A novel regulatory role of gp49B on dendritic cells in T-cell priming†
Abbreviations
BMMCbone marrow-derived cultured mast cells
LILRleukocyte Ig-like receptor
PIRpaired Ig-like receptor
Tconv cellconventional T cell
Abstract
Dendritic cells (DC) play pivotal roles in the induction and regulation of both innate and acquired immunity. DC express several cell-surface immune inhibitory receptors. However, little is known about their potential immunoregulatory functions in the context of T-cell activation. Here we report that murine gp49B, a member of the immunoglobulin superfamily, harboring immunoreceptor tyrosine-based inhibitory motifs, is expressed on DC and downregulates cellular activity to prevent the excessive activation of T cells in vitro and in vivo. Bone marrow-derived DC (BMDC) from newly generated gp49B-deficient (gp49B−/−) mice induced enhanced proliferation and IL-2 release of antigen-specific CD4+ and CD8+ T cells compared with BMDC from wild-type mice, in a cell–cell contact manner. The enhanced proliferation by gp49B−/− BMDC was also observed in allogeneic CD4+ and CD8+ T cells. Moreover, the transfer of allogeneic BALB/c splenocytes into C57BL/6 gp49B−/− mice induced severe acute graft-versus-host disease with an augmented upregulation of CD86 on CD11c+ splenic gp49B−/− DC, while transfer of C57BL/6 gp49B−/− splenocytes into BALB/c mice did not, suggesting the exacerbation of the disease was due, at least in part, to augmented activation of recipient gp49B−/− DC. These findings demonstrate a novel regulatory role of gp49B in the function of DC.
Introduction
Dendritic cells (DC) are professional antigen-presenting cells that recognize a diverse repertoire of antigens and present them to naïve T cells for the induction of acquired immunity 1. DC are also related to innate immunity by producing cytokines such as both type I and II IFN, or by activating NK cells 2, 3. In addition, recent reports revealed that a subset of DC maintains peripheral tolerance in the steady state 4, showing that DC play a pivotal role in the induction of immune responses including autoimmunity.
On the DC surface, a variety of structurally different immunoreceptors that regulate DC and T-cell functions have been found, including CD80, CD86, CD40, and various Ig superfamily and lectin family molecules 5. Among them, a group of paired Ig superfamily receptors, termed Ig-like receptors, have been attracting the interest of many researchers because they are expressed on various subsets of DC and are considered to contribute to the development or activation of DC 6-10. Generally, Ig-like receptors are subdivided into two types, namely activating and inhibitory receptors, and in some cases they are expressed on hematopoietic cells in a pair-wise manner 11-14. Most activating receptors associate with homodimeric subunits containing ITAM, such as the FcR common γ chain and KARAP/DAP12 15, 16, and deliver positive signals into the cells through interaction with their ligands 17. For instance, the uptake of immune complexes through activating FcR for IgG augments DC maturation, leading to efficient antigen presentation 18-20. On the other hand, the inhibitory receptors harbor ITIM in their cytoplasmic portions and initiate inhibitory signaling by recruiting protein phosphatases, such as SHP-1 or SHIP, to phosphotyrosylated ITIM 13. Therefore, the deletion of inhibitory receptors leads to enhanced antigen presentation 21, 22. DC lacking paired Ig-like receptor (PIR)-B induce polarized Th2 responses 8, and PIR-B-deficient mice show an exacerbated lethal GVH disease due to augmented activation of recipient DC 23. In addition, overexpression of inhibitory receptors such as leukocyte Ig-like receptor (LILR)B2 or LILRB4 (also known as ILT4/LIR-2/MIR-10/CD85d or ILT3/LIR-5/HM18/CD85k, respectively) reduces T-cell activation 7. Although some of the functions of these receptors on DC in T-cell responses have been determined, the roles of many others in the physiologic regulation of DC as well as of immune responses remain unclear.
Murine gp49 molecules are 49-kDa Ig-like receptors expressed on the surfaces of cells involved in innate and acquired immunity such as NK cells, mast cells, neutrophils, macrophages, and activated T cells 12, 24, 25. The two major subtypes, gp49A and gp49B, are encoded by two different genes adjacent to each other 12, 26, 27. gp49A has a short cytoplasmic tail and does not harbor any specific motif for signal transduction nor bind to the FcR common γ subunit, while gp49A is considered to be expressed as a homodimer and elicits activation signals in vitro 28. In contrast, gp49B contains two ITIM in its cytoplasmic portion, and inhibits activation signals upon cellular engagement with other activating receptors by recruiting SHP-1 to phosphotyrosylated ITIM 27, 29. gp49B binds to integrin αv and β3 chains (αvβ3), which are expressed on a variety of cells including platelets, lymphocytes, and endothelial cells 30-33. Colligation of gp49B with the high-affinity receptor for IgE or the type I FcγR by antibodies inhibits degranulation of mast cells or production of TNF-α by macrophages, respectively 29, 34. Recently, it was reported that gp49B expression can be induced on activated T cells and NK cells stimulated by IL-2, or viral or bacterial infection, and that it regulates IFN-γ production and NK cell-mediated cell lysis 35, 36. In in vivo studies, gp49B-deficient (gp49B−/−) mice exhibited increased severity of passive and active cutaneous anaphylaxis 26. gp49B−/− mice also showed a severe thrombohemorrhagic response due to increased neutrophil reaction to LPS 25. These results showed that gp49B has a non-redundant role in the regulation of immune responses. It remains unclear, however, as to whether or not gp49B is expressed on DC and regulates DC activation.
