Toll-like receptors’ two-edged sword: when immunity meets apoptosis
Abstract
Toll-like receptors (TLR) have emerged as key players in the detection of pathogens and the induction of anti-microbial immune response. TLR recognize pathogen-associated molecular patterns, and trigger anti-microbial innate immune responses ranging from the secretion of pro-inflammatory mediators to the increase of natural killer cell cytotoxicity. Besides activating the innate immune response, TLR engagement also shapes the adaptive immune response. Indeed, the broad diversity of signaling pathways initiated by TLR is progressively unraveled. Recent reports suggested that among the anti-microbial defenses they initiate, members of the TLR family can induce apoptosis. This review focuses on this newly described function of TLR, and emphasizes the similarities and differences between the different apoptosis-signaling pathways described downstream of TLR. The functional relevance of TLR-triggered apoptosis is also discussed, as therapeutic applications are likely to ensue in the near future.
Abbreviations:
-
- HMGB1:
-
high mobility group protein 1
-
- HR-PCD:
-
hypersensitive response programmed cell death
-
- PAMP:
-
pathogen-associated molecular pattern
-
- PRR:
-
pattern-recognition receptors
-
- RIP1:
-
receptor interacting protein 1
Introduction
Originally identified as components of developmental pathways in the fruit fly, members of the Toll family are now recognized as major pathogen-associated molecular pattern (PAMP) recognition receptors (PRR). The Drosophila Toll receptor was first shown to be involved in protection against fungal and gram-positive bacteria infection of the fly 1. The first mammalian Toll-like receptor (TLR) (currently known as TLR4) was identified in 1997 2 and shown to recognize lipopolysaccharides (LPS), a major cell wall component of gram-negative bacteria 2. In humans, ten functional TLR have been cloned and recognized as major players of the innate immune responses. The natural ligands identified so far for those receptors are mainly microbial components, although a few potential endogenous ligands have also been detected (i.e., HSP70 and high mobility group protein 1 (HMGB1)). Molecular events triggered by TLR are progressively elucidated and shown to rely on two proximal signal transducers, Myd88 and TRIF, which activate signaling cascades ultimately leading to the activation of NF-κB and to the initiation of innate inflammatory immune responses 3. In addition, TLR influence the adaptive immune response both indirectly, by modulating antigen presentation by dendritic cells (DC) and directly, by inflecting the T and B lymphocytes that express several TLR 4. Recently, several reports have shown that TLR engagement could also result in the induction of apoptosis. Apoptosis is a form of “programmed cell death” that results from the sequential activation, through proteolytic cleavage, of a family of cysteine proteases, the caspases. Two major apoptotic pathways have been described: the “extrinsic pathway” typically engaged after death receptor ligation and mediated by the initiator caspase-8, and the “intrinsic pathway”, typically triggered by DNA-damage-inducing agents that bring about mitochondrial membrane depolarization, cytochrome c release in the cytoplasm and activation of the initiator caspase-9 (for review see 5). A link between immune defense and apoptosis was reported in plant biology, with cell death being recognized as a strategy to confine pathogens at the site of infection. In mammals, although apoptosis often contributes to microbial pathogenicity, it may also limit innate and adaptive immune responses that would otherwise cause immune pathology. Examples include the activation-induced cell death of effector T lymphocytes, which may prevent the overwhelming innate immune response known as toxic shock syndrome, or the elimination of autoreactive B cells arising as a consequence of somatic hypermutation, which would otherwise trigger autoimmune damage. Interestingly, in both examples the antigen-specific receptors are directly involved in the triggering of apoptosis.
Pathogen-triggered apoptosis is a conserved innate immune defense strategy
While the innate immunity triggered upon engagement of PRR by pathogen products always comprises various degrees of inflammation, it may also rely on apoptosis of infected cells as a way to prevent microbe spreading throughout the entire organism. This strategy has been conserved during evolution. For instance, the hypersensitive response-programmed cell death (HR-PCD) in plants is characterized by the rapid death by apoptosis of plant cells at the site of pathogen infection 6. HR-PCD is typically triggered upon recognition of a pathogen-encoded avirulence protein by a cognate plant resistance Apaf-1-related R protein that fulfills the role of PRR 7. Of note, members of the class 3 subgroup of R proteins show a striking similarity with TLR, as they contain a region of leucine-rich repeats and an N-terminal region with similarity to the N terminus of the Toll and IL-1R (TIR) proteins 8. In Drosophila, where the Toll and Drosophila immune deficiency (Imd) pathways appear to be the major regulators of the immune response 9, fungal or bacterial infection triggers the production of anti-microbial peptides and reactive oxygen species (ROS), the engulfment of microorganisms by blood cells and the entrapment of microorganisms by deposition of melanin. Moreover, Imd was shown to be a death domain protein that can promote apoptosis 10 and dfadd and Dredd, the homologues of mammalian FADD and caspase-8, respectively, both act downstream of Imd and have been implicated in apoptosis 11. Nematodes can also induce a cell suicide program in infected cells, as the innate immune response of Caenorhabditis elegans against Salmonella thyphimurium is dependent on apoptosis mediated by CED-4, a member of the NOD family that is related to Apaf-1 12. This response is initiated by an unidentified PRR, requires intact LPS and is mediated by MAPK signaling pathway 13.
