Therapy-induced antitumor vaccination by targeting tumor necrosis factor-α to tumor vessels in combination with melphalan
Abstract
Treatment of tumor-bearing mice with mouse (m)TNF-α, targeted to tumor vasculature by the anti-ED-B fibronectin domain antibody L19(scFv) and combined with melphalan, induces a therapeutic immune response. Upon treatment, a highly efficient priming of CD4+ T cells and consequent activation and maturation of CD8+ CTL effectors is generated, as demonstrated by in vivo depletion and adoptive cell transfer experiments. Immunohistochemical analysis of the tumor tissue demonstrated massive infiltration of CD4+ and CD8+ T cells 6 days after treatment and much earlier in the anamnestic response to tumor challenge in cured mice. In fact, the curative treatment with L19mTNF-α and melphalan resulted in long-lasting antitumor immune memory, accompanied by a mixed Th1/Th2-type response and significant in vitro tumor-specific cytolytic activity. Finally, the combined treatment reduced the percentage and absolute number of CD4+CD25+ regulatory T cells in the tumor-draining lymph nodes of mice responding to therapy, and this was associated with the establishment of protective immunity. These findings pave the way for alternative therapeutic strategies based on the targeted delivery of biological and pharmacological cytotoxic compounds that not only kill most of the tumor cells but, more importantly, trigger an effective and long-lasting antitumor adaptive immune response.
Abbreviation:
-
- B-FN:
-
ED-B-containing fibronectin isoform
Introduction
Although several immunotherapeutic approaches have been successfully used in the activation and expansion of tumor-specific T lymphocytes in animal models 1, 2, to date their application in cancer patients has been rather disappointing. Many studies have highlighted the potent and diversified capacity of different tumors to evade antitumor immune responses, among which are the loss of tumor-associated surface antigens 3, the down-regulation of MHC class I expression 4, the accumulation of inhibitory immature myeloid cells 5 and the release of inhibitory cytokines or immunosuppressive factors, e.g. IL-10 and TGF-β 8, 9. More recently a distinctive T cell subpopulation with a CD4+CD25+ phenotype, designated regulatory T cells (Treg), has been described to have an important function in the regulation of peripheral tolerance and in the suppression of antitumor effector responses 8, 9.
Induction of antitumor cytolytic T lymphocytes (CTL) displaying a CD8+ phenotype is a fundamental part of the effector mechanisms against the tumor, as these cells are able to recognize and kill, in an MHC class I-restricted fashion, the cancer cells both in vitro and in vivo. However, their activation and expansion are largely dependent on the action of antigen-specific CD4+ T helper (Th) cells 10–12. Thus, current approaches to cancer vaccination are aimed at triggering both Th and CTL tumor-specific immune responses 13, 14 and at eliminating inherent suppressive mechanisms that can impact these cells 15.
Activation of CD4+ Th cells is often accompanied by polarization into two distinct functional phenotypes, Th1 and Th2, depending mainly on the pattern of cytokine production. It has been shown that IFN-γ, a Th1-type cytokine, plays a central role in the regulation of cell-mediated antitumor immunity 16, 17. However, the view that a Th1-type response is the unique helper activity guiding effective antitumor CTL priming has been challenged by several reports demonstrating that Th1/Th2 mixed immune responses also strongly correlate with the induction of potent and effective antitumor CTL responses and with tumor rejection processes 18–20.
Tumor necrosis factor alpha (TNF-α) is a pleiotropic homotrimeric cytokine 21, 22 that exerts its major antitumor effects via a preferential toxicity for the endothelial cells of the tumor-associated vasculature, with extensive thrombosis and destruction of tumor vasculature resulting in extensive tumor necrosis, and through an increase in the antitumor immune response 23–25. TNF-α increases vascular permeability 26 and reduces the tumor's interstitial fluid pressure 27, a process pivotal to facilitating the penetration of antitumor agents at the tumor site. Although TNF-α is one of the most potent antitumor cytokines, its unacceptable toxic side effects have prevented its systemic administration at therapeutically effective doses. To date, the clinical use of TNF-α has been limited to loco-regional applications, such as “Isolated Limb Perfusion” (ILP) in combination with melphalan for the treatment of non-resectable high-grade sarcoma and melanoma 28, 29.
We previously demonstrated that the ED-B-containing fibronectin isoform (B-FN) is a marker of angiogenesis 30, 31 and that endothelial cells invading tumor tissues migrate along extracellular matrix (ECM) fibers containing B-FN 32. The possibility to selectively target tumor vasculature using a human recombinant antibody specific for B-FN, L19(scFv), in both experimental animal models and cancer patients 32–35 paved the way for the antibody's use in vivo for both diagnostic and therapeutic purposes. Indeed, the selective targeted delivery of cytokines to the B-FN using L19(scFv) dramatically enhances their anticancer properties 36–38.
We generated the fusion protein L19mTNF-α 38, consisting of mouse TNF-α (mTNF-α) and the human antibody fragment L19(scFv) directed to the B-FN. When injected intravenously (i.v.) into tumor-bearing mice, this fusion protein selectively accumulates around the tumor vasculature and, 48 h after injection, the dose of L19mTNF-α in the tumor is roughly 35 times higher than the dose achieved with a control fusion protein in which mTNF-α is conjugated to an irrelevant scFv.
A single systemic treatment with L19mTNF-α and melphalan has been shown to be highly effective in two histologically unrelated mouse tumor models, WEHI-164 fibrosarcoma and C51 colon carcinoma, implanted in syngeneic BALB/c mice 39. Importantly, a long-lasting T cell-mediated immune response against tumors involving both CD4+ and CD8+ T cells is induced by the combined therapy, and this correlates with the capacity to reject new tumor challenges and metastases, even of different histological origins 39.
Here we focused our investigation on characterization of the early events of the antitumor immune response after combined therapy with L19mTNF-α and melphalan in order to understand the regulatory mechanisms and the cell subpopulations involved in the triggering and maintenance of the protective antitumor immunity.
