Donor double-negative Treg promote allogeneic mixed chimerism and tolerance
Abstract
Bone marrow (BM) transplantation is an efficient approach to develop donor-specific tolerance and prevent chronic rejection. Allogeneic BM transplantation is limited by donor T cell-mediated graft-versus-host disease, requirement of cytoreduction and high numbers of BM cells. In addition of these drawbacks, recent studies demonstrate that not only T cells, but also NK cells can mediate BM rejection, and long-term mixed chimerism depends on NK cell tolerance. Thus, NK cell is another potential barrier against engraftment of BM and an important target in efforts to induce transplant tolerance. We have previously identified a novel type of Treg with the phenotype TCRαβ+CD3+CD4–CD8– (double-negative, DN). We and others have demonstrated that DN-Treg can effectively suppress anti-donor T cell responses. In this study, we found that donor-derived DN-Treg can suppress NK cell-mediated allogeneic BM graft rejection in both parent-to-F1 and fully MHC-mismatched BM transplantation models. Perforin and FasL in DN-Treg play important roles in the suppression of NK cells. Furthermore, adoptive transfer of DN-Treg can promote a stable mixed chimerism and donor-specific tolerance without inducing graft-versus-host disease. These results demonstrate a potential approach to control innate immune responses and promote allogeneic BM engraftment.
Abbreviations:
-
- B6:
-
C57BL/6
-
- DN:
-
TCRαβ+CD3+CD4–CD8– (double-negative)
-
- GVHD:
-
graft-vs.-host disease
-
- GZM:
-
granzyme
-
- PFN:
-
perforin
-
- TCD:
-
T cell-depleted
Introduction
Establishing donor-specific transplant tolerance is beneficial for several reasons: it can prevent chronic rejection and avoid side effects related to long-term immunosuppressive drug treatment. It also can help patients to preserve their own natural immunity against infections and tumors. Achieving mixed chimerism via BM transplantation is an efficient approach that directs the immune system to regard the donor as ‘self’. By generating specific immunologic tolerance to donor transplants, chronic rejection as well as the need for ongoing immunosuppression can be avoided 1–3.
Rejection of MHC-mismatched allografts is largely mediated by a recipient's CD4+ and CD8+ T cells. Recent studies, however, have demonstrated that NK cells are another potential barrier in allograft rejection and tolerance 4–7. Furthermore, studies have shown that NK cells can mediate BM rejection 8, 9. This notion has been well addressed in the hybrid resistance model, in which parental BM cells, not solid organs, are vigorously rejected by an F1 hybrid 10, 11. This rejection is mediated solely by a recipient's NK cells, not by T cells, NKT cells or B cells 12–14. Recent studies have also shown that NK cells can mediate allogeneic BM graft rejection in costimulatory blockade-treated recipients 15 and that long-term mixed chimerism depends on NK cell tolerance 15–18. Thus, the NK cell is another barrier against engraftment of BM and an important target in efforts to induce successful transplant tolerance.
Besides the problem of graft rejection mediated by recipient immunity, donor BM transplantation is limited by frequent graft-vs.-host disease (GVHD), which is mainly caused by donor T cells. However, depleting donor T cells results in poor engraftment of donor BM. Hence, it will be intriguing to search for a comprehensive approach that can control recipient immunoresponses and simultaneously prevent GVHD following BM transplantation.
Treg play an important role in the regulation of immune responses to self and to allogeneic antigens as demonstrated in a number of in vivo models of autoimmunity, infection, inflammatory disease and transplantation 19. CD4+ Treg have the ability to suppress conventional CD4+ and CD8+ T cells as well as to help regulate the functions of APC. Furthermore, studies have demonstrated that CD4+CD25+ Treg can inhibit anti-donor BM NK cell responses and promote mixed chimerism 20, 21.
In recent studies, we found that TCRαβ+CD3+CD4–CD8– (double-negative, DN) T cells possess immune-regulatory functions and play an important role in the development of tolerance post-transplantation 22–25. We have demonstrated that mouse DN-Treg specifically can eliminate activated syngeneic anti-donor CD4+ T cells, CD8+ T cells, and B cells 22, 23, 25, 26. Furthermore, adoptive transfer of DN-Treg leads to significant prolongation of donor-specific skin and heart allografts in a single MHC-mismatched murine model 22, 23, 27.