In mammals, both anti-viral and anti-bacterial responses include two reciprocal cellular programs: cell survival with production of protective cytokines 14 and apoptosis to eliminate infected cells. Antiviral defense is triggered by at least three PRR that recognize double-stranded (ds) viral RNA, the protein kinase PKR 15, and two helicase domain-containing proteins, RIG-I and Mda-5, recently shown to initiate apoptosis and/or IFN-I secretion during viral infection 16, 17. The broad range of tools adopted by viruses to prevent or delay the apoptosis of infected cells emphasize the importance of these defense mechanisms 18. Regarding pathogenic bacteria, they also activate PRR that can induce different forms of cell death, including necrosis, apoptosis, pyroptosis, and autophagy, some of which may contribute to host defense 19. For example, detection of specific glycopeptides derived from cell wall peptidoglycans by members of the NOD family of intracellular sensors triggers the activation of caspase-8-dependent apoptosis, thereby enhancing intracellular bacteria removal 20. Likewise, pyroptosis, the caspase-1-mediated form of cell death triggered by NOD family member in Salmonella or Shigella-infected macrophages, combines the elimination of the intracellular bacteria with the alarming effect of proinflammatory cytokines release. Furthermore, PRR-triggered apoptosis can also participate to the defense against extracellular bacteria, as illustrated by the role of TLR4-dependent apoptotic response of alveolar macrophages to pneumolysin in the control of pneumococcal disease 21.
TLR: A family of innate immunity receptors that can trigger cell death
Global gene expression profiling of PBL treated with different TLR ligands showed the expected “inflammatory and pro-survival gene signature” reflecting the transcriptional activation of NF-κB-dependent genes 22. Besides this, however, several genes primarily involved in apoptosis were also up-regulated 23, suggesting a link between TLR activation and apoptosis, which forms the focus of the following paragraphs. To date, pro-apoptotic properties have been established or suggested for seven out of the ten human TLR. TLR2 was the first family member to be described as a death-inducing receptor, either after transfection 24, or directly in macrophages 25, neutrophils 26, trophoblasts 27, Schwann cells 28 and microglia cells 29. In these studies, the involvement of TLR2 in apoptosis induced by cognate ligands was established using blocking anti-TLR2 antibodies or TLR2–/– cells. More recently, the participation of TLR2 in virus- and fungus-triggered apoptosis was also demonstrated 30. TLR3 overexpression induces poly(ADP-ribose) polymerase (PARP) cleavage, a hallmark of caspase-mediated apoptosis 31. Furthermore, the involvement of TLR3 in dsRNA-triggered apoptosis of breast and multiple myeloma human cancer cells was demonstrated by RNA interference 32, 33. Other reports also suggested pro-apoptotic properties of dsRNA, but without formally addressing the direct implication of TLR3 34–36. The long-known LPS-triggered apoptosis (for review, see 37) was only recently demonstrated to depend on TLR4, using C3H/HeJ mice that lack functional TLR4 2, 34, 37. LPS induces apoptosis in macrophages 36, endothelial 37 and microglial cells 38. Chlamydia HSP60 also induces trophoblast cell apoptosis through TLR4 39. In intestinal epithelial cells that highly express TLR5 on their basolateral membrane, purified flagellin simultaneously triggers TLR5-mediated pro-apoptotic and pro-inflammatory signaling 40. Consistently, when activation of either NF-κB or Akt was blocked, TLR5 ligands triggered the apoptosis of epithelial cells 40. Furthermore, intracolonic flagellin administration in mice caused severe apoptosis in colonic pre-sensitized epithelium 41. The TLR7 ligand Imiquimod induces the apoptosis of basal carcinoma cells, of transformed keratinocytes and of melanoma metastases both in vitro and in vivo 42, 43. TLR8 was shown to trigger neuronal apoptosis 44. Lastly, CpG-rich oligonucleotides not only trigger TLR9-mediated death of transfected cells 45, but also of glioma and colon cancer cell lines 46, 47.