In the present study, we demonstrate that gp49B, but not gp49A, is expressed on bone marrow-derived DC (BMDC) and splenic DC. BMDC from our newly generated gp49B−/− mice showed enhanced antigen presentation through direct interaction with T cells. Moreover, gp49B−/− mice showed an accelerated, lethal acute GVH disease due to the augmented activation of recipient DC. These results indicate that gp49B negatively regulates DC functions in vitro and in vivo.
Results
BMDC and splenic DC express gp49B
To investigate the inhibitory potential of gp49B in vivo, we generated gp49B−/− mice by standard gene targeting techniques. We constructed a targeting vector for which the first exon and the promoter region had been deleted (Fig. 1A). Southern blot analysis of genomic DNA confirmed disruption of the gp49B gene in the targeted mice (Fig. 1B). We confirmed that the targeting was specific for gp49B by analyzing the expression of the mRNAs for gp49A and gp49B in BM-derived cultured mast cells (BMMC) (Fig. 1C). The targeted gp49B−/− mice were grossly normal and fertile as described 37, and were backcrossed to the C57BL/6 (B6) genetic background for more than 12 generations.
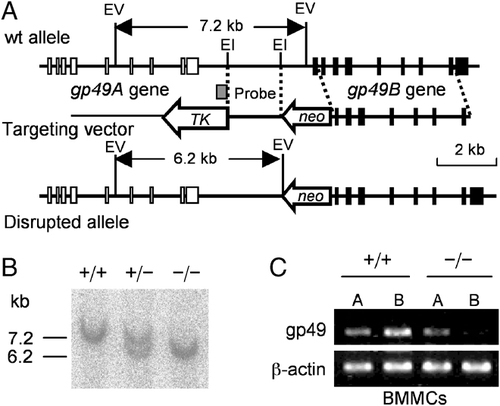
Targeted disruption of the gp49B gene. (A) Structure of the gp49 gene (top), targeting vector (middle), and disrupted allele (bottom). Exons are indicated as filled boxes. The neomycin resistance gene (neo) and thymidine kinase (TK) gene are shown. The probe used for Southern blotting is indicated as a shaded box. The 1.7-kb fragment containing the promoter region and the initial codon of the gp49B gene was replaced by neo. The targeted mice were grossly normal and fertile, as described by others 26. (B) Southern blot analysis of EcoRV-digested genomic DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mutant mice. The wild-type allele is a 7.2-kb fragment, and the target allele is a 6.2-kb one. (C) RT-PCR analysis of the expression of gp49A and gp49B in BMMC from wild-type (+/+) and gp49B−/− (−/−) mice. β-Actin was used as the control.
Although it has been shown that gp49A or gp49B is expressed on myeloid cells including mast cells, macrophages and neutrophils, and on activated T cells and NK cells 12, 25, it remains to be determined whether or not gp49A or gp49B is expressed on DC. We examined the surface expression of gp49A and gp49B on BMDC and splenic CD11c+ DC either from B6 or gp49B−/− mice as well as on the BMDC after stimulation with LPS or addition of OVA by flow cytometry with mAb H1.1, which recognizes a common epitope on gp49A and gp49B 35. As shown in Fig. 2A and B top row, apparent gp49A/B expression was observed clearly on B6 BMDC and weakly on splenic DC. However, no gp49A expression was detected on gp49B−/− BMDC and splenic DC, even after LPS stimulation of, or OVA administration to BMDC (Fig. 2A and B), showing that BMDC and splenic DC preferentially express gp49B on their surface. We also assessed various surface markers on gp49B−/− BMDC and B6 BMDC in the 24 h presence of LPS or OVA. As shown in Fig. 2B second row, there was no difference in maturation marker I-A/E, CD80, or CD86 expression between BMDC from gp49B−/− and B6 mice. This was also the case for BMDC after OVA administration: I-A/E, CD80, CD86, CD40, or ICAM-1 expression was not different between BMDC from gp49B−/− and B6 mice (Fig. 2B, third and bottom rows). The expression level of gp49B ligand 30 integrin αvβ3 was also comparable between gp49B−/− and B6 BMDC after OVA stimulation (Fig. 2B, bottom row).