Results
In vivo depletion of CD4+ or CD8+ T cells abolishes the therapeutic efficacy of L19mTNF-α and melphalan
We previously showed that the treatment of tumor-bearing mice with L19mTNF-α and melphalan cures about 80% of WEHI-164 and 30% of C51 tumor-bearing BALB/c mice and that the host's immune system is fundamental for treatment efficacy and induction of the specific antitumor memory that protects the animals from subsequent tumor challenges 39.
To study the contribution of distinct lymphocyte subpopulations in the early processes leading to tumor cure, groups of ten animals were deprived of CD4+ T cells, CD8+ T cells or NK cells by injecting specific antibodies intraperitoneally. Antibodies were injected at days –2, 0 and +2 from WEHI-164 tumor cell injection (day 0) (Fig. 1). Treatment was performed on days 6 and 7. Removal of either CD4+ or CD8+ T cells completely abrogated the therapeutic effects (Fig. 1A, B), thus demonstrating that both CD4+ and CD8+ T cells are needed for tumor rejection. Conversely, removal of NK cells did not affect the efficacy of the treatment, since 80% of NK-deprived animals were cured of WEHI-164 tumor (Fig. 1C), as were the animals treated with control rat antibodies (Fig. 1D) or rabbit serum (data not shown).
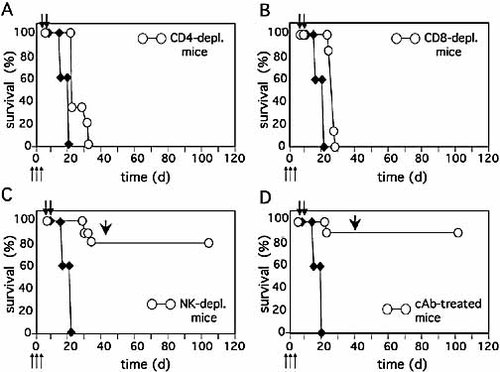
Antitumor efficacy of L19mTNF-α and melphalan in in vivo-depleted mice. Antibodies specific for CD4+ T cells (A), CD8+ T cells (B) or NK cells (C) or irrelevant antibodies (D, cAb) were administered to groups of ten mice (white circles) on days –2, 0 and +2 (thin arrows) with respect to s.c WEHI-164 tumor cell (3 × 106) injection. Systemic treatments with L19mTNF-α and melphalan were carried out on days 6 and 7 (thick arrows). The survival of untreated animals is indicated by black diamonds. The arrowheads in panels (C) and (D) indicate a homologous tumor challenge on day 40.
Primed CD4+ T cells trigger naive CD8+ T cells into antitumor effectors in vivo
In light of the results of the in vivo cell depletion experiments described above (Fig. 1), additional experiments were carried out to better evaluate the relevance of the host's CD4+ and CD8+ T cells in the triggering of the antitumor effector functions. Naive animals were in vivo-deprived of either CD4+ or CD8+ T cells by i.p. injections of specific antibodies at days –2, 0, and +2. At day 0 WEHI-164 tumor cells were co-injected s.c. with either CD4- or CD8-depleted immune spleen cells.
We then compared the in vivo protection from WEHI-164 tumor growth exerted by CD4-depleted immune spleen cells in immunocompetent or CD4-deprived mice and the protection by CD8-depleted immune spleen cells in immunocompetent or CD8-deprived mice. Using CD4-depleted immune splenocytes, no survival differences were observed between the immunocompetent and the in vivo CD4-depleted mice (Fig. 2A); indeed, 20% of tumor-free mice (1/5), were found in both groups 2 months after cell transfer. In contrast, dramatic differences were found using CD8-depleted immune splenocytes, since these cells were able to protect 80% (4/5) of immunocompetent mice and 0% (0/5) of in vivo CD8-depleted mice (Fig. 2B). These results indicate that CD4+ T cells cannot be regarded as effectors per se but rather as classical helper cells necessary to trigger naive CD8+ T cells in the host for effector functions.
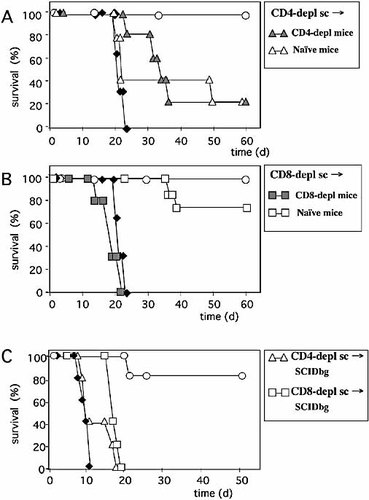
Winn assay experiments using in vitro-depleted immune splenocytes in in vivo-depleted BALB/c or SCIDbg mice. (A) Survival of groups of five immunocompetent (white triangles, naive mice) or in vivo CD4-depleted (grey triangles, CD4-depl mice) BALB/c mice s.c. co-injected with WEHI-164 tumor cells and CD4+ T cell-depleted immune splenocytes (CD4-depl sc). (B) Survival of groups of five immunocompetent (white squares, naive mice) or in vivo CD8-depleted (grey squares, CD8-depl mice) BALB/c mice s.c. co-injected with WEHI-164 tumor cells and CD8+ T cell-depleted immune splenocytes (CD8-depl sc). (C) Survival of groups of five SCIDbg mice s.c. co-injected with WEHI-164 tumor cells and CD4-depleted (white triangles, CD4-depl sc) or CD8-depleted (white squares, CD8-depl sc) immune splenocytes. The survival of groups of naive BALB/c (panels A, B) or SCIDbg (panel C) mice that received WEHI-164 tumor cells alone (black diamonds) or were co-injected with total immune spleen cells (white circles) is also reported.
