IL-35 is schematically presented. IL-35 was expressed in CHO cells and purified by agarose 4B columns. The purity of the murine IL-35 was examined by Coomassie blue staining (B) and Western blot with anti-human Fc antibody (C). (D) Human IL-35 was similarly constructed and the linked heterodimeric protein was examined by silver staining and by Western blot with anti-EBI3 and anti-IL-12 p35 antibodies.
IL-35 expanded CD4+CD25– and CD4+CD25+ T cells with costimulation
We next determined the functions of IL-35 in vitro. We purified CD4+CD25– and CD4+CD25+ T cells from spleen and lymph nodes of BALB/c mice and cultured them in vitro with plate-bound anti-CD3 and anti-CD28 antibodies, a condition to produce maximal T cell activation. IL-35 markedly expanded the proliferation and IFN-γ synthesis of CD4+CD25– T cells under these culture conditions (Fig. 2A, B). IL-35 had little or no effect on IL-4 synthesis. Thus, IL-35 appeared to preferentially polarize Th1 cells. Consistent with this, IL-35 activated T-bet but not GATA3 mRNA expression (data not shown). CD4+CD25+ T cells were cultured with IL-35 in the presence of plate-bound anti-CD3 antibody for 3 days, and IL-2 and anti-CD28 antibody were added at 24 h after the start of the culture. Under these culture conditions, IL-35 also induced marked proliferation of CD4+CD25+ T cells with significant elevation of the IL-10 concentration in the culture supernatant (Fig. 2C, D). However, the percentage of Foxp3+ cells remained constant at 75% and the intensity of Foxp3 staining was unchanged, suggesting that IL-35 expanded the CD4+CD25+ T cells without induction of additional Foxp3 expression. The IL-35-expanded CD4+CD25+ T cells remained highly suppressive against the effector CD4+CD25– T cells (Fig. 2E). These results show that, under strong inductive conditions with polyclonal TCR activation and costimulatory signals (anti-CD28 antibody), IL-35 is capable of expanding Th1 cells and natural Treg cells. The IL-35-expanded Treg cells retained their suppressive function.

IL-35 induced CD4+CD25– and CD4+CD25+ T cell proliferation under polyclonal TCR activation and costimulation. CD4+CD25– and CD4+CD25+ T cells were purified from spleen and lymph nodes of BALB/c mice and cultured with plate-bound anti-CD3 antibody and soluble anti-CD28 antibody in the presence of graded concentrations of IL-35 or human Fc. IL-35 induced CD4+CD25– T cells to proliferate (A) and to produce IFN-γ (B) in a dose-dependent manner. IL-35 also induced CD4+CD25+ T cells to proliferate (C) and to produce IL-10 (D). There was no difference in the response of cells cultured with Fc or medium alone (data not shown). (E) The IL-35-expanded CD4+CD25+ T cells [as in (C), with 100 ng/mL IL-35] were then cultured with freshly purified CD4+CD25– T cells for 72 h. Cell proliferation was determined by [3H]thymidine uptake. Data are means ± SEM, n = 4, and are representative of at least three independent experiments. *p <0.05, **p <0.01 compared to controls: Fc, or CD25– group in (E).
IL-35 suppressed CD4+CD25– effector T cells
We then investigated the effect of IL-35 under different culture conditions using soluble anti-CD3 antibody and mitomycin C-treated antigen-presenting cells (APC), or OVA peptide and APC (for T cells from OVA-TCR-transgenic DO.11 mice) 8. Under these culture conditions, IL-35 had little or no effect on CD4+CD25+ T cells, which gave a minimal proliferative response (data not shown). In contrast, IL-35 markedly inhibited the proliferation and IFN-γ production by CD4+CD25– T cells activated with anti-CD3 antibody and APC compared to a control culture in the presence of Fc protein (Fig. 3A, C). IL-35 also suppressed the proliferation and IFN-γ production by OVA-specific CD4+CD25– T cells from DO.11 mice stimulated with OVA peptide and APC (Fig. 3B, D). These data therefore demonstrate that IL-35 can directly suppress the proliferation of CD4+CD25– effector cells under more “physiological” conditions.