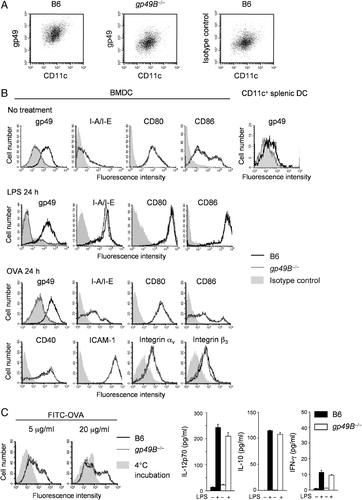
Expression of gp49B on DC and characterization of BMDC from gp49B−/− mice. (A) Flow cytometric analysis of gp49B expression on BMDC from B6 WT (left) and B6 gp49B−/− mice (middle). BMDC were double-stained with CD11c and H1.1 mAb. Right panel shows BMDC stained with isotype-matched control mAb (Hamster IgG3) and CD11c. (B) Expression of gp49B on BMDC and splenic CD11c+ DC, and various cell-surface molecules on BMDC before or after stimulation with LPS or administration of OVA. BMDC at day 6 culture or freshly isolated splenic DC from B6 (black lines) or gp49B−/− (gray lines) mice were stained. In splenic DC staining, MFI values were as follows: B6, 21.0; gp49B−/−, 10.4; isotype control, 13.8. BMDC were incubated with LPS (second row) or OVA (third and bottom row) for 24 h and stained for gp49A/B (H1.1), MHC class II (I-A/I-E; M5/114.15.2), CD80 (16-10A1), CD86 (GL1), CD40 (3/23), ICAM-1 (3E2), integrin αv (RMV-7), or integrin β3 (2C9.G2), followed by flow cytometric analysis. Filled gray lines represent cells stained with isotype-matched control mAb. Representative data from two or three independent experiments with similar results are shown. (C) Antigen uptake by BMDC. BMDC from B6 (black lines) or gp49B−/− (gray lines) mice were pulsed with 5 mg/mL or 20 mg/mL of FITC–OVA at 37°C or 4°C for 3 h. After washing, FITC–OVA uptake was monitored by flow cytometry. Filled gray lines represent cells incubated at 4°C. Representative data for three independent experiments with similar results are shown. (D) Cytokine production by BMDC. BMDC from B6 (closed columns) or gp49B−/− (open columns) mice were incubated with OVA in the presence or absence of LPS for 24 h. Supernatants of the culture medium were assessed by means of the multi-plex cytokine assay system. Data are shown as means±SD. Representative data from three independent experiments with similar results are shown.
Next, to determine the ability of DC as to the internalization of antigen, BMDC were cultured with FITC-conjugated OVA. The uptake of OVA by DC was measured by flow cytometry. As shown in Fig. 2C, the fluorescence intensity for OVA incorporation after 3 h incubation was comparable between gp49B−/− and B6 BMDC, indicating that the antigen uptake was not defective in gp49B−/− BMDC. We also examined cytokine production from gp49B−/− and B6 BMDC during the antigen uptake. Cytokine levels were measured at 48 h after administration of OVA or OVA plus LPS. As is shown in Fig. 2D, the production of Th1/Th2 polarizing cytokine, IL-10, IL-12, or IFN-γ was comparable between gp49B−/− and B6 BMDC in the presence of OVA or OVA plus LPS. When we stimulated BMDC with anti-CD40 mAb in the presence of OVA, gp49B−/− BMDC produced various cytokines, including IL-12p70, IL-10, TNF-α, IFN-γ, and IL-2, whose levels were comparable to those of B6 BMDC (data not shown). These data indicate that the deletion of gp49B does not impair the maturation of, antigen uptake by, and cytokine production by BMDC.
DC lacking gp49B show augmented antigen presentation to OT-I and OT-II T cells
To investigate MHC class I or class II-restricted antigen presentation by gp49B−/− DC, we first checked the expression levels of gp49B ligand integrin αvβ3 chains on OT-II CD4+ T cells and OT-I CD8+ T cells by flow cytometry. As shown in Fig. 3A, a substantial proportion of, or most of the OT-II CD4+ T cells expressed the integrin αv or β3 chain, respectively. On the other hand, while the majority of OT-I CD8+ T cells showed expression of the integrin β3 chain, their αv chain expression was low, as essentially described previously 38, suggesting that this gp49B ligand is more abundant in OT-II CD4+ T cells than OT-I CD8+ T cells.
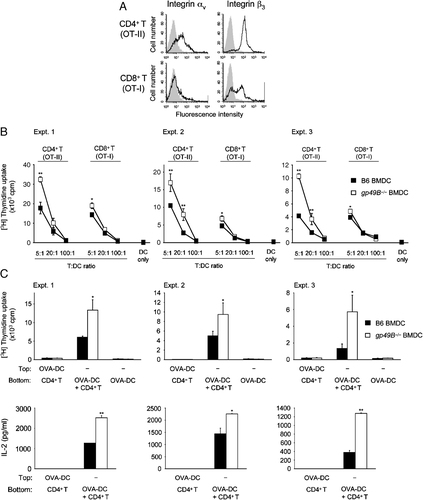
Augmented activation of antigen-specific T cells by gp49B−/− BMDC. (A) Freshly isolated OT-II CD4+ or OT-I CD8+ T cells were stained for integrin αv or integrin β3 chain (solid lines), followed by flow cytometric analysis. Filled gray lines represent cells stained with isotype-matched control mAb. Representative data from three independent experiments with similar results are shown. (B) MHC class II or class I-restricted antigen presentation. OT-II CD4+ or OT-I CD8+ T cells (1×105) were cultured with the indicated ratio of OVA-pulsed B6 BMDC (closed squares) or gp49B−/− BMDC (open squares), and then proliferation was determined by [3H]thymidine uptake on day 3. Data from three independent experiments are shown with mean±SD of triplicate samples from each experiment. *p<0.05, **p<0.01. (C) T-cell proliferation stimulated by B6 and gp49B−/− BMDC is dependent on direct DC–T interaction. A total of 1×104 OVA-pulsed B6 BMDC (closed columns) or gp49B−/− BMDC (open columns) were co-cultured with 5×104 OT-II CD4+ T cells in transwell plates. OT-II CD4+ T cells were placed in the bottom chambers. BMDC were placed into the top or bottom chambers. After 3 day co-culture, the upper chambers were removed, and proliferation was determined by [3H]thymidine uptake and IL-2 concentration was determined by ELISA, respectively. Data from three independent experiments are shown with mean± SD of triplicate samples from each experiment. *p<0.05, **p<0.01. nd, not detected.