In support of our findings, a recent study demonstrated that CD4+ T cells, converted to DN-Treg, were highly potent in suppressing alloimmunity both in vitro and in vivo in an antigen-specific manner 28. Others have reported that DN-Treg can down-regulate CD8+ T cell-mediated immune responses in autoimmune and infectious disease models 29, 30. Interestingly, DN-Treg, derived from human peripheral blood, possess similar immune-regulatory functions as those we demonstrated 31. Collectively, these findings indicate that DN-Treg are able to down-regulate immune responses, both to self and to foreign antigens.
In this study, we have found that donor-derived DN-Treg could suppress NK cell-mediated allogeneic BM graft rejection and did not induce GVHD. Adoptive transfer of DN-Treg promoted mixed chimerism and induced allogeneic graft tolerance in a fully MHC-mismatched transplantation model. These results demonstrate a potential approach that would use DN-Treg to control innate immune responses and promote allogeneic BM engraftment.
Results
DN-Treg suppress NK cell-mediated BM rejection
First, we determined the potential of DN-Treg to suppress NK cells in vivo using the hybrid resistance model. In this model, only NK cells in F1 mice could vigorously mediate the rejection of parental BM cells 10, 11. We used a C57BL/6 green fluorescent protein-transgeneic (B6-GFP, H-2b)-to-CB6F1 (B6 × BALB/c, H-2b/d, F1 generation) combination in our experiment. DN-Treg were purified from B6 mice spleen and lymph nodes, then activated by anti-CD3 antibody in presence of IL-2 (50 IU/mL) overnight before being used for adoptive transfer. CB6F1 mice were given sub-lethal irradiation (6.5 Gy) before receiving B6-GFP T cell-depleted (TCD) BM and DN-Treg. Seven days later, recipient spleen cells were collected for CFU assay.
As shown in Fig. 1, CB6F1 mice that received B6 BM and DN-Treg co-transplantation exhibited a much greater number of CFU in comparison to those that had received only donor BM transplantation (n=3), both in terms of frequency per well (Fig. 1A) and total colony numbers in spleen (Fig. 1B). All CFU cells were collected and analyzed using flow cytometry; about 90% of cells were GFP+ (Fig. 1C), confirming the donor origin of CFU cells. To confirm the dominant role of NK cell-mediated BM rejection in this model, NK cells were depleted by anti-Asialo GM1 treatment (50 μL/mouse i.p., 3, 0 and 2 days before and after BM transplantation), and these mice were found to have high numbers of CFU formations after BM transplantation alone (Fig. 1A, B). These results indicate that donor DN-Treg can suppress NK-mediated rejection of parent-to-F1 BM transplantation.
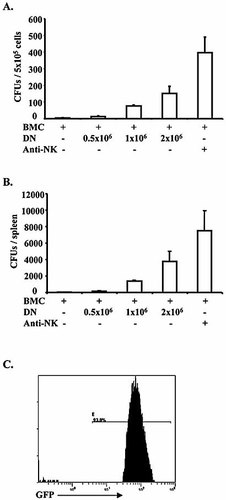
DN-Treg promote development of donor hematopoietic progenitor cells, as indicated by CFU, in parent-to-F1 BM transplantation. Recipient CB6F1 (H-2b × d) mice were sub-lethally irradiated (6.5 Gy), then given different doses of donor BM cells and DN-Treg. DN-Treg were purified from B6 mice and activated by anti-CD3 antibody and IL-2 (50 IU/mL) for 24–48 h. B6-GFP BM cells (107) were depleted of T cells by anti-CD90 MACS beads. NK cells were depleted using anti-Asialo GM1 antibody (50 μL/mouse) on days –3, –1, 0 and 2 before and after BM transplantation. Seven days after transplantation, spleen cells from recipient mice were collected and cultured for 5 days in triplicate wells, allowing for the detection of early hematopoietic progenitor cells of donor origin. Any colony consisting of over 50 cells was counted and the number of colonies was averaged from triplicate wells. (A) Averages of CFU per well (5×105) from three mice. (B) Averages of total CFU per spleen from three mice. (C) CFU cells were collected and analyzed on a flow cytometer to detect donor-derived GFP+ cells. The data represent a CFU assay from one mouse.