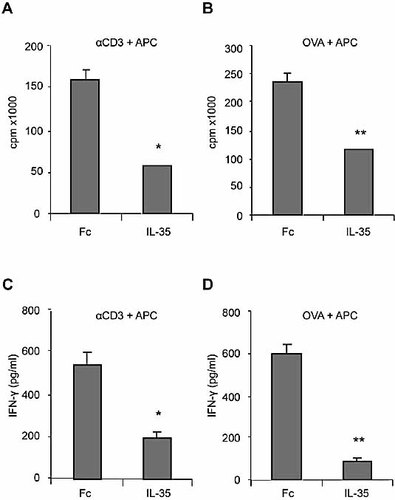
IL-35 suppressed the proliferation and IFN-γ production by CD4+CD25– T cells from BALB/c mice and DO.11 mice. CD4+CD25– T cells were purified from spleen and lymph nodes of the mice and cultured for 72 h with soluble anti-CD3 antibody and APC in the presence of 100 ng IL-35 or Fc. IL-35 markedly suppressed the proliferation (A, B) and IFN-γ production (C, D) of CD4+CD25– T cells. There was no difference in the response of cells cultured with Fc or medium alone (data not shown). Data are means ± SEM, n = 4, and are representative of three independent experiments. *p <0.05, **p <0.01 compared to Fc control.
IL-35 suppressed the differentiation of Th17 cells
We then investigated the effect of IL-35 on the differentiation of Th17 cells. CD4+ T cells were purified from BALB/c mice and cultured with plate-bound anti-CD3 and anti-CD28 antibodies and a cocktail of reagents (TGF-β, IL-1β, IL-23) as described in the Materials and methods section for the induction of Th17 cell differentiation 9–11. IL-35 or control EBI3-Fc was added at the start of the culture. IL-35 markedly suppressed the differentiation of Th17 cells compared to medium alone (Fig. 4). EBI3-Fc had no effect on IL-17 synthesis compared to cells cultured with medium alone.
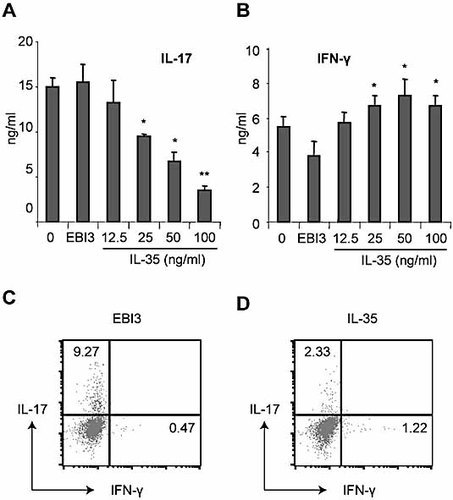
IL-35 suppressed Th17 cell but promoted Th1 cell differentiation. CD4+ T cells were purified from BALB/c mice and cultured for 3 days with plate-bound anti-CD3 antibody and soluble anti-C28 antibody in the presence of TGF-β, TNF-α, IL-1β and IL-6. Concentrations of IL-17 and IFN-γ in the culture supernatants were determined by ELISA (A, B) and the cells were stained for intracellular IL-17 or IFN-γ (C, D). Data are means ± SEM, n = 4, and are representative of three independent experiments. *p <0.05 compared with cells cultured with medium alone.
IL-35 attenuated CIA
Since both Treg 12–14 and Th17 cells 15, 16 are closely associated with the control of rheumatoid arthritis, we next investigated the effects of IL-35 in the CIA model in DBA/1 mice. Mice were primed on day 0 with type II collagen (CII) in complete Freund's adjuvant (CFA) and then boosted with CII in PBS on day 21, as previously described 17. Mice were injected intraperitoneally (i.p.) with 2 µg IL-35 or PBS daily for 10 days from day 24, when disease was already established. Control mice treated with PBS developed the expected disease progression. In contrast, mice treated with IL-35 displayed a significant reduction in incidence (Fig. 5A) and in number of arthritic paws (Fig. 5B). Histological analysis showed that mice treated with PBS exhibited mononuclear and polymorphonuclear cell infiltration into the joint compartment, synovial hyperplasia, and adjacent cartilage and bone erosion (Fig. 5C, D). Each of these parameters was markedly suppressed in the mice treated with IL-35 (Fig. 5E). The histological scores are summarized in Fig. 5F. Together, these data demonstrate that IL-35 potently suppressed the development of CIA, and such activity can prevent progression of articular damage. Serum of IL-35-treated mice contained significantly higher concentrations of IL-10 compared to the PBS-treated mice (156 ± 45 pg/mL vs. 25 ± 5 pg/mL, p <0.01). There was no significant difference in the levels of TNF-α, IFN-γ, IL-6, IL-12 and IL-1Rα, or anti-CII antibody of IgG1 or IgG2a isotypes in the serum between the two groups of mice. We also analyzed the spleen cell populations for cells producing IL-17 and IFN-γ by intracellular FACS. Spleen cells from IL-35-treated mice produced IL-17-secreting cells at a lower frequency compared to that of the PBS-treated control mice. Interestingly, the IL-35-treated mice also produced IFN-γ at higher frequency compared to PBS-treated mice. These results therefore suggest that IL-35 preferentially increased IL-10 production and suppressed Th17 cell development in CIA, consistent with the effect of IL-35 on Treg cell expansion and inhibition of Th17 cell differentiation in vitro.