Next, OVA-primed and irradiated BMDC from gp49B−/− and B6 mice were co-cultured with OT-II CD4+ T cells and OT-I CD8+ T cells at the indicated T-cell: DC ratios, and the proliferative response of T cells was determined as [3H]thymidine uptake on day 3. gp49B−/− BMDC showed augmented antigen presentation to both OT-II CD4+ T cells and OT-I CD8+ T cells compared with B6 BMDC (Fig. 3B). In particular, gp49B−/− BMDC significantly induced the OT-II CD4+ T-cell proliferative response. Employing the transwell assay, we confirmed that the increased proliferative response of CD4+ OT-II T cells caused by gp49B−/− BMDC was dependent on the direct DC–T-cell interaction (Fig. 3C). OT-II CD4+ T cells were placed in the bottom chambers of the transwell plates, and then OVA-pulsed gp49B−/− or B6 BMDC were added to either the bottom chambers or the top chambers. After 3-day culture, proliferation and the IL-2 concentration in the supernatant were assessed. The proliferative response and IL-2 production were enhanced when gp49B−/− BMDC were placed in direct contact with OT-II CD4+ T cells, but not in the top chambers of the transwell plates.
DC lacking gp49B induce augmented stimulation of allogeneic T cells
To further evaluate the roles of gp49B in DC functions, we also analyzed the ability of gp49B−/− BMDC to stimulate allogeneic CD4+ T cells, CD8+ T cells, or splenocytes by means of a primary MLR assay. Allogeneic CD4+ or CD8+ T cells, or total splenocytes isolated from BALB/c mice were co-cultured for 3 days with irradiated gp49B−/− and B6 BMDC at the indicated T:DC ratios, in which allo-stimulation was determined as proliferation. As shown in Fig. 4, gp49B−/− BMDC induced enhanced proliferation of allogeneic CD4+ T cells, CD8+ T cells, and splenocytes compared with B6 BMDC. These results indicate that gp49B−/− BMDC augment both syngeneic (Fig. 3B and C) and allogeneic (Fig. 4) T-cell responses.
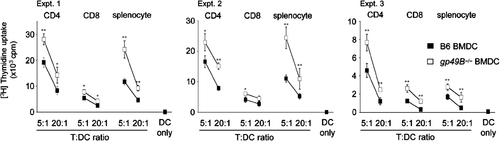
DC lacking gp49B induce augmented stimulation of allogeneic T cells. CD4+, CD8+ T cells, or total splenocytes (5×104) from BALB/c mice were cultured with the indicated ratio of gp49B-sufficient B6 BMDC (closed squares) or gp49B−/− BMDC (open squares). Data from three independent experiments are shown with mean±SD of triplicate samples from each experiment. *p<0.05, **p<0.01.
Treg-mediated suppression of proliferation was not impaired
Recent reports indicated the involvement of putative human relatives of gp49B, LILRB2, and LILRB4, in CD4+CD25+ regulatory T-cell (Treg) priming 39. Since there were no differences in the antigen uptake, maturation, or cytokine production between gp49B−/− and B6 BMDC (Fig. 2A–D), we examined whether the enhanced T-cell proliferation caused by gp49B−/− BMDC was due to insufficient Treg cell activation or Treg cell-mediated suppression. OVA-specific conventional CD4+CD25− T (Tconv) cells isolated from OT-II transgenic mice and Treg from B6 mice were mixed with irradiated OVA-pulsed BMDC at various ratios, and then T-cell proliferation was assessed. As shown in Fig. 5, the proliferative responses decreased as the number of Treg increased. However, the proliferation curve profile showed no gross difference between gp49B−/− and B6 BMDC. In addition, the proportions of Tconv and Treg among the splenocytes from gp49B−/− and B6 mice were comparable, as assessed by flow cytometry (data not shown). These findings suggest that augmented T-cell activation by gp49B−/− BMDC does not correlate with the altered strength of Treg-mediated suppressive effect.
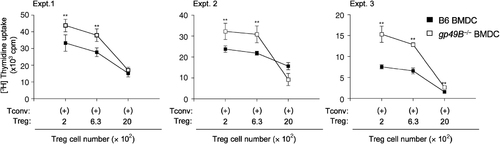
Treg-mediated suppression of conventional T-cell proliferation was comparable. OT-II CD4+CD25− conventional T (Tconv) cells (2×104) were mixed with the indicated numbers of B6 CD4+CD25+ Treg, followed by co-culture with 1×104 OVA-pulsed B6 BMDC or gp49B−/− BMDC (open squares). Proliferation was determined by [3H]thymidine uptake on day 3. We did not observe a reduced suppressive activity of Treg in mixed culture with Tconv cells and gp49B−/− BMDC. Data from three independent experiments with mean±SD of triplicate samples from each experiment. **p<0.01.