Perforin plays a critical roles in DN-Treg-mediated NK cell suppression
Previous studies from our laboratory and other groups have demonstrated that alloreactive DN-Treg suppress CD8+ T cells by means of Fas-FasL interactions 22, 23, 29. Interestingly, in our recent study, xenoantigen-activated DN-Treg were found to target syngeneic B and T cells by a perforin/granzyme (PFN/GZM)-dependent mechanism, both in vivo and in vitro 26. It has never been studied whether DN-Treg-mediated NK suppression is Fas-FasL- or PFN/GZM-dependent, or whether it involves other mechanisms. In general, cytotoxic T cells kill target cells mainly through Fas-FasL interaction or PFN/GZM-dependent mechanism. Therefore, we initiated this study using FasL-mutant (gld) and PFN-deficient (PFN–/–) mice.
DN-Treg were purified from gld and PFN–/– mice and activated for 24 h with anti-CD3 in the presence of IL-2 (50 IU/mL) before i.v. injection into sub-lethally-irradiated (6.5 Gy) CB6F1 mice that had received TCD B6 BM cells on the same day. After 7 days, spleens from the recipients were collected for CFU assay.
As shown in Fig. 2A, both CFU frequency and total number in the spleens of PFN–/– DN-Treg-treated mice were significantly decreased (p<0.01, Student's t-test). In contrast, gld DN-Treg-treated mice presented similar results as wild-type B6 DN-Treg-treated mice in CFU assays (p>0.1, Student's t-test, Fig. 2A). However, although reduced, a significant level of donor CFU remains in PFN–/– DN-Treg-treated mice. Hence, our data suggest that DN-Treg-mediated NK cell suppression may occur through a PFN/FasL-dependent pathway.
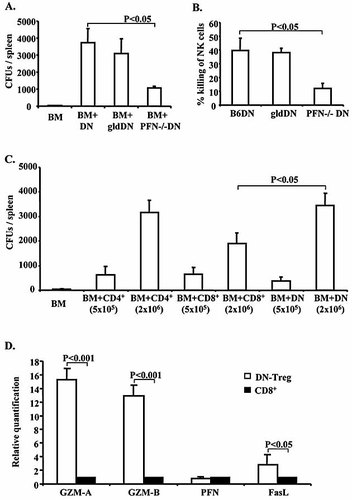
DN-Treg-mediated NK cell suppression involves PFN and FasL. DN-Treg were purified from wild-type B6, gld or PFN–/– mice and activated for 24–48 h with anti-CD3 in presence of IL-2 (50 IU/mL). B6 TCD BM cells were prepared as described in Fig. 1. Recipient CB6F1 (H-2b × d) mice were sub-lethally irradiated (6.5 Gy) and given 107 donor BM cells and 2×106 DN-Treg. After 7 days, spleens from recipients were collected for CFU assay as described in Fig. 1. CD4+ and CD8+ T cells were purified from B6 mice and activated by plate-coated anti-CD3/CD28 (1 μg/mL) with IL-2 (50 IU/mL) for 24–48 h. NK cells were purified from BALB/c mice by anti-CD49b selection beads and activated by IL-2 (1000 IU/mL) for 4–7 days. Contaminating other type cells were removed by MACS beads. [3H]thymidine was added during the last 24 h to label NK cells. (A) Averages of total CFU per spleen from four mice. (B) DN-Treg-mediated NK cell killing in vitro. 3H-labeled BALB/c NK cells were mixed with activated DN-Treg (1:5) purified from wild-type B6, gld or PFN–/– mice in triplicate in 96-well plates. After co-culture for 5 h, the cells were harvested, counted in a β-scintillation counter, and specific cell lysis calculated. The experiment was repeated once and a similar result was obtained. (C) The average total CFU per spleen from four mice that received DN-Treg or CD4+ or CD8+ T cells with donor BM cells. (D) Real-time PCR to quantify the expression level of killing related genes. Total RNA was extracted from the DN-Treg and CD8+ T cells after activation for 3–4 days, and cDNA synthesis and real-time PCR performed. The relative quantification is calculated based on the normalized delta threshold cycle value. The endogenous control is β-actin and the relative quantification was compared to B6 CD8+ T cells. Data represent the average result from four mice.