IL-35 suppressed disease development in CIA in DBA/1 mice. Mice were primed on day 0 and boosted on day 21 with CII and then treated i.p. with IL-35 (2 µg per day for 10 days) or PBS from day 24 post immunization. IL-35-treated mice developed significantly less arthritic incidence (A) and less severe disease score (B) compared to control mice treated with PBS. Data are means ± SEM, n = 10. Histological examination also shows markedly less cellular infiltration, cartilage and bone erosion and hyperplasia in the diseased joints (C–E). Photomicrographs are representative of five mice and the histological scores are from five mice per group. The spleen cells from the IL-35-treated mice also show a significantly reduced frequency of IL-17-producing cells but a higher frequency of IFN-γ-producing cells (F). *p <0.05, **p <0.01, ***p <0.001 compared with the IL-35-treated group.
The effect of IL-35 on CIA was comparable to that of soluble TNFR
We further characterized the therapeutic effect of IL-35 on CIA. First, we excluded the possibility that the effect of IL-35 was due to the Fc fragment of the molecule. DBA/1 mice primed and boosted with CII were treated i.p. daily for 10 days with IL-35, EBI3-Fc or PBS from day 24. Mice treated with IL-35 developed significantly less severe disease compared to PBS-treated mice. Mice treated with EBI3-Fc developed disease indistinguishable from the PBS-treated mice (Fig. 6A). These results demonstrate that the effect of IL-35 was not due to the Fc fragment of the molecule. Furthermore, EBI3-Fc alone had no effect on CIA, consistent with the in vitro results showing that EBI3-Fc had no effect on Th17 cell differentiation (Fig. 4). We then carried out a dose-response study on the therapeutic effect of IL-35 on CIA. Mice were primed and boosted with CII and treated as above with graded doses of IL-35. A daily dose of 10 µg per mouse of IL-35 was found to produce the best therapeutic effect, although a daily dose of 2 µg of IL-35 was effective (Fig. 6B). We then compared the effect of subcutaneous (s.c.) vs. i.p. administration of IL-35 on CIA and also the effect of administration of IL-35 at a later stage of the disease. Mice primed and boosted on day 21 with CII were treated daily for 10 days with IL-35 injected s.c. or i.p. from day 27. IL-35 administered s.c. had a similar attenuating effect on CIA as IL-35 injected i.p. Finally, we compared the efficacy of IL-35 with soluble IL-15Rα 18 and soluble TNFR (Enbrel), the current gold standard of treatment for clinical rheumatoid arthritis. Mice were primed and boosted with CII as before and injected i.p. daily for 10 days from day 27 with 2 µg/mouse of IL-35, soluble IL-15Rα, soluble TNFR, or a combination of IL-35 and soluble TNFR. Although detailed dose-response studies are needed, on a weight for weight basis, IL-35 was as effective as Enbrel in treating an established CIA. These results therefore demonstrate that IL-35 may be an effective alternative therapeutic agent against clinical rheumatoid arthritis.