Exacerbated GVH disease in gp49B−/− mice
Recent reports suggested that the close relatives of gp49B on DC, murine PIR-B, and human LILRB, participate in the regulation of GVH disease 23, 39. It is well accepted that the recipient's DC trigger a GVH reaction through interaction with allo-reactive donor CD4+ and CD8+ T cells 40. Thus, to examine the possibility of negative regulation by gp49B in the allogeneic reaction in vivo, we employed a lethal GVH disease model, for which sublethally irradiated gp49B−/− or B6 (H-2b) recipient mice received BALB/c (H-2d) splenocytes intravenously. As shown in Fig. 6A, all gp49B−/− recipient mice developed acute GVH disease in a few days, and died within 10 days, while B6 recipients developed less severe GVH disease and half of the animals survived for at least 20 days. When we measured IFN-γ production by host splenocytes stimulated with T-cell-depleted syngeneic or allogeneic splenocytes, we observed that the IFN-γ production was significantly higher in splenocytes from gp49B−/− recipients than those from B6 recipients (Fig. 6B), suggesting that the recipient gp49B−/− DC augment allo-reactive T-cell activation.
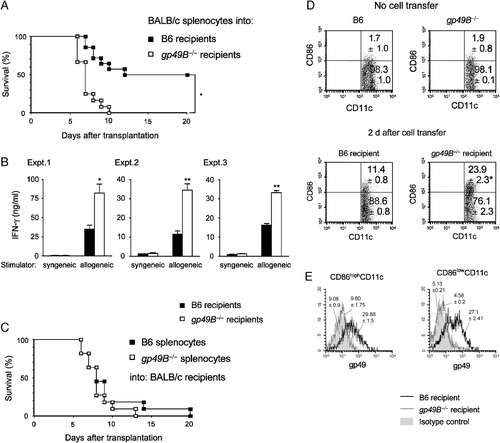
Induction of lethal GVH disease in gp49B−/− mice. (A) Survival curves for gp49B-sufficient B6 (closed squares, n=14) or gp49B−/− recipients (open squares, n=12) with GVH disease. BALB/c splenocytes (5×107) were injected into 9 Gy-irradiated B6 or gp49B−/− recipients. Mortality was assessed every 24 h for 20 days. *p<0.05, gp49B−/−versus B6 mice. (B) IFN-γ production of allo-reactive splenocytes after GVH disease induction. BALB/c splenocytes were injected into B6 or gp49B−/− recipients as in (A). Splenocytes were harvested (n=3/group) from B6 or gp49B−/− recipients at 4 days after transplantation. A total of 1×105 splenocytes (responder) were cultured with 15 Gy-irradiated 2×105 T-cell-depleted splenocytes (stimulator) from syngeneic BALB/c mice or allogeneic B6 mice for 24 h. IFN-γ in the supernatant was measured by ELISA. IFN-γ production was higher in gp49B−/− recipients than in B6 recipients. Data from three independent experiments are shown with mean±SD of triplicate samples from each experiment. *p<0.05, **p<0.01. (C) Lethal GVH disease induced by B6 (closed squares) or gp49B−/− (open squares) splenocytes in BALB/c recipients (n=11 for each splenocyte transfer). Donor B6 or gp49B−/− splenocytes (5×107) were injected into 6 Gy-irradiated BALB/c recipients. Mortality was assessed every 24 h for 20 days. (D) Surface expression of CD86 on H-2Dd-negative CD11c recipient splenocytes was analyzed before (upper) and 2 days after (lower) transplantation. Representative data for three independent experiments with similar results are shown. Numbers indicate the percentages of cell populations, expressed as means±SD% from three independent experiments. *p<0.05. (E) Flow cytometric analysis of gp49B expression on CD86high (left) or CD86low (right) H-2Dd-negative CD11c+ splenocytes isolated from B6 (black line) or gp49B–/– (gray line) recipients on day 2 after transplantation. Numbers above the histograms represent means±SD of MFI values from three independent experiments.
On the other hand, it has been reported that gp49B expression is induced on activated T cells after IL-2 stimulation or viral infection 36. Therefore, to check the involvement of inducible gp49B on donor T cells in GVH disease, we next examined GVH disease with the opposite combination, i.e. donor gp49B−/− or B6 splenocytes were transferred to sublethally irradiated BALB/c recipients. As shown in Fig. 6C, the mortality rate was not different between the recipient mice that received gp49B−/− splenocytes and those that received B6 splenocytes. These findings suggest that the recipient gp49B−/− DC contributed to the increased mortality of the gp49B−/− recipients, and also indicate that gp49B, which might be expressed on allo-reactive, activated T cells, did not affect the function of the T cells in this GVH disease model.
Finally, to verify the involvement of recipient DC in the exacerbated GVH disease, we examined the level of expression of co-stimulatory molecule CD86 on recipient splenic DC before and after the induction of GVH disease (Fig. 6D, Table 1). CD86 expression was not significant on either gp49B−/− or B6 splenic DC before the induction of GVH disease (Fig. 6D upper panels). In contrast, concomitant with DC activation after the transfer of allogeneic splenocytes, the expression of CD86 was elevated on recipient gp49B−/− or B6 splenic DC (Fig. 6D lower panels compared with upper panels). In particular, the population of H-2Dd− CD86+ DC was significantly increased in gp49B−/− recipients compared with B6 recipients (Fig. 6D lower panels), showing the augmented DC activation in gp49B−/− recipients. These splenic CD86highCD11c+ and CD86lowCD11c+ DC populations from B6 recipient mice were demonstrated to be positive for gp49B surface expression as shown in flow cytometric profiles of Fig. 6E. Collectively, these data indicate that the GVH disease induced in gp49B−/− mice was greater than in B6 recipients associated with augmented activation of recipient DC with excessive activation of donor cells possibly including allo-reactive cytotoxic T lymphocytes.
| CD86high | CD86low | |
|---|---|---|
| B6 | 1.7±1.0 | 98.3±1.0 |
| gp49B−/− | 1.9±0.8 | 98.1±0.1 |
| B6 recipient | 11.4±0.8 | 88.6±0.8 |
| gp49B−/− recipient | 23.9±2.3* | 76.1±2.3 |
- a a)Data are the mean±SD (%) of three independent experiments of flow cytometric analysis in Fig. 6D. Statistical analyses were performed using Student's t test: *p<0.05 between B6 and gp49B−/− recipients' CD86high splenic CD11c+ cells.