The multiple apoptosis signaling pathways triggered by TLR
Among the five TIR domain-containing adaptors involved in TLR signaling, only MyD88 and TRIF function as transducers (reviewed in 48). TIRAP and TRAM act as bridging adaptors, while SRAM negatively regulates TRIF. Apoptosis can result from triggering TLR that use exclusively Myd88 (TLR2, TLR7, TLR8 and TLR9), TRIF (TLR3), or both (TLR4). This indicates that Myd88 and TRIF can activate apoptosis independently and suggests that cross-talk between the two signaling pathways can lead to apoptosis as well.
The Myd88 adaptor and apoptosis
The Myd88-dependent pathway initiated by TLR starts with the recruitment of Myd88 and TIRAP through TIR-TIR homophilic interaction. Myd88 then forms a complex with two members of the IL-1R-associated kinases (IRAK4 and IRAK1) and with the E3 ubiquitin ligase TRAF6. Activated TRAF6 activates the TGF-β-activated kinase 1 (TAK1)-TAB1/2/3 complex leading to the activation of the canonical NF-κB pathway and the MAP kinases. Because overexpression of Myd88 induces very low level of apoptosis 49, its involvement in cell death was suggested to be mostly indirect, through the production of pro-apoptotic intermediates such as TNF-α 50 or NO 29. However, apoptosis triggered by TLR2 and TLR4 (in endothelial cells) appears to depend on Myd88 association with FADD 49.
The TRIF adaptor and apoptosis
TRIF is recruited directly or through the TRAM adaptor to TLR3 and TLR4, respectively. The C-terminal region of TRIF then interacts with the receptor interacting protein 1 (RIP1), a serine/threonine kinase that can enhance survival through NF-κB and MAPK activation, but that can also promote apoptosis by recruiting FADD and by activating caspase-8 51. Interaction of the N-terminal region of TRIF with TRAF6 also leads to the activation of NF-κB, while the binding of TRAF3 triggers the phosphorylation of IRF3 and the secretion of IFN-β. Transfection experiments have shown that TRIF overexpression in macrophages and fibroblasts is sufficient to trigger apoptosis 36, 51, while TRIF deficiency protects murine macrophages against poly(I:C)- and LPS-induced apoptosis 34, 36. Moreover, TLR3-triggered apoptosis of macrophages and breast cancer cells strictly depends on TRIF 33, 36 like TLR4-induced macrophage apoptosis, which is independent of Myd88 36.
Different pathways can signal apoptosis from TLR
Different signaling pathways can lead to apoptosis depending on the TLR under consideration. TLR2 recruits to its TIR domain both the bridging adaptor TIRAP and the signaling transducer MyD88 (Fig. 1A). In turn, the MyD88 death domain (DD) can associate with the DD of FADD, which in turn recruits caspase-8 via its death effector domain (DED). Activated caspase-8 cleaves and activates caspase-3, which then executes the apoptotic program 49. In some conditions, the autocrine secretion of inflammatory factors such as TNF-α 52 or the up-regulation of FasL 27 may contribute to TLR2-triggered apoptosis. Moreover, TLR2 activation induces ROS generation 24 and activation of apoptosis signal-regulating kinase 1 (ASK1). The ensuing sustained p38 MAPK phosphorylation leads to the activation of NF-κB and AP-1 as well as to the enhancement of cell death 53.
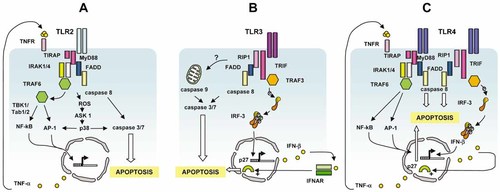
Different signaling pathways link TLR2, 3 and 4 to apoptosis. (A) TLR2 recruits FADD through Myd88 and activates caspase 8. It also upregulates the NF-κB/AP-1-dependent expression of FasL and the production of TNF-α that both contribute to cell death. (B) TLR3 recruits FADD through TRIF/RIP1 and activates caspase-8. It also induces the IRF3-dependent secretion of IFN-I which prevents cell cycle entry through stabilization of p27. The participation of the mitochondrial apoptotic pathway remains uncertain. (C) TLR4 recruits and activates caspase-8 through Myd88 and/or TRIF/RIP1. It may also use FasL upregulation and autocrine secretion of TNF-α and IFN-I to enhance cell death.