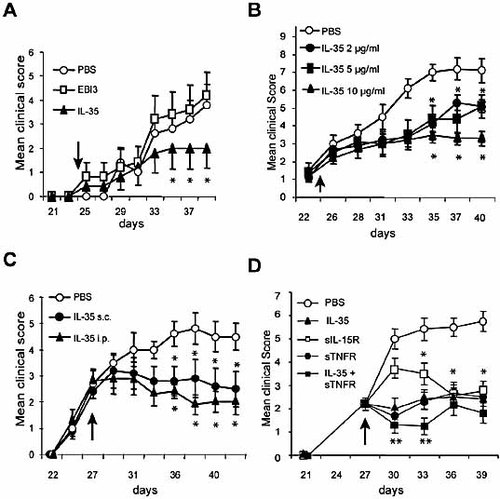
Further characterization of the effect of IL-35 on CIA. (A) IL-35 but not EBI3-Fc suppressed CIA. DBA/1 mice were primed and boosted with CII as in Fig. 5. The mice were treated i.p. with IL-35 or EBI3-Fc (2 µg daily for 10 days) from day 24 as indicated by arrow. (B) Dose-response study of the effect of IL-35 on CIA. (C) IL-35 administrated s.c. or i.p. was equally effective in suppressing CIA. (D) The therapeutic effect of IL-35 was comparable to those conferred by soluble IL-15Rα (sIL-15R) or soluble TNF receptor (sTNFR). Data are means ± SEM, n = 10, *p <0.05, **p <0.01 compared to PBS-treated controls.
Discussion
The IL-35 reported here is a heterodimer consisting of EBI3 and IL-12 p35, both of which are present in EBV-transformed B cells. IL-12 p35 is constitutively expressed in most tissues, while EBI3 is mainly expressed in hematopoietic cells. In human lymphoid tissues, EBI3 message was found in tonsil cells, spleen cells and at high levels in full-term placenta of normal births 1. EBI3 is expressed in the mucosa of patients with ulcerative colitis 19. In EBV infection, the expression of a Th1 cytokine profile, and the associated cell-mediated immune response, results in clearance of EBV-infected cells 20. EBI3 is strongly expressed in Hodgkin lymphoma 21. There is also good evidence that expression levels of EBI3 and IL-12 p35 are both up-regulated in placental trophoblasts 22 and that EBI3 associates with p35 in the extract of the trophoblast components of human full-term normal placenta 7. Trophoblasts are thought to play a key role in maternal tolerance to the semi-allogeneic fetus, in part through cytokine production 23–26. We demonstrate here that IL-35 may play an important role in suppressing the inflammatory response by expanding Treg cells and in dampening the differentiation of Th17 cells.
IL-35 is a member of the IL-12 family, which includes IL-23 and IL-27. Our IL-35 construct is akin to those for IL-23 and IL-27 with Fc fusion protein 5, 6. It is of interest to note that the two members of this family (IL-27 and IL-35) that share EBI3 are immune suppressive (27, 28 and data reported here), whereas the other two members (IL-12 and IL-23) that do not have EBI3 generally activate immune responses. However, we show here that EBI3 on its own is not immune suppressive in vitro or in vivo. The interactive role of EBI3 with its cytokine partners, the receptor for IL-35 and the IL-35 signaling pathway remain to be explored.
Like IL-27, IL-35 can induce the proliferation as well as anti-proliferation of CD4+ T cells in vitro, depending on the culture conditions. Early studies showed that IL-27 was directly involved in Th1 cell differentiation 29, 30. In contrast, other studies demonstrated that IL-27 was an antagonist of Th17 cell activity 27, 28. Thus, it was postulated that IL-27 is able to induce Th1 cell differentiation of naive CD4+ T cells, but is also able to suppress the production of pro-inflammatory cytokines such as IL-17 31. Our results thus far demonstrate a similarity between IL-35 and IL-27 function. The dual functional role of IL-35 may depend closely on the mode of T cell activation. IL-35 may preferentially activate Th1-like cells in the present of strong antigenic challenge such as acute infections, as demonstrated in vitro by a combination of plate-bound anti-CD3 and anti-CD28 antibody costimulation. However, under chronic infections or inflammation, IL-35 may selectively suppress effector cells, including Th17 cells, as demonstrated in vitro by soluble anti-CD3 antibody and APC, or OVA-specific activation with APC. This could result in the attenuation of inflammatory autoimmune diseases such as rheumatoid arthritis, exemplified here by CIA. There is, however, a crucial difference between IL-35 and IL-27. While IL-35 was effective in suppressing an established CIA, IL-27 could only do so at the onset of the disease (W. Niedbala et al., unpublished). The preferential suppression of IL-17 while elevating IFN-γ synthesis during the suppression of CIA by IL-35 in vivo is consistent with recent reports that Th17 cells rather than Th1 cells are the key pathogenic drivers of arthritic diseases 15, 16.