Discussion
To demonstrate the inhibitory role of gp49B on various cells in the immune system such as NK cells and mast cells, we generated gp49B−/− mice and backcrossed them with B6. While conducting at our backcross work, we noted studies on targeted disruption of the gp49B gene from two other groups, in which gp49B−/− mice did 30 or did not 37 show altered NK cell and mast cell development and functions. On the other hand, Katz and colleagues further reported that gp49B−/− mice were sensitive to LPS-induced microangiopathy 25, and IgE- or stem cell factor-induced anaphylaxis 30 or tissue swelling 41, respectively, due to the enhanced function of gp49B-deficient neutrophils and mast cells. Considering these situations, we focused on the versatility of the inhibitory role of gp49B in other myeloid cells such as DC both in vitro and, particularly, in vivo.
DC express various immune receptors via which positive or negative signaling regulates the development, maturation, and activation of DC, leading to modulation of the functional status of DC 10. Although several inhibitory Ig-like receptors including LILRB1/B2/B4 and PIR-B contribute by maintaining adequate DC development or functions 8, 39, 42, 43, little is known about their physiological roles. In addition, it should be further investigated whether these inhibitory receptors regulate cellular responses at the interface between DC and T cells. Our present study demonstrated for the first time that a murine inhibitory Ig-like receptor, gp49B, is expressed on DC, and that gp49B on DC negatively regulates antigen-specific T-cell activation in vitro and in vivo. Our findings provide a novel insight into the regulatory mechanisms involving gp49B in the antigen presentation phase in vivo, in addition to the existing knowledge on the physiological and pathological roles of gp49B in regulation of adaptive and innate immune responses 25, 30, 41.
Previous studies by others revealed that gp49B attenuates cytokine and chemokine production by mast cells or macrophages 29, 34. On the other hand, our present study demonstrated that, despite the comparable maturation statuses of or cytokine production by gp49B−/− and B6 BMDC, gp49B−/− BMDC showed augmented MHC class I and class II-restricted antigen presentation compared with B6 BMDC in vitro. Our transwell study confirmed that the direct DC–T interaction, at least in the initial phase, was necessary for effective antigen presentation by gp49B−/− DC.
A ligand for gp49B, integrin αvβ3 30, is expressed on T cells 38, and several reports have indicated that integrin αvβ3-mediated signaling can activate T cells 44, 45. Moreover, integrin αvβ3 expression is upregulated on activated T cells 32, suggesting that integrin αvβ3 positively regulates immune responses at the interface between DC and T cells. Our present data showed that gp49B−/− DC can induce the proliferation of CD4+ T cells better than that of CD8+ T cells. CD4+ T cells express a relatively high level of integrin αvβ3 compared with CD8+ T cells. Therefore, the enhanced CD4+ T-cell proliferation may depend on the higher level of expression of the gp49B ligand. It is likely that the regulation of DC activity upon interaction between gp49B on DC and integrin αvβ3 on T cell then negatively feeds back to the T-cell activation. Aimed at obtaining evidence of the interaction between gp49B on DC and αvβ3 on T cells in vitro, we prepared a recombinant gp49B-Fc fusion protein by means of a standard technique. We initially attempted to demonstrate the binding of the recombinant gp49B-Fc to activated T cells as well as to other cultured T-cell lines. However, we failed to obtain any convincing evidence on the binding assessed by flow cytometry (data not shown). Although the DC–T interaction via gp49B–αvβ3 binding might still be a reasonable scenario, it remains to be demonstrated.
Our present data also demonstrated the physiological role of gp49B on DC, i.e. gp49B−/− recipients showed exacerbated GVH disease compared with B6 recipients (Fig. 6A). The donor T-cell–host antigen-presenting cell interaction is essential for triggering of the GVH reaction 40. Therefore, in the induction phase of the GVH reaction, it is likely that gp49B on host DC interacts with donor T cells via integrin αvβ3 or other unidentified ligand(s), and thereby delivers negative signals into the host DC, and then limits the excessive allogeneic T-cell response. Indeed, we found here that after the induction of GVH disease, the population of CD86+ host DC was increased among splenocytes in gp49B−/− recipients (Fig. 6D). CD86 is critical for the activation of allo-reactive T cells 46, 47, gp49B−/− host DC thus further activate donor T cells, leading to the high lethality in gp49B−/− recipients. Although in vitro analyses showed no difference in DC maturation between gp49B−/− and B6 BMDC, the in vivo data provide a possible mechanism by which gp49B negatively regulates DC activation through interaction with T cells. In contrast to the observation in gp49B−/− recipients, gp49B−/− donor T cells could not induce accelerated GVH disease lethality compared with B6 donor T cells (Fig. 6C). Unlike IL-2 stimulation or viral infection, it is possible that gp49B on T cells does not negatively regulate allogeneic responses at least in our GVH disease model. Therefore, gp49B could render DC capable of regulating allogeneic T cells at least via the suppression of DC activation in vivo.