TLR3-triggered apoptosis appears to combine a direct TRIF-dependent signaling pathway with the indirect pro-apoptotic effect of IFN-I (Fig. 1C). Upon ligand recognition, TLR3 recruits TRIF via TIR-TIR domain interaction. TIRF is indeed strictly required for apoptosis. TLR3-triggered apoptosis appears to be initiated primarily by caspases-8 and executed by caspase-3 51. The protein kinase RIP-1, which interacts with TRIF through its RHIM domains, plays a pivotal role in engaging FADD and caspase-8 35. However, several aspects of the signal transduction from TLR3 to apoptosis, including the potential role NF-κB remain unclear 33, 35. DsRNA-triggered apoptosis was inhibited by p65 NF-κB suppression in breast cancer cell lines 33, but was independent of NF-κB transcriptional activity in hepatoma cells 54. TLR3 also stimulates IFN-β production through TRIF-mediated activation of IRF3/7 transcription factors 55. A role for IFN-I in TLR3-mediated apoptosis was suggested by the contribution of STAT-1 to dsRNA-induced apoptosis in primary pancreatic beta-cells 56, and by the observation that the paracrine/autocrine secretion of IFN-β in response to TLR3 triggering was necessary but insufficient for apoptosis to occur in human breast cancer cells 33. Insight into the mechanisms of IFN-I contribution to TLR3-induced apoptosis was recently provided by showing how this cytokine inhibits TLR3-stimulated cell cycle entry and favors cell death by antagonizing the TRIF-dependent and CDK-cyclin/AKT-mediated phosphorylation and subsequent degradation of p27kip1 57.
Data regarding TLR4-triggered apoptotic pathways are more complex, since both the adaptors involved and the nature of the programmed cell death (extrinsic versus intrinsic) appear to vary with cell type. On one hand, the well-characterized LPS-induced apoptosis of endothelial cells is mediated by Myd88 and relies mainly on the extrinsic, death receptor pathway. It is indeed initiated by the recruitment of TIRAP 58 and Myd88 59 to TLR4, which triggers a signaling cascade involving FADD, IRAK-1, TRAF6 and c-Jun NH2-terminal kinase (JNK) 59, 60. It culminates with the activation of caspases-8 and -3. Similar to the prototypic death receptor TNFα-R, apoptosis is inhibited by cFLIP 37. However, LPS exposure also increases Bax and p53 expression in endothelial cells 61, therefore suggesting the contribution of the intrinsic apoptotic pathway in TLR4-induced, Myd88-mediated cell death. On the other hand, in macrophages, TLR4-induced apoptosis is independent of Myd88 but is mediated by TRIF and involves cytochrome c release into the cytosol and caspase-9 activation, without detectable activation of caspase-8 34. However, in another study analyzing the involvement of TRIF in TLR4-triggered cell death in macrophages 36, the overexpression of this TIR-containing adapter induced apoptosis through the extrinsic pathway, since it required FADD and was independent on Bcl-2, as described by others in fibroblasts 35, 51. Nevertheless, it must be stressed that triggering cell death through the endogenous TLR4-TRIF-FADD pathway in macrophages induces cleavage of Bid by caspase-8 36, which potentially leads to activation of the mitochondrial pathway, as shown in murine macrophages where LPS induces caspase-9 activation 62. Lastly, LPS-induced cell death of microglial cells appears to result from a cooperation between Myd88 (for caspase activation) and TRIF (for induction of IFN-β and NO production) downstream of TLR4 38. It is, therefore, difficult to assign a unique apoptotic pathway to TLR4, and it is difficult to ascertain whether the different apoptotic pathways triggered by this receptor depend only on the cell type, or whether experimental parameters such as transfected versus endogenous molecules and metabolic conditions also determine the intracellular cascade that ultimately lead to cell death.
Lastly, when TLR9-transfected fibroblasts were exposed to CpG ODN, de novo expression of pro-apoptotic genes resulted in activation of Bax and caspase-3 followed by apoptosis and/or sensitization to apoptosis by other stimuli 45. Although both TLR2 and TLR9 rely on the same single transducing adapter Myd88, the signaling pathways to apoptosis downstream of these two receptors are clearly distinct, as FADD and caspase-8 are required in TLR2-induced but are not involved in TLR9-dependent apoptosis 45, 49.