To further study DN-Treg-mediated NK cell suppression, we performed an in vitro cytotoxic assay. Activated DN-Treg from B6, gld and PFN–/– mice were mixed with IL-2-activated BALB/c NK cells (5:1). As shown in Fig. 2B, B6 DN-Treg and gld DN-Treg exhibited a stronger capacity than PFN–/– DN-Treg to kill NK cells (p>0.05 B6 DN-Treg vs. gld DN-Treg, p<0.01 B6 DN-Treg vs. PFN–/– DN-Treg, Student's t-test). Hence, DN-Treg could use PFN to target NK cells in vitro.
We were interested in studying whether DN-Treg, CD4+ and CD8+ T cells possess a similar function after adoptive transfer with donor BM cells. It was surprising that DN-Treg showed a much stronger capacity to suppress NK cell-mediated donor BM rejection than CD8+ T cells (p<0.05, Student's t-test), but similar to CD4+ T cells (p>0.05) in a dose-dependent manner (Fig. 2C). We further analyzed the cytotoxic-related gene expression in both cells by real-time PCR. As shown in Fig. 2D, DN-Treg express higher levels of GZM and FasL than CD8+ T cells. Hence, these data suggest that high levels of GZM or FasL expressions might ensure that DN-Treg can effectively attack NK cells. It is unknown whether CD4+ and CD8+ T cells use a similar mechanism or different mechanisms to suppress NK cells in this study.
DN-Treg inhibit NK cell-mediated fully MHC-mismatched BM rejection without induction of GVHD
The above experiments demonstrate that DN-Treg can suppress NK cell-mediated BM rejection in the parent-to-F1 model. Next, we extended our study to fully MHC-mismatched allogeneic hematopoietic BM transplantation. Different doses of TCD BM cells were purified from B6-GFP mice (H-2b), then i.v. injected into sub-lethally irradiated (6.5 Gy) BALB/c mice (H-2d). The recipient mice, divided into several groups, received either donor BM cells alone or donor BM cells with DN-Treg (1×106 or 2×106). After 7 days, spleens from the recipients were collected for CFU assay to determine the reconstitution of donor hematopoietic progenitors in the recipients.
Both the frequency and the total number of CFU were greatly increased in recipients that had received co-transplantation of BM and DN-Treg vs. those that had received BM transplantation alone (Fig. 3A). Treatment using CD4 (YTS191.1) and CD8 (YTS169.4) depletion antibodies did not further enhance CFU numbers (Fig. 3A). The control group that received NK cell depletion antibody (anti-Asialo GM1) was found to have high numbers of CFU formations after BM transplantation alone, indicating the role of NK cells in donor BM rejection (Fig. 3A). Interestingly, although significantly reduced (p<0.05, Student's t-test), B6 DN-Treg helped C3H BM cells to survive in BALB/c recipients (Fig. 3A), suggesting that adoptive transfer of B6 DN-Treg can help third-party BM engraftment. CFU cells collected from one mouse for flow cytometry to detect donor-derived GFP+ cells indicate that about 87% of CFU cells were GFP+ (Fig. 3B). Collectively, these results suggest that adoptive transfer of DN-Treg can promote early engraftment of fully MHC-mismatched allogeneic BM transplantation.