No human gp49B homologue has been identified yet. Phylogram studies on gp49B and related Ig superfamily members by others 48, 49 and us (data not shown) suggest that human LILRB molecules are closely related to gp49B. In particular, LILRB4 and gp49B exhibit common characteristics in that they have two highly homologous C2-type Ig-like domains. In addition, while the murine gp49B gene is located on a different chromosome with no apparent synteny, the genes for human LILRB4 and rat gp49B are located within the syntenic chromosomal region, the leukocyte receptor complex 48, 49, suggesting that LILRB4 is not a perfect counterpart molecule but the closest homologue of gp49B. Regarding the function of LILRB4 on DC, several reports have shown that LILRB4 expression is upregulated on tolerogenic DC 7. Moreover, a soluble recombinant LILRB4 protein can repress T-cell proliferation 50, suggesting that LILRB4 regulates DC activation or immune responses at the interface between DC and T cells. Recent reports have also shown that LILRB4 on tolerogenic DC contributes to the differentiation of Treg 39. Although this is still controversial because other groups have demonstrated that LILRB4 on 1, 25-dihydroxyvitamin D3-treated tolerogenic DC is dispensable for the induction of Treg 51, our present data showed that the deletion of gp49B on DC does not affect the proliferation of Treg (Fig. 5). Unlike gp49B, a ligand for LILRB4 has not been identified. Therefore, further investigation is required as to whether or not gp49B is a functional homologue of LILRB4.
In summary, we found here that gp49B on DC attenuates antigen presentation in vitro and in vivo. It is widely accepted that DC also have an essential role in innate immunity by monitoring for the presence of infectious microorganisms 52. Tissue inflammation induced by infectious agents such as bacteria induces DC migration and maturation 1. Furthermore, Toll-like receptor engagement on DC directs polarization of the T-cell response 53, 54. gp49B suppresses LPS-induced intravascular neutrophil adhesion in vivo 25, gp49B could thus also regulate DC migration to the site of inflammation. The suppressing DC functions via gp49B might be a novel mechanism regulating both innate and adaptive immunity. On the other hand, the gp49B ligand integrin αvβ3 is broadly expressed on various tissues. Although it remains to be determined why gp49B on DC or other immune cells recognizes the ubiquitous ligand, like inhibitory MHC class I receptors such as PIR-B 23 and LILRB1/B2 55, gp49B could maintain an adequate threshold for cellular activation, thereby regulating immune responses including allergy, inflammation, transplantation tolerance, and autoimmunity.
Materials and methods
Generation of gp49B−/− mice
gp49B−/− mice were generated initially with the 129/SvJ and C57BL/6 (B6, H-2b) hybrid background as described 56, and backcrossed with B6 for more than 12 generations. Briefly, the gp49B genomic DNA was isolated from the 129/Sv mouse genomic library (Stratagene, La Jolla, CA). A targeting vector was designed to replace the promoter region and the initial codon of the gp49B gene with a neo (neomycin resistance gene) expression cassette. The targeting vector was electroporated into RW4 embryonic stem (ES) cells and selected with G418 (Invitrogen, Carlsbad, CA) and FIAU (Wako Pure Chemical Industries, Osaka, Japan). Homologous recombinants were identified by Southern hybridization using the 0.4-kb fragment intervening the gp49A and gp49B gene. Southern hybridization detected a 7.2-kb StuI fragment of the endogenous gp49B allele and a 6.2-kb fragment of the targeted allele. The identified targeted ES clones were microinjected into the blastocysts of B6 mice (Charles River Laboratories, Japan; Yokohama, Japan). Chimeric mice were crossed with B6 females to generate heterozygous mice. Heterozygous mice were intercrossed to obtain homozygous mice. Homozygous mice were backcrossed with B6 for more than 12 generations. B6 mice were used as gp49B-sufficient wild-type mice. BALB/c (H-2d) mice were purchased from CLEA, Japan (Tokyo, Japan). OT-I and OT-II mice with the B6 genetic background were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were housed and bred in the Animal Unit of The Institute of Development, Aging and Cancer (IDAC, Tohoku University, Sendai, Japan), an environmentally controlled and specific pathogen-free facility, according to the guidelines for the care and use of laboratory animals of Tohoku University, and animal protocols were reviewed and approved by the IDAC Animal Studies Committee. All experiments involved 8- to 12-wk-old age-matched male and female mice. Wild-type B6 or BALB/c mice were acclimated in our facility for 2–4 wk before being subjected to experiments.
Southern blot analysis and RT-PCR analysis
For Southern blot analysis, DNA was isolated from G418-resistant ES cells digested with Eco RV. Hybridization was performed with 32P (Amersham Biosciences)-radiolabeled 3′ probes indicated in Figure 1. For RT-PCR analysis, total RNA was isolated from BMMC by RNeasy Mini Kit (QIAGEN), and then cDNA was synthesized by 1st Strand cDNA Synthesis Kit (Roche, Basel Switzerland). Primers used were forward 5′-ACCAAGTTCAAAATTCGATTT-3′ and reverse 5′-GTGTGGATGAGCTGCACT-3′ to detect the gp49A gene, and forward 5′-CAAGTTCAACATTCCAAGC-3′ and reverse 5′-CTATGGATGAGCTGCAAC-3′ to detect the gp49B gene. Amplification was performed by 35 cycles of 40 s at 94°C, 30 s at 52°C, and 20 s at 68°C.