To confirm this helper role of primed antitumor CD4+ T cells for both unprimed and primed CD8+ T cells, the Winn assay was performed in immunodeficient SCIDbg mice (Fig. 2C). While total immune splenocytes from tumor-cured donors were able to protect 80% (4/5) of SCIDbg mice from WEHI-164 tumor growth, no protection was achieved with immune spleen cells depleted of CD4+ T cells in vitro. Similarly, injection of in vitro-depleted antitumor CD8+ effector T cells in this system also resulted in a lack of protection from tumor growth.
Tumor tissue correlates of immunity in the priming and memory phases
The phenotype of tumor-infiltrating leukocytes was evaluated on WEHI-164 tumor cryostat sections at different times after tumor cell injection in naive mice (Fig. 3A) and in mice that had previously rejected a first tumor injection (Fig. 3B, cured mice). In the priming phase (Fig. 3A), CD4+ T cells were observed already at day 6 after tumor cell inoculation, just before treatment. By contrast, CD8+ T cell infiltration was barely visible at this time. A remarkable increase in the number of CD4+ and CD8+ T cells infiltrating the tumor was seen at day 12 after tumor cell injection in treated animals (day 6 after treatment) as compared to untreated animals. A similar behavior was observed for B cells (B). In contrast, macrophage (Mϕ) and NK cell (NK) infiltration, particularly prominent at day 7 after tumor cell injection, showed no appreciable differences between treated and untreated animals at most time points analyzed, with the possible exception of macrophages on day 9. In untreated animals, granulocyte infiltration (Gr) was low at all time points analyzed. In contrast, a massive and dramatic granulocyte infiltration was observed already 24 h after the first treatment (day 7 after tumor cell inoculation). This correlated with a rapid induction of tumor necrosis by L19mTNF-α, as previously observed 38. Granulocyte infiltration diminished in the following days to increase again dramatically at day 12 after tumor cell inoculation, the time at which the rapid and dramatic increase in CD4+ and CD8+ T lymphocytes was also observed.
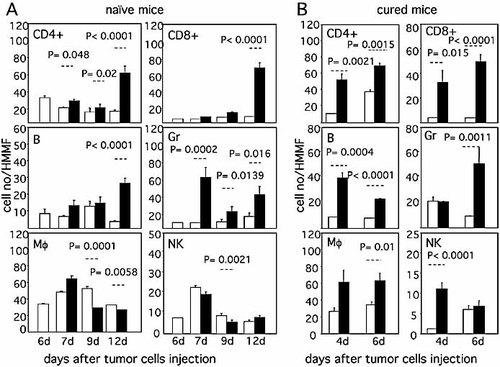
Immunohistochemical assessment of tumor infiltrates for the presence of CD4+ T cells (CD4+), CD8+ T cells (CD8+), B cells (B), granulocytes (Gr), macrophages (Mϕ) and NK cells (NK). (A) Cellular infiltrates in WEHI-164 tumors from naive mice analyzed at the indicated times (days) after s.c. tumor cell injection (white columns: untreated mice; black columns: mice treated with L19mTNF-α and melphalan on days 6 and 7). (B) Cellular infiltrates in WEHI-164 tumors analyzed at the indicated times (days) after implantation in control mice (white columns) or in tumor-cured mice (black columns). Three mice per group were used. Results are expressed as cell number (mean ± SE) per high magnification microscopic field (HMMF). The results of Mann-Whitney statistical analysis between the groups connected by dotted lines are also reported.
The characteristics of the tumor cellular infiltrate were then analyzed in mice that had been cured of WEHI-164 tumor following combined treatment and that were further challenged with a tumorigenic dose of 3 × 106 homologous tumor cells (cured mice, Fig. 3B). Comparison was carried out with tumor tissues taken 4 and 6 days after tumor cell injection in control naive animals. CD4+ and CD8+ T cells rapidly and massively infiltrated tumor tissues of rejecting mice, and their numbers increased from day 4 to day 6 after tumor cell inoculation. A similar behavior was observed for B cells (B), although in this case the number of infiltrating cells was higher at day 4 as compared to day 6. Macrophage infiltration (Mϕ) was also increased in tumor-rejecting mice with respect to control animals at the two time points analyzed. NK cell infiltration (NK) was strongly increased in rejecting mice, but only at day 4, while granulocyte infiltration (Gr) was strongly and statistically significantly increased only at day 6.