TLR-induced apoptosis in cancer therapy
BCG has been used for over 30 years to treat superficial bladder tumors 63, but it was only recently that TLR2 and TLR4 ligands present in the mycobacterial cell wall were shown to make up the active components of this treatment 64. The use of molecularly identified TLR agonists in cancer therapy are under intense scrutiny and several phase I and phase II clinical trials are underway (reviewed by Kanzer et al. 65). The antitumor clinical activity of TLR ligands may be brought about by several mechanisms, including the direct killing by apoptosis of TLR-positive tumors, which provides the rationale for screening TLR agonists for pro-apoptotic activity against cancers 66. However, several questions must be addressed to optimize the utilization of TLR-induced apoptosis for cancer treatment.
First, the capacity of tumor cells to respond to TLR ligands minimally requires the presence of the receptor, but the repertoire of TLR constitutively or inducibly expressed in different types of tumors remains poorly documented. Second, the sensitivity of TLR-positive tumors cells to TLR-triggered apoptosis appears to vary not only with the type of tumor but even between individual tumors of the same origin. The level of expression of TLR by tumor cells may represent a determinant factor for the sensitivity to apoptosis. In this regard, a retrospective analysis of an old clinical trial of the TLR3 ligand poly-AU in breast cancer patients revealed an improved 20-year survival rate in the subset of patients whose tumor cells overexpressed TLR3 67. Our own observations show that constitutive or inducible TLR3 expression on melanoma cancer cell lines was required for apoptosis, albeit insufficient by itself to predict sensitivity versus resistance to apoptosis in vitro 68. Third, although apoptosis has been clearly demonstrated downstream of TLR in different cell types (see above), recent data showed that, under certain circumstances, stimulation of these receptors in cancer cells might be detrimental to the host either by dampening the immune responses as described for TLR4 69 or by promoting tumor growth directly through TLR2 70 or indirectly through TLR3 32. Cancer cells often up-regulate pro-survival molecules, including Bcl-2 or PI3K, which render them less susceptible to apoptosis. Therefore, whether a tumor cell will survive or die after TLR triggering will depend on the balance of antagonizing signals that are tuned by key molecular switches such as NF-κB 71 and RIP-1 72. The dual capacity of TLR to activate pro-survival and pro-apoptotic pathways is reminiscent to what has been described for TNFR, which efficiently activates apoptosis only in particular situations (prolonged stimulation, inhibition of NF-κB, inhibition of MAPK). These considerations thus suggest the potential benefit of combining TLR agonists with inhibitors of p38 or PI3K, two kinases contributing to survival downstream of TLR 73, or with chemotherapy or radiotherapy, to engage synergistic mechanisms of apoptosis. Finally, the immunogenicity of dying cells, which varies with the death-inducing factor, appears to be correlated with the rapid translocation of calreticulin to cell membrane 74 and with the release of HMGB1 out of the cell 75, 76. Preliminary data suggest that TLR-induced cell death can be accompanied by calreticulin cell surface expression (B.S., unpublished data), and treatment of murine macrophages with either LPS or poly(I:C) or plasmacytoid DC with CpG ODN induces the secretion of HMGB1 62, 77. If the immunogenicity of TLR-induced cell death were to be confirmed, this could represent an important therapeutic advantage. Indeed, TLR agonists should then be considered as multifunctional adjuvants for cancer therapy, combining the induction of immunogenic cell death, the provision of tumor antigens in situ and the promotion of cross-priming by DC 71. Those additive effects might improve quantitatively and qualitatively the antitumor immune response.
Concluding remarks
The TLR turn out not to differ from other immune receptors in their dual capacity to launch both cell defenses and cell death. While our understanding of the signaling cascades from TLR down to cell activation has rapidly progressed during the last few years, the molecular pathways leading to TLR-induced apoptosis remain ill defined. Current data suggest that the latter are as diverse as the former, varying with the TLR, with the cell type and with the metabolic condition of the cell. Moreover, TLR triggering leads to pro-survival and pro-apoptotic signals that may have different relative impact on normal versus transformed cells, with an increased tendency to death in cancer cells. Whether and how those new twists in our understanding of TLR biology could be translated into targeted therapies for cancer represent exciting challenges for the near future.
Acknowledgements
The authors want to thank Dr. T. Renno, G. Jego and D. Rimoldi for discussion and advices during the writing of the manuscript. This work was partially supported by grants from Oncosuisse (B.S.), from the Association de Recherche contre le Cancer (B.S.) and from the “Cancer Immunology and Immunotherapy project” of the European Union, FP6 (P.R.).
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commencial conflict of interest.