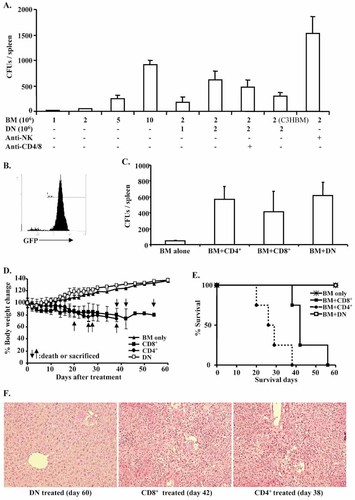
DN-Treg promote donor hematopoietic progenitor cell development in fully MHC-mismatched BM transplantation without induction of GVHD. BALB/c mice (H-2d) were sub-lethally irradiated (6.5 Gy) before transfusion of B6-GFP BM cells and B6 DN-Treg. Some recipients received co-transplantation with activated DN-Treg (2×106). Seven days after transplantation, spleen cells from recipient mice were collected and counted before being used for the CFU assay. Third-party BM cells were purified from C3H mice. CD4 (YTS191.1, 0.2 mg/mouse) and CD8 (YTS169.4, 0.2 mg/mouse) depleting antibody were co-injected subcutaneously at days –8, –5, and –3 before DN-Treg and BM cell transfusion. NK cells were depleted using anti-Asialo GM1 (50 μL) on days –3, –1, 0 and 2 before and after BM transplantation. (A) The averages of total CFU per spleen were calculated based on six mice. (B) CFU cells were analyzed on a flow cytometer to detect donor-derived GFP+ cells. (C) Activated CD4+ (2×106) and CD8+ (2×106) T cells were co-transfused with B6 BM cells (2×106). The average of total CFU per spleen was obtained from four mice from each group. (D) Adoptive transfer of donor DN-Treg does not induce GVHD. DN-Treg (107) and BM cells (2×106) were co-transfused into BALB/c mice (n=4) that had been sub-lethally irradiated (6.5 Gy) 1 day before. Control mice received activated CD4+ (2×106) or CD8+ (2×106) T cells together with 2×106 B6 BM cells (n=4). Body weight was averaged from four mice in each group. (E) Mortality or GVHD termination record. (F) Pathology analysis of liver grafts. H&E staining is shown after DN-Treg, CD4+ T cell or CD8+ T cell treatment. Hepatocytes, liver cell cords, portal and venous structures are normal with no evidence of GVHD in DN-Treg-treated mice. Infiltrating mononuclear cells, proliferation in bile ducts, and abnormal portal and venous structure, a typical lesion of chronic GVHD, occurred in both CD4+ and CD8+ T cell-treated mice. Magnification ×200.
Next, we determined whether CD4+ and CD8+ T cells have a similar effect as DN-Treg in promoting early mixed chimerism. Anti-CD3/CD28-activated B6 CD4+ or CD8+ T cells were co-transfused with donor BM cells. Both donor CD4+ and CD8+ T cells showed a similar effect as DN-Treg in CFU assay (Fig. 3C).
Our previous studies and those of others have demonstrated that adoptive transfer of donor DN-Treg can attenuate GVHD induced by donor CD8+ T cells in sub-lethally irradiated SCID mice, and anti-host DN-Treg can inhibit CD8+ T cell-mediated autoimmunity 29, 32, 33, thus indicating that donor DN-Treg do not play a role in inducing GVHD. To determine whether adoptive transfer of DN-Treg played a role in GVHD in the current model, we transferred B6 DN-Treg (107) with B6 BM cells (2×106) into conditioned (6.5 Gy) BALB/c mice.
As shown in Fig. 3D–F, all mice (n=4) that received up to 107 DN-Treg survived beyond 60 days without a decrease in body weight or signs of GVHD. Pathology analysis showed that hepatocytes, liver cell cords, and portal and venous structures were normal with no evidence of GVHD. In contrast, control mice that received activated CD4+ T cells (2×106) developed severe GVHD with weight loss and mortality. Infiltrating mononuclear cells, proliferation in bile ducts, and abnormal portal and venous structure, a typical lesion of chronic GVHD, was evident. Although the mice that received CD8+ T cells (2×106) had extended survival, pathology indicated that these mice developed GVHD, as tissue damage was found in the liver (Fig. 3F).
Co-transplantation of BM cells and DN-Treg induces stable mixed chimerism and allograft tolerance
Our above data indicate that donor DN-Treg inhibit rejection during the early stage of BM transplantation. We were interested in assessing whether mixed chimerism and tolerance could be induced in these mice.