L19mTNF-α and melphalan induce a persistent mixed Th1/Th2 polarization
To study in more detail the initial triggering of the antitumor immune response, particularly the early events of T cell priming, we investigated the frequency, phenotype and functional importance of the CD4+ T cell subpopulations (Th1 and Th2) in WEHI-164 tumor-bearing mice before and soon after therapy with L19mTNF-α and melphalan. The results of ELISPOT assays measuring the frequency of ex vivo spleen cells producing IFN-γ or IL-4 (Fig. 4A, B) in response to in vitro stimulation with the same (WEHI-164) or a different (C51) tumor cell line, showed that in untreated WEHI-164 tumor-bearing mice (UT), the number of spleen cells secreting IL-4 in response to both homologous and heterologous tumors was high, while IFN-γ-producing cells were almost undetectable. A similar pattern of the Th2-type response was found in tumor-bearing mice treated with melphalan alone. In contrast, as compared to untreated tumor-bearing mice, treatment with L19mTNF-α alone induced high frequencies of IFN-γ-producing spleen cells and a remarkable reduction in IL-4-producing spleen cells in response to both tumor cells. When melphalan was given in combination with L19mTNF-α, a mixed Th1/Th2-type response, with high numbers of both IFN-γ- and IL-4-secreting spleen cells, again to both tumor cell lines, was observed (Fig. 4A and B). Moreover, the mixed Th1/Th2 response was still very strong after 1 month and persisted, albeit at lower frequencies, at least up to 5 months after WEHI-164 tumor cure (Fig. 4A, B). At this time, a new WEHI-164 tumor challenge strongly increased the frequency of spleen cells displaying a Th1/Th2-type response to both WEHI-164 and C51 tumor cells (anamnestic immune response; Fig. 4A, B) 2 wk after challenge. Importantly, both IFN-γ- and IL-4-secreting spleen cells responding to tumor challenge were mostly of the CD4-positive phenotype, and their function was blocked by pre-treatment of the cells with anti-MHC class II antibodies but not anti-MHC class I antibodies (Fig. 4A, B, right panels)
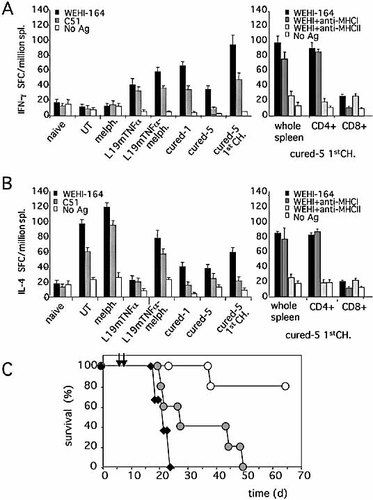
Th1- and Th2-type responses induced by treatment with L19mTNF-α and melphalan in tumor-bearing mice. Splenocytes were obtained from naive BALB/c mice (naive) as well as BALB/c mice 2 wk after WEHI-164 tumor cell injection and untreated (UT) or treated with melphalan alone (melph.), L19mTNF-α alone (L19mTNFα) or a combination of the two (L19mTNFα-melph.). IFN-γ- (A) and IL-4- (B) producing Splenocytes were assessed by ELISPOT analysis after incubation with WEHI-164 or C51 cell antigens or no antigen (left panels, mean ± SD). The frequencies of IFN-γ- and IL-4-producing splenocytes were also evaluated in cured mice after 1 month (cured-1) and 5 months before (cured-5) and after (cured-5 1stCh) the first WEHI-164 tumor challenge. In the latter animals, purified CD4+ or CD8+ T cells were also analyzed for IFN-γ (A, right panel) or IL-4 (B, right panel) production in presence of mAb specific for either MHC class I (MHCI) or class II (MHCII) molecules. (C) Survival of WEHI-164 tumor-bearing wild-type (white circles) and IFN-γ–/– (grey circles) BALB/c mice after treatment with L19mTNF-α and melphalan at the times indicated by the arrows. Groups of five mice were used. The survival of untreated tumor-bearing mice is also reported (black diamonds).
To evaluate the in vivo role of IFN-γ produced by Th1 cells, IFN-γ–/– mice bearing WEHI-164 tumors were treated with L19mTNF-α and melphalan. As shown in Fig. 4C, no tumor cure was achieved in this group of mice (0/5), underlining the key role exerted by this cytokine in the early events of the protective antitumor response generated after treatment.
A down-modulation of CD4+CD25+ Treg is induced by therapy
In order to evaluate the involvement of CD4+CD25+ Treg in the process of tumor growth and in the antitumor immune response induced by L19mTNF-α/melphalan treatment, both the quantitative variations and the function of this T cell subset in the draining lymph nodes of WEHI-164 tumor-bearing mice were analyzed after various modalities of treatment. Table 1 summarizes the quantitative results. In naive BALB/c mice, CD4+CD25+ Treg cells represented about 6% of the lymph node cells. This value rose to more than 11% in untreated mice bearing growing WEHI-164 2 wk after tumor cell injection and dropped to ⩽4.6% in those mice undergoing the various therapeutic protocols resulting in reduction of tumor growth at the same time point. In contrast, in mice with WEHI-164 tumor growth despite therapy (non-responders, NR), a much higher percentage of CD4+CD25+ Treg was observed (mean 7.2%). Noteworthy is that in two mice killed 1 month after WEHI-164 tumor cure with L19mTNF-α and melphalan, CD4+CD25+ Treg still represented only 3.4% of the total cells in the lymph nodes. The percentage of CD25+ and FoxP3+ (an additional marker of Treg) double-positive cells closely paralleled the percentage of CD4+CD25+ cells. The total cell number in the lymph nodes of untreated mice with growing tumors (compared to naive mice) was reduced by about 30%, and a 20–53% reduction was observed after the different treatments (Table 1), returning to the level of naive mice 1 month after cure. The absolute number of CD4+CD25+ Treg cells in untreated tumor-bearing mice was 30% higher than in naive mice, while it was reduced by more than 50% after all the treatments, with the exception of non-responding mice. Very similar results were obtained in C51 tumor-bearing mice undergoing the same therapeutic treatments (data not shown).
|
Treatmenta) |
mice no. |
CD4+CD25+ (% ± SD) |
FoxP3+CD25+ (% ± SD) |
CD4+ (% ± SD) |
cell no. (x106)± SD |
CD4+CD25+ (x106)± SD |
CD4+ (x106)± SD |
|---|---|---|---|---|---|---|---|
|
Untreated |
4 |
11.3±0.6 |
10.6±0.6 |
33.8±3.7 |
3.0±0.4 |
0.339±0.05 |
1.0±0.2 |
|
L19mTNF-α/melph |
4 |
4.3±0.2 |
3.9±0.4 |
46.5±6.2 |
2.2±0.5 |
0.095±0.02 |
1.0±0.4 |
|
L19mTNF-α |
4 |
4.6±0.3 |
4.5±0.2 |
55.0±7.0 |
2.0±0.3 |
0.092±0.03 |
1.1±0.5 |
|
Melphalan |
4 |
3.6±0.1 |
3.3±0.4 |
47.3±3.8 |
3.4±0.4 |
0.122±0.02 |
1.6±0.3 |
|
NRb) |
7 |
7.2±0.7 |
6.6±0.5 |
40.8±2.4 |
2.8±0.1 |
0.202±0.02 |
1.0±0.2 |
|
1-L19mTNF-α/melphc) |
2 |
3.4 |
3.0 |
56.5 |
4.0 |
0.136 |
2.3 |
|
Naive miced) |
4 |
6.0±1.3 |
5.8±0.8 |
57.7±3.9 |
4.2±0.5 |
0.255±0.04 |
2.4±0.3 |
- a) Tumor-bearing mice were treated at days 6 and 7 after tumor injection, and tumor-draining lymph nodes were analyzed 7 days after treatment.