The dual role of T cell regulation by IL-35 may be of considerable evolutionary interest. During acute infections (such as EBV infection), IL-35 is formed by the association of EBI3 and IL-12 p35. IL-35 could markedly induce Th1 cells which help clearing the infection. IL-35 could also expand Treg cells under acute infection. The Treg cells would, during the ensuing chronic infective phase, prevent collateral damage by suppressing the residual effector cells. IL-35 could also suppress Th17 cell differentiation even during acute infection, to prevent the onset of excessive autoimmune responses. Furthermore, by its conspicuous presence in the trophoblasts, IL-35 could also play an important role in preventing rejection of the fetus by suppressing the potentially harmful effector cells. It is now possible to explore further the key physiological and patho-physiological roles of IL-35 in health and disease.
We have also constructed human IL-35, which has similar in vitro functions as the murine IL-35 (unpublished). Together, our results suggest that IL-35 may represent an alternative effective therapeutic agent against inflammatory disease in general, and arthritis in particular.
Materials and methods
Animals and reagents
BALB/c mice and male DBA/1 mice obtained from Harlan Olac (Bicester, UK) were used at 8–10 wk of age and maintained at the Biological Services Facilities, University of Glasgow. OVA-TCR-transgenic mice (DO.11) were bred in our animal facilities. All animal experiments conducted in this study were carried out in accordance with the UK Home Office animal guidelines.
Construction of IL-35
A mammalian cell expression vector (pS-L-Fc) was constructed to be used for two proteins linked with a linker peptide (3×GGGGS) followed by fusion with the human IgG1 Fc region. Briefly, two complementary oligonucleotides were designed and synthesized with 5′ end phosphorylation. Sense: 5′-GATCC GGT GGT GGT GGT TCT GGT GGT GGT GGT TCT GGT GGT GGT GGT TCT G. Antisence: 5′-AATTC AGA ACC ACC ACC ACC AGA ACC ACC ACC ACC AGA ACC ACC ACC ACC G. The formation of restriction enzyme sites for Bam HI and Eco RI was included. After annealing, the double-stranded oligonucleotide fragment, which encodes the 3×GGGGS linker, was inserted into the pSecTag2A vector (Invitrogen) at the Bam HI and Eco RI sites to create a pS-Linker vector. The cDNA of the human IgG1 Fc fragment including the hinge region was amplified by RT-PCR on total RNA extracted from human PBMC. Xho I and Sal I sites were introduced into PCR primers with the following sequences: sense: 5′-GAG CCT CGA GCC GAG CCC AAA TCT TGT GA; anti-sense: 5′-AGA AGT CGA CTT ATT TAC CCG GGG ACA GG. The DNA fragment was inserted into the pSec-Linker vector to generate the pS-L-Fc vector. The sequence was confirmed by sequencing. IL-35 was constructed by linking EBI3 and IL-12 p35 in fusion with human IgG1 Fc in the pSec-L-Fc vector. Briefly, mouse EBI3 and IL-12 p35 cDNA PCR fragments were amplified from an LPS-activated J774 cell cDNA library, using primer sequences as follows: EBI3 sense: 5′-CCC CGG ATC CCA CTG AAA CAG CTC TCG TGG CTC T, and EBI3 anti-sense: 5′-CGG GAT CCC TTA TGG GGT GCA CTT TCT ACT TGC C. IL-12 p35 sense: 5′-GGC CGA ATT CAT TCC AGT CTC TGG ACC TGC CA, and IL-12 p35 anti-sense: 5′-GGC GGC GGC CGC ATA GCC CAT CAC CCT GTT GA. A Bam HI site was introduced into the EBI3 primers. Eco RI and Not I sites were introduced into the IL-12 p35 sense and anti-sense primers, respectively. Human EBI3 and IL-12 p35 cDNA fragments were amplified from the LPS-stimulated KG-1 cell cDNA library, using hEBI3 sense: 5′-TCT GAG ATC TCT GCC CGC CCT GCA GTG GAA GG, and anti-sense: 5′-CTT GAG ATC TGC CCA GGC