Flow cytometric analysis
The following mouse-specific antibodies were used for flow cytometric analysis: FITC-, PE- or biotin-conjugated antibodies to CD4 (anti-CD4; L3T4), anti-CD8a (Ly-2), anti-CD11c (HL3), anti-CD40 (3/23), anti-CD54 (ICAM-I) (3E2), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-MHC class II (I-A/I-E) (M5/114.15.2), anti-CD51 (RMV-7), anti-CD61 (2C9.G2), and anti-H-2Dd (34-2-12). All antibodies were from BD Pharmingen (San Diego, CA). Alexa Fluor goat anti-hamster IgG (Molecular Probes, Eugene, OR) was used to stain purified anti-gp49 (H1.1) (BD Pharmingen, also a kind gift from Wanye M. Yokoyama, Washington University School of Medicine, St. Louis, MO). Cell-surface staining was performed according to the standard techniques, and flow cytometric analysis was conducted with a BD LSR and CellQuest software (Becton, Dickinson and Company, Franklin Lakes, NJ).
DC culture
For BMDC, bone marrow cell suspensions from wt and gp49B−/− mice were treated with RBC lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) to remove RBC. The remaining cells were further subjected to depletion of CD4-, CD8- and MHC class II+ cells by MACS (Miltenyi Biotech). The cells were cultured in complete culture medium consisting of RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 50 μM 2-ME, and 5% heat-inactivated FBS with antibiotics in the presence of mouse rGM-CSF (PeproTech, Rocky Hill, NJ). The medium was changed on days 4 and 6. On day 6, the cells expressed MHC class II, CD40, CD80, CD86, and CD11c, and consisted of immature DC 57. To induce DC maturation, 50 μg/mL of OVA or 100 μg/mL of LPS was added to the DC culture 24 h before the harvesting of cells. After stimulation, the culture supernatants were collected and the amounts of mouse cytokines in the supernatants were measured with Bio-Plex Mouse Cytokine Th1/Th2 Assay (Bio-Rad Laboratories, Hercules, CA); the cells were then stained for CD40, CD54, CD80, CD86, and MHC class II, and analyzed by flow cytometry. For antigen uptake by DC, cells were incubated with FITC-conjugated OVA (Molecular Probes) at 37°C or 4°C for 3 h, washed and then stained with PE-anti-CD11c. The uptake of FITC–OVA by DC was assessed by flow cytometry.
T-cell proliferation assay
Splenic CD4+ or CD8+ T cells were purified by MACS (Miltenyi Biotec). CD4+CD25− conventional T (Tconv) cells and CD4+CD25+ Treg were separated by MACS using a CD4+CD25+ Regulatory T-Cell Isolation Kit (Miltenyi Biotec). The purity was consistently >95% for the CD4+ and CD8+ T cell, and >90% for the CD4+CD25+ T-cell preparation. The cells were co-cultured with BMDC from B6 or gp49B−/− mice, at least in quadruplicate, in 96-well plates in complete culture medium modified by the addition of 10% heat-inactivated FBS and MEM non-essential amino-acid solution. For MLR assay, either CD4+, CD8+ T cells or total splencocytes from BALB/c mice were co-cultured with BMDC from B6 or gp49B−/− mice as described above. For the transwell experiments, the cells were cultured in 96-well plates on the top or bottom of 0.2 μm Cell Culture Inserts (Nunc, Roskilde, Denmark). After 3 days stimulation, the culture supernatant was collected and cytokines present in it were measured by Bio-Plex. The cells were incubated for 3 days and [3H]thymidine was added 18 h before cell harvest.
Induction of GVH disease
For host survival analysis, 5×107 spleen cells from BALB/c mice were injected intravenously into sublethally irradiated (9 Gy) B6 or gp49B−/− B6 mice. In the reverse model, 5×107 spleen cells from B6 or gp49B−/− B6 mice were injected intravenously into sublethally irradiated (6 Gy) BALB/c mice. These irradiation doses were determined by prior estimation as to the sublethality for each mouse strain. Mortality after cell injection was assessed every 24 h for 20 days. For flow cytometric analysis, splenocytes from mice before or 2 days after treatment were analyzed as previously described 23.
ELISA
For the measurement of IFN-γ production, spleen cells were harvested and pooled from three recipient mice per group at 4 days after transplantation. A total of 1×105 spleen cells were incubated with 15 Gy-irradiated, T-cell-depleted splenocytes (2×105 cells) from syngeneic BALB/c mice or allogeneic B6 mice for 24 h at 37°C on a 96-well flat-bottomed plate (BD bioscience). The T-cell depletion was performed with MACS (Miltenyi Biotec). IFN-γ concentration in the supernatant was measured by ELISA MaxTM Set Standard (BioLegend, San Diego, CA) according to the manufacturer's protocol.
Statistical analysis
Differences in the survival of the treatment groups were analyzed using the log-rank test. Other comparisons for statistical significance involved the two-tailed Student's t-test. p<0.05 was considered to be statistically significant.
Acknowledgements
This work was supported in part by the CREST Program of the Japan Science and Technology Agency, a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the 21st century COE program “Center for Innovative Therapeutic Development Towards the Conquest of Signal Transduction Diseases.”
Conflict of interest: The authors declare no financial or commercial conflict of interest.