- b) Mice not responding to therapy with L19mTNF-α/melphalan.
- c) Tumor-draining lymph nodes analyzed 1 month after tumor cure.
- d) Lymph nodes from the same anatomical site of naive mice not receiving tumor cells and untreated.
To study and compare the function of CD4+CD25+ Treg of cured mice with that of untreated tumor-bearing mice, their capacity to inhibit responder T cell proliferation in vitro in a unidirectional mixed lymphocyte reaction (MLR) was assessed. The results showed that addition of increasing numbers of Treg (up to half the number of responder CD4+CD25– T cells) from either untreated tumor-bearing mice or from cured mice 2 wk after tumor cell inoculation displayed similar inhibitory capacity on syngeneic T cells proliferating in the MLR (Fig. 5A). The functional capacity of Treg in vivo was also evaluated by transferring the cells into WEHI-164 tumor-bearing animals 2 days after L19mTNF-α/melphalan treatment. As shown in Fig. 5B, Treg substantially counteracted the antitumor protective immunity generated by the therapy. Indeed, only 50% of animals injected with Treg displayed tumor cure after therapy in contrast to a cure rate of 80% achieved in animals not receiving Treg. Thus, the quantitative reduction of Treg but not their intrinsic function is an important event generated by the establishment of protective immunity after L19mTNF-α and melphalan treatment.
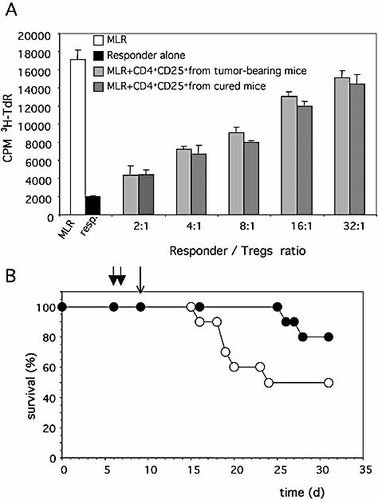
Effect of Treg on the proliferation of T lymphocytes in a MLR (A) and on the efficacy of L19mTNF-α/melphalan therapy in vivo (B). (A) CD4+CD25– spleen cells from naive BALB/c mice were incubated with equal numbers of irradiated spleen cells from naive C57BL/6 mice in the absence (MLR) or in the presence of the reported proportion (y-axis) of purified Treg from the tumor-draining lymph nodes of untreated (tumor-bearing) or from L19mTNF-α/melphalan-treated (cured) WEHI-164 tumor-injected mice. Proliferation was evaluated in triplicate wells by a [3H]-thymidine 18-h pulse (CPM 3TdR) at the end of 5 days of culture. (B) Effect of adoptive Treg transfer (thin arrow) on the survival of WEHI-164 tumor-bearing mice treated with L19mTNF-α/melphalan (thick arrows). Groups of ten mice were i.v. injected with 0.5 × 106 Treg/mouse (white circles) or 0.5 × 106 naive spleen cells (black circles).
Rejection of tumor challenges correlates with therapy-induced long-lasting memory CTL
In order to characterize in detail the antitumor cytolytic T cell function generated after L19mTNF-α/melphalan treatment of WEHI-164 tumor-bearing mice, the ability of the immune spleen cells to kill different tumor cell lines in a standard in vitro 4-h 51Chromium release assay was evaluated. Already 1 month after cure, high CTL responses specific for WEHI-164 and the heterologous C51 tumor cells were detected (not shown). The specific lytic ability remained high 2 months after tumor cure (Fig. 6A) and gradually declined but was still significant after 5 months (Fig. 6B). Re-challenge of cured mice with a tumorigenic dose of homologous cells 5 months after cure resulted in boosting of CTL activity against both homologous (WEHI-164) and heterologous (C51) tumor cells, which was MHC class I-restricted as shown by strong inhibition of lysis in presence of anti-MHC class I mAb (Fig. 6C) but not anti-MHC class II mAb (data not shown). This activity was associated with fast and complete tumor rejection in vivo and was still present 10 months after WEHI-164 tumor cure, as 4/4 cured mice were able to reject WEHI-164 tumor, thus demonstrating a very long-lasting antitumor immune memory (Fig. 6D). No CTL activity was found in spleen cells from mice bearing growing WEHI-164 tumors or in naive mice (data not shown).
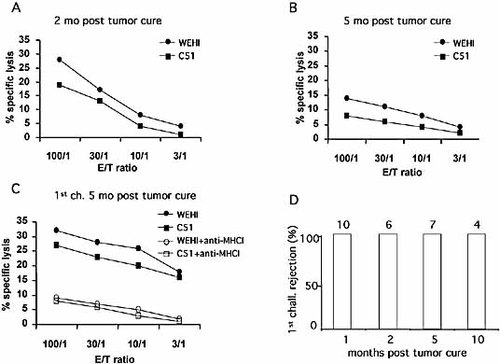
Specific cytolytic activity of immune splenocytes at different times after WEHI-164 tumor cure (A–C) and the persistence of antitumor memory (D). The cytotoxic activity of immune splenocytes from mice 2 months (A) or 5 months (B) after tumor cure and 2 wk after a first tumor challenge carried out 5 months after tumor cure (C) was tested against WEHI-164 and C51 tumor cells. mAb specific for MHC class I (MHCI) inhibited the lysis of both WEHI and C51 tumor cell lines (C). Results are representative of three independent 51Chromium-release experiments with similar results. (D) Ability of mice cured from WEHI-164 tumor to reject the first tumor challenge at different times after cure. The number of challenged mice is indicated above the columns.