TCA TTG TGG CAG TG; and IL-12 p35 sense: 5′-TTT GCG GCC GCA CCT CCC CGT GGC CAC TCC AG, and anti-sense: 5′-TTT GCG GCC GCA TTC AGA TAG CTC ATC ACT CT. The PCR fragments were then inserted into the Bam HI site of the pS-L-Fc vector in the right orientation to form pEBI3-L-Fc, followed by IL-12 p35 insertion into the Eco RI and Not I sites for mouse IL-12 p35, and the Not I site for human IL-12 p35, to form pEBI3-L-p35-Fc. The reading frame of EBI3-p35-Fc was sequenced to confirm that no signal mutations had occurred during PCR amplification. The EBI3-p35-Fc expression construct was then transfected into CHO cells. The permanently transfected cell lines were selected using 700 μg/mL Zeocin in 10 days of culture. The clones with the highest expression were screened using a specific human IgG1 Fc ELISA with purified rabbit anti-human IgG1 (Sigma) as capture antibody and biotinylated anti-human Fc-specific antibody as detecting antibody (Sigma). Recombinant proteins were purified with a protein A agarose 4B column (Amersham, UK). The molecular weight and purity of recombinant mouse IL-35 was examined by SDS-PAGE and Coomassie blue gel staining (Invitrogen, UK) and Western blot with biotinylated anti-hFc antibody (Sigma). The human IL-35 was examined by silver staining using the SilverSNAP stain kit (Pierce) and by Western blot with antibodies to human EBI3 (clone 2G4H6) 23 or human IL-12 p35 (R&D Systems). Binding of antibodies was detected using horseradish peroxidase-conjugated IgG antibodies (1 : 2000 dilution; Cell Signaling) and enhanced chemiluminescence reagents (Pierce). The EBI3-Fc expression vector was constructed by EBI3 PCR fragment insertion into the Bam HI and Eco RI sites of pS-L-Fc to create an in-frame fusion of EBI3 and the human IgG1Fc region.
Effect of IL-35 on T cells in vitro
Spleen and lymph node cells were harvested from mice and separated into CD4+CD25+ and CD4+CD25– T cells by MACS (Miltenyi Biotec) with routine purity >95%. The cells were cultured under two different conditions. For the first culture condition, cells were cultured in full culture medium [RPMI 1640 supplemented with 100 IU/mL penicillin, 100 µg/mL streptomycin, 25 mM HEPES buffer and 10% FCS (all Invitrogen, Paisley, UK)] in flat-bottom 96-well plates (Nunc) with plate-bound anti-CD3 antibody (2-5 μg/mL) and soluble anti-CD28 antibody (1-2.5 μg/mL) with or without various concentrations of IL-35, or control human Fc. IL-2 (50 ng/mL) was added to the culture of CD4+CD25+ cells 24 h after the start of the culture. The cells were cultured for 3–4 days and supernatants were harvested for the determination of cytokine concentrations by ELISA. Cell proliferation was measured by [3H]thymidine (GE Healthcare, Little Chalfont, UK) incorporation. Cells were also harvested at various times for intracellular cytokine and Foxp3 staining. For the second culture condition, cells were cultured with full medium in round-bottom 96-well plates (Nunc) in the presence of soluble anti-CD3 antibody (1 μg/mL) and mitomycin C-treated normal spleen cells (APC). IL-35 or Fc was added at the beginning of the culture. Supernatants were harvested at 72 h, and cellular proliferation and intracellular staining were carried out as above. In some experiments, T cells from the OVA TCR-transgenic mice (DO.11) were used. The cells were cultured as above. For condition 2, OVA peptide was used instead of soluble anti-CD3 antibody. In some other experiments, IL-35-expanded CD4+CD25+ T cells were harvested, washed and co-cultured with freshly purified CD4+CD25– T cells in round-bottom plates with soluble anti-CD3 antibody and APC (condition 2). Cell proliferation was determined at 72 h.