Discussion
The therapeutic combination of L19mTNF-α and melphalan induces potent stimulation of the host immune response that can endow the host with antitumor immunity even against heterologous tumors of distinct histological origin 39. The cellular basis of this therapy-induced antitumor vaccination entails the strong priming of the CD4+ T cell compartment, an absolutely necessary prerequisite for subsequent triggering of the activation and maturation of the major antitumor effector mechanism represented by CD8+ CTL. The above conclusions are based on results from in vivo cell-depletion experiments, which have clearly demonstrated that elimination of CD4+ T cells from naive BALB/c mice makes the animals incapable of rejecting primary tumors after L19mTNF-α/melphalan treatment. In vivo depletion of CD8+ T cells but not NK cells produces the same effect (Fig. 1).
We previously demonstrated that total immune splenocytes derived from WEHI-164 tumor-cured mice and injected into naive animals protect 100% of the mice from WEHI-164 tumor growth. The protection against tumor growth dropped to 25% when the immune splenocytes were in vitro-depleted of the CD4+ T cells before cell transfer but remained 75% after depletion of CD8+ T cells 39. These results suggested that primed CD4+ T cells are more effective than primed CD8+ T cells in inducing rejection of the tumor when injected into immunocompetent unprimed animals. In the present study, we investigated this aspect in more detail. We found that adoptive transfer of CD8-depleted T cells derived from animals cured of the tumor after treatment with L19mTNF-α/melphalan were incapable of inducing tumor rejection when injected into CD8-depleted naive animals (Fig. 2B), strongly suggesting that the major protective role of primed antitumor CD4+ T cells lies in triggering CD8+ naive T cells to become functionally mature antitumor CTL effectors. Moreover, adoptive transfer experiments in immunodeficient SCIDbg mice underlined the important role of primed CD4+ T cells in stimulating and maintaining optimal effector function of primed CD8+ T cells in vivo. Taken together these results demonstrate the key role of CD4+ T cells in orchestrating adaptive antitumor immunity in our experimental model of tumor therapy by L19mTNF-α/melphalan treatment.
Immunohistochemical analysis (Fig. 3A) demonstrated dramatic infiltration of the tumor early after treatment, first by CD4+ T cells and then by CD8+ T cells. Impressive also was the de novo infiltration of granulocytes immediately after the tumor necrosis generated by the treatment and later, at the time of the greater infiltration of CD4+ T cells. The kinetics of CD4+ and CD8+ T cell infiltration at the tumor site was even accelerated in cured mice rejecting a tumor challenge (Fig. 3B) and, again, this infiltration was accompanied by a similar infiltration of granulocytes and macrophages. B cells were also largely present in the tumor infiltrate both after tumor treatment and in cured mice after tumor challenge, following kinetics similar to that of CD8+ T cells. While we cannot rule out involvement of B cells in the antitumor response, it is no doubt marginal compared to the role played by CD4+ and CD8+ T cells. Thus, the major lymphocyte populations involved in the antitumor priming and tumor rejection in vivo were indeed also the major populations observed in the tumor tissue of those animals undergoing therapeutic treatment with L19mTNF-α and melphalan as well as in cured mice rejecting tumor challenge.
These results strongly suggest that the massive necrosis induced by L19mTNF-α/melphalan and the earlier infiltration of granulocytes contributing to tumor cell killing and digestion may play a critical role in fueling professional antigen-presenting cells (APC), such as dendritic cells and macrophages, with large amounts of tumor-associated antigens. By this reasoning, APC can then stimulate specific antitumor CD4+ Th cells and CD8+ effector CTL, leading to the establishment of a critical reservoir of memory effector cells responsible for the accelerated rejection of the tumor upon challenge.
Further phenotypic and functional characterization of the CD4+ T cells involved in the priming of the antitumor immune response following treatment with L19mTNF-α/melphalan revealed that both MHC class II-restricted Th1-type and Th2-type cells were triggered (Fig. 4A, B). Interestingly, while melphalan preferentially triggered IL-4-secreting Th2-type cells, L19mTNF-α triggered exclusively CD4+ T cells secreting IFN-γ. This Th1-derived cytokine was indeed instrumental in the process of priming and maturation of the immune effector functions, since IFN-γ–/– knockout mice were no longer curable by the combined therapeutic treatment and were, thus, unable to reject the tumor (Fig. 4C). Nevertheless, treatment with either melphalan or L19mTNF-α alone was insufficient to generate efficient priming of the antitumor immune response 38, 39. Taken together these results emphasize the role of both Th1 and Th2 cells in the process leading to an efficient in vivo antitumor response in our experimental setting. Although in some tumor systems a polarized Th2 response promotes rather than inhibits tumor growth and spread 40 and is associated to lack of protective antitumor immune response 41, in other tumor models a mixed Th1/Th2 immune response correlates with the tumor rejection 18–20. Indeed, in antitumor vaccination approaches, Schuler et al. 19 showed that IL-4, the prototype cytokine of Th2 polarization, is pivotal in the early priming processes that lead to protective antitumor CTL responses. Future studies using IL-4-knockout mice will clarify this aspect in our experimental model. Our results suggest, however, that Th2 cells and their secreted cytokines may play an important role in both the recruitment of inflammatory cells of the innate immunity such as neutrophils to the tumor site as well as in their activation 42, which may contribute to the optimal activation of APC by providing excess amounts of tumor-associated antigens after digestion of necrotic tumor tissue. Thus, the rather abused notion that a polarized in vivo Th1 response is synonymous with a protective antitumor immune response, whereas a polarized Th2 response may even favor tumor growth, warrants seriously rethinking.
The very efficient CD4+ T cell priming promoted by combined L19mTNF-α/melphalan treatment, an instrumental step for the activation and maturation of antitumor CD8+ effector CTL, resulted in the acquisition of long-lasting specific immune memory, since cured mice still displayed a potent anamnestic rejection response 10 months after treatment (Fig. 5D). The tumor-specific cytotoxic T effector activity (Fig. 5A–C) was present at a high level soon after therapy, decreased over the next 5 months, and rebounded after an in vivo homologous tumor challenge, showing a strong correlation with the in vivo rejection capacity. This finding strongly suggests that our therapy-induced antitumor vaccination generated a stable and protective antitumor immune reservoir from which CD4+ memory T cells could easily be rescued, act both on primed and unprimed CD8+ T cells (favoring the rapid manifestation of effector function of the first and the maturation toward effector cells of the second) and finally counteract tumor take-up and growth upon challenge. This also indicates that the therapy-induced antitumor vaccination efficiently counteracted possible events of immune polarization toward a favorable pro-tumor environment.
This interpretation is also supported by the results of characterization of CD4+CD25+ Treg in tumor-bearing mice, untreated and treated with L19mTNF-α and melphalan. It is widely accepted that Treg are involved in the control of activation and effectiveness of T cell immune responses and in the immune tolerance to tumors 8, 9, 40, 43, 44. Moreover, and relevant for our studies, the number of peripheral blood Treg is increased in rheumatoid arthritis patients treated with anti-TNF-α 45, whereas in tumor-bearing hosts the number and function of Treg are down-regulated by low doses of the alkylating drug cyclophosphamide, resulting in potentiation of the antitumor immune responses 46. Here we show that tumor-bearing mice treated with L19TNF-α and the alkylating drug melphalan, both alone and in combination, showed a significant reduction of Treg in their draining lymph nodes as compared to either untreated or naive mice (Table 1). This down-modulation was induced in both WEHI-164 and C51 tumor-bearing mice, was consistent with the FoxP3 expression (Table 1) and persisted at least 1 month after tumor cure. Moreover, those mice not responding to combined therapy with tumor volume reduction (non-responding mice) presented significantly higher levels of Treg compared to responding mice. In vitro and in vivo functional studies further suggested that the quantitative reduction of Treg but not their intrinsic function was associated with the establishment of protective antitumor immunity. In fact, Treg from untreated tumor-bearing mice and cured mice were equally efficient in inhibiting T cell proliferation of syngeneic cells in MLR, and Treg from untreated tumor-bearing mice could inhibit the therapeutic effect of L19mTNF-α and melphalan in vivo.
Because all the reported treatments reduced Treg to a similar extent, it is possible that the establishment of protective immunity generated by treatment with L19mTNF-α and melphalan also acts via the quantitative, but not the qualitative, reduction of Treg. Thus, down-modulation of CD4+CD25+ Treg, although insufficient per se in inducing a protective antitumor response, may nevertheless participate in the complex series of cellular and molecular events that generate a stable and polarized antitumor immune response in our model.
In conclusion, these findings demonstrate that the targeted delivery of L19mTNF-α in conjunction with melphalan not only results in killing of most of the tumor cells but, more importantly, triggers an effective and long-lasting antitumor adaptive immune response.
Materials and methods
Animal tumor models
WEHI-164 mouse fibrosarcoma (ECACC, Sigma Aldrich, Milan, Italy) and C51 mouse colon adenocarcinoma (kindly provided by Dr. M. P. Colombo, Department of Experimental Oncology, Istituto Nazionale per lo Studio e la Cura dei Tumori, Milan, Italy), both of BALB/c origin, were cultured in D-MEM medium supplemented with L-glutamine and 10% fetal bovine serum (FCS) in a 5% CO2 atmosphere at 37°C.
WEHI-164 (3 × 106) and C51 (25 × 104) cells were subcutaneously (s.c.) implanted in the left flank of 8- to 10-week-old immunocompetent syngeneic BALB/c mice or severely immunocompromised beige (SCIDbg) mice (Harlan UK, Oxon, UK). IFN-γ–/– (BALB/c) mice 47 were purchased from The Jackson Laboratory (Bar Harbor, ME). The tumor volume was determined using the following formula: (d)2 × D × 0.52, where d and D are the short and long dimension (cm) of the tumor, respectively, measured with a caliper. Mice were killed when the tumor reached a volume of 1.5 cm3. Housing, treatment and sacrifice of animals followed national legislative provisions (Italian law no. 116 of 27 January, 1992).
Tumor therapy
Groups of tumor-bearing mice (∼0.2 cm3 tumor volume) received an injection in their tail vein of 100 μL phosphate-buffered saline (PBS; 20 mM NaH2PO4, 150 mM NaCl, pH 7.4) containing the fusion protein L19mTNF-α (0.7 pmol/g in the combined treatments; 1 pmol/g as a single therapeutic) obtained and purified as described 38. The control group received PBS only. Melphalan (Alkeran, Glaxo Smith Kline, Research Triangle Park, NC, USA), reconstituted in the solvent provided by the manufacturer immediately before use and further diluted in PBS, was given i.p. (4.5 μg/g in 400 μL) 24 h later. The weight loss was recorded daily and never exceeded 5% within 72 h of the treatments. The tumor growth curves were recorded and the results of the treatment expressed as percent of survival versus time.
In vivo and in vitro cell subset depletion
Cell subset in vivo depletions were performed as previously described 41 by i.p. injections of anti-CD4 (GK1.5; ATTC, Rockville, MD), anti-CD8 (2.43; ATTC) rat mAb and anti-asialo-GM1 (anti-NK rabbit serum; Wako Chemicals GmbH, Dusseldorf, Germany). Control animals received normal rabbit serum or an irrelevant rat mAb, as described 41. Depletion efficiency for each cellular subset was always >90% as assessed by immunofluorescence and FACS analysis (Becton Dickinson, Milan, Italy) on splenocytes of two euthanized mice derived from each group. For in vitro depletion, spleen cells were treated with either anti-CD8 or anti-CD4 antibodies following a two-step procedure, as already described 39. Cell depletion efficiency was assessed by immunofluorescence and cytofluorimetric analysis by direct staining for CD4 (FITC-conjugated YTS 191.1.2 mAb; Immunotools, GmbH, Germany) or CD8 (PE-conjugated YTS 169.4 mAb; Immunotools) and was always >95%.