Induction of CIA and assessment of arthritis
CIA was elicited in mice as previously described 17. Briefly, mice (n = 10) were immunized by intradermal injection of 200 µg acidified bovine CII (Sigma) emulsified in CFA (Difco, Detroit, MI). Mice were boosted i.p. with CII (200 µg in PBS) on day 21. Mice were monitored for signs of arthritis as described 17. Scores were assigned based on erythema, swelling or loss of function present in each paw on a scale of 0–3, giving a maximum score of 12 per mouse. Paw thickness was measured with a dial-calliper (Kroeplin, Munich, Germany). For histological assessment, mice were sacrificed and the hind limbs removed, fixed in 10% neutral-buffered formalin, and 5-µm sections were stained with hematoxylin and eosin or toluidine blue (both Sigma). The quantification of arthritis was by ‘treatment-blind’ observer, and a score was assigned to each joint based on the degree of inflammation, synovial hyperplasia and erosion as described 17. To investigate the effect of IL-35 in murine CIA, DBA/1 mice were injected i.p. daily from day 24 or day 27 for 10 days with IL-35 (2–10 µg/mouse). Control mice received similar amounts of EBI3-Fc or PBS.
Collagen-specific in vitro culture
Spleen and lymph nodes were removed on day 32 or day 42 after primary immunization. Single-cell suspensions were prepared and cultured in triplicate (2 × 106 cells/mL) in full medium with graded concentrations of CII in flat-bottom 96-well plates (Nunc). Supernatants were collected after 72 or 96 h and stored at –20°C until assayed for cytokine concentration. Proliferation assays were performed in parallel cultures in U-bottom 96-well plates (Nunc) for 96 h and were pulsed with [3H]thymidine during the last 6 h of culture.
Measurement of cytokines and anti-collagen antibody levels
All cytokines and anti-collagen antibody levels were detected by ELISA. The antibody pairs for TNF-α, IFN-γ, IL-5, IL-6 and IL-10 were obtained from BD PharMingen, and assays were performed according to the manufacturer's instructions. Detection limits were as follows: IL-5, IL-6 and TNF-α all at 10 pg/mL; IL-10 and IFN-γ both at 20 pg/mL. Serum anti-collagen II antibody titers of individual serum were detected with biotin-conjugated anti-mouse IgG1 or IgG2a (BD PharMingen), followed by conjugated avidin peroxidase (Sigma) and developed with tetramethylbenzidine substrate (Kirkegaard and Perry, Gaithersburg, MD).
Polarization of Th17 cells and the effect of IL-35 in vitro
CD4+ T cells were purified from spleen and lymph nodes of naive mice using MACS (Miltenyi Biotec) with purity routinely >95%. Cells were cultured with plate-bound anti-CD3 and anti-CD28 antibodies, TGF-β, IL-6, and IL-1β, as previously described 9. IL-35 (12.5–100 ng/mL) or EBI3-Fc (100 ng/mL) was added at the beginning of culture. Cells were harvested at 72 h and assayed for intracellular cytokines. Culture supernatants were analyzed for cytokine concentrations by ELISA.
Intracellular staining for IL-17, IFN-γ and Foxp3
Single-cell suspensions were stimulated for 4 h with 50 ng/mL PMA (Sigma) and 500 ng/mL ionomycin (Sigma). GolgiStop (BD Biosciences) was added for the final 3 h of culture. Cells were first stained extracellularly with anti-CD4 antibody, then fixed and permeabilized with Perm/Fix solution (eBiosciences), and finally stained intracellularly with anti-IFN-γ (BD Bioscience), anti-IL-17, and anti-Foxp3 antibodies (both eBioscience). Directly conjugated isotype-matched rat anti-mouse antibodies (eBioscience) were used as controls for non-specific staining.
Statistical analysis
Disease and histological scores were analyzed with the non-parametric Mann-Whitney U-test. Differences between cumulative incidences at a given time point were analyzed by the Chi-square contingency analysis. Cytokine and collagen-specific IgG levels were compared using Student's t-test.
Acknowledgements
We thank Mr. Rod Ferrier and Mr. James Reilly for histological slide preparations. We also thank Dr. Odile Devergne (INSERM, Clamart, France) for the gift of anti-human EBI3 antibody for Western blots. This work was supported by The Wellcome Trust, Medical Research Council, UK; Arthritis Research Campaign, UK; The Chief Scientist's Office, Scotland; and the European Union. W.N. was also supported by the Ministry of Science and Higher Education of Poland (Grant 2PO 5BO 8527).Conflict of interest: The authors declare no financial or commercial conflict of interest.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