Immunohistochemical analyses
Cryostat sections (6 μm thick) were air-dried and fixed in cold acetone for 10 min. Immunostaining was performed using a previously described procedure 30 and the following primary antibodies: anti-CD4 (GK1.5), anti-CD8 (2.43), anti-granulocyte Ly-6G (Gr-1; clone RB6–8C5), anti-macrophage (clone MOMA-1; ImmunoKontact), anti-B (clone RA3–3A1/6.1; ATCC) and anti-NK (anti-asialo-GM1). Quantitative studies of stained sections were performed independently by three researchers in a blind fashion. Cell counting was carried out in 8–12 randomly chosen fields under a Leica Wetzlar light microscope (Germany) at 400× magnification, 0.180 mm2/field. The results are expressed as cell number per high magnification microscopic field (cell no./HMMF, mean ± SE). Mann Whitney U-test was used to evaluate statistically significant differences between the groups.
Adoptive immunity transfer experiments (Winn assay)
WEHI-164 tumor-cured mice were s.c. boosted in the contralateral flank with the same tumor cells (3 × 106). After 12 days the mice were killed, their spleen was removed, and a spleen cell suspension was prepared 48. Total or in vitro-depleted splenocytes (see section In vivo and in vitro Cell Subset depletion) were mixed with 3 × 106 WEHI-164 tumor cells (1/1 ratio) and co-injected into naive animals 39. The results are expressed as percent of survival versus time.
ELISPOT assay
Frequencies of IFN-γ- or IL-4-producing spleen cells were determined using an enzyme-linked immunospot (ELISPOT) assay performed with ex vivo total splenocytes or, as specified, with purified CD4+ and CD8+ T cells co-cultured with irradiated WEHI-164 or C51 tumor cells as antigen, as described 41. Purification was carried out using an immunomagnetic procedure. Briefly, negative selection was performed with a pool of mAb specific for all cells except CD4- or CD8-positive cells (CD4+ and CD8α+ T cell isolation kit, respectively; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Purity was >95%, as assessed by immunofluorescence. In the case of testing with purified CD4+ T cells, irradiated naive feeder spleen cells were added 1/1 to the effector cells. A >2-fold increase in the number of spots over the control (splenocytes cultured in the absence of tumor cells) was considered a positive response. In certain experiments, mAb specific for either MHC class I (34.1.2S clone, 50 μg/mL) or class II (K24.199 and K22.42.2 clones, 50 μg/mL) antigens were added during the co-culture with irradiated tumor cells. Data are expressed as number of spot-forming cells per million spleen cells.
Cell-mediated cytotoxicity assay
The cell-mediated cytotoxicity assay was performed using splenocytes from naive, WEHI-164 tumor-cured or WEHI-164 tumor-rejecting mice. Splenocytes were co-cultured with irradiated WEHI-164 cells for 5 days and then tested in a standard 4-h 51Chromium-release assay on WEHI-164 or C51 target cells, as reported 39, in the absence or presence of anti-MHC class I (34.1.2S clone, 50 μg/mL) or anti-MHC class II (K24.199 and K22.42.2 clones, 50 μg/mL) antibody after having additionally preincubated the target cells for 30 min with either antibody.
Identification of CD4+CD25+ Treg cells and assessment of their function
The presence and the proportion of CD4+CD25+ Treg cells in tumor-draining lymph nodes were assessed. Cell suspensions were first incubated with anti-CD16 mAb to block Fc receptor, washed and stained with anti-CD4 FITC-conjugated and anti-CD25 PE-conjugated mAb (Immunotools) and analyzed by flow cytometry. Expression of FoxP3, an additional marker of Treg, was assessed in permeabilized cells using a FITC-labeled anti-FoxP3 rat mAb (clone FJK-16s; eBioscience, Italy). A FITC-labeled rat IgG2a mAb (eBioscience) was used as a negative control. The function of Treg was evaluated both in vitro and in vivo. CD4+CD25– or CD4+CD25+ cell fractions were isolated using a two-step immunomagnetic procedure (CD4+CD25+ regulatory T cell isolation kit, Miltenyi Biotec) following the manufacturer's instructions. Purity was >95%, as assessed by immunofluorescence. For evaluation in vitro, the capacity of the Treg to inhibit syngeneic T cell proliferation in MLR was analyzed. Briefly, purified naive CD4+CD25– spleen cells from BALB/c mice were incubated with equal numbers of irradiated spleen cells from C57BL/6 mice in the presence of various numbers of purified Treg from tumor-draining lymph nodes of WEHI-164-bearing mice untreated or treated with L19mTNF-α/melphalan. Proliferation was measured after 5 days of culture and an additional 18 h pulse with 0.5 μCi/well [3H]-thymidine (Amersham Bioscences). For in vivo evaluation of Treg and their impact on the efficacy of L19mTNF-α/melphalan therapy, Treg were purified from 2-wk WEHI-164 tumor-bearing mice and i.v. transferred (0.5 × 106 Treg/mouse) into treated WEHI-164 tumor-bearing mice 2 days after therapy. The tumor growth curves were recorded and the results of the treatment expressed as percent of survival versus time.
Acknowledgements
We are indebted with Professor Luciano Zardi for scientific discussion and support. We thank Mr. Thomas Wiley for manuscript revision. This work was supported by the following grants: I.S.S. National Research Project on A.I.D.S. n° 40F.1 and 45G.1 (R. S. A.), Fondi di Ateneo per la Ricerca (FAR) 2006 (R. S. A. and G. T.), Italian Association for Cancer Research (AIRC) (E. B., F. S., P. C., B. C. and L. B.); CE Contract: LSHC-CT-2003–503233 / STROMA (S. F.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of Interest: The authors declare no conflict of interest.