TCD B6-GFP BM cells (2×106) were co-transplanted with B6 DN-Treg (2×106) into sub-lethally irradiated (6.5 Gy) BALB/c mice. Control mice received only BM cells (2×106). Peripheral blood of treated mice was monitored on different days to detect donor-derived (H-2kb+ or GFP+) lymphocytes. Donor-derived lymphocytes (H-2kb+) in the recipient periphery could be detected from 30 to 180 days. The range of donor cells was 3–88% (n=12, Fig. 4A), whereas no significant donor-derived lymphocytes were detected in recipients treated only with donor BM cells (n=6, Fig. 4A). Four chimera mice were sacrificed on day 100 to assess lymphoid and myeloid lineages by detecting donor-derived GFP+ cells. On an average 50% of BM cells and 36% of spleen cells were GFP+ (Fig. 4B). Donor-derived CD3+, CD19+ and CD11b+ (myeloid lineage) cells in these spleens are correlated with the chimera (Fig. 4C).
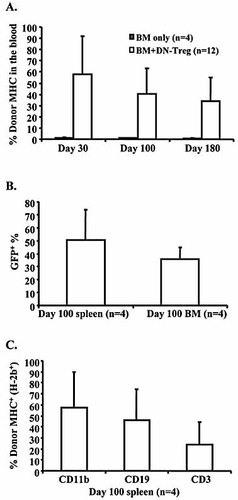
Allogeneic hematopoietic chimerism after co-transfer of donor DN-Treg and BM cells. B6-GFP TCD BM cells and DN-Treg were prepared as described in Fig. 1. BALB/c mice were sub-lethally irradiated (6.5 Gy) 1 day before receiving a co-transfusion of 2×106 B6 DN-Treg and 2×106 B6-GFP TCD BM cells. (A) Peripherial blood was collected according to indicated time points to detect donor-derived lymphocytes. (B) Four chimeric mice were sacrificed on day 100 to detect donor-derived lymphocytes in spleen and BM by analysis of GFP+ cells. (C) The spleens were then stained with anti-H-2kb, anti-CD3, anti-CD19 and anti-CD11b. Data represent % of donor-derived H-2kb+ cells among CD3+ (or CD11b+ or CD19+) cells in the recipients’ spleens.
Next, we studied whether mixed chimerism would lead to acceptance of the donor graft. Heart grafts from BM donor strain B6 mice were transplanted into chimeric BALB/c mice and control mice. The control mice were treated with sub-lethal irradiation only, or BM plus sub-lethal irradiation, or DN-Treg plus irradiation, then kept for 40–60 days before receiving their graft. Heart grafts survived longer than 180 days in chimeric mice, but were rejected in control mice (Fig. 5A).
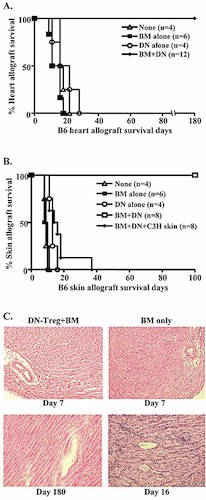
Allograft tolerance in recipients of co-transfused BM cells and DN-Treg. BALB/c mice received treatment as described in Figs. 3 and 4. Heart grafts from BM donor strain B6 mice were transplanted into chimeric BALB/c mice after 40–60 days. The control groups (n=4 or 6/group) were treated by irradiation only, or by irradiation plus BM, or by irradiation plus DN-Treg, then kept 40–60 days before receiving B6 heart grafts. Cessation of beating heart was defined as the end point of rejection (A). At 60–100 days after co-transfusion of BM cells and DN-Treg, chimeric BALB/c mice (n=8) received second grafts (skin) from donor B6 and third-party C3H mice (B). (C) Heart grafts from chimeric mice were analyzed after 180 days by H&E staining. The data represent one chimeric mouse and one control mouse for each time point (magnification ×200).
To determine whether chimeric mice corroborate donor-specific tolerance, second grafts (skin grafts) from BM donor strain B6 and third-party control C3H (H-2k) mice were transplanted onto chimeric BALB/c mice 60–100 days after BM transplantation. Graft survival results are concluded in Fig. 5B. Skin grafts from control mice were rejected shortly after grafting (all mean survival time <20 days). Skin grafts from donor B6 mice were not rejected, surviving over 100 days in chimeric mice without any sign of rejection (Fig. 5B). In contrast, skin grafts from the third-party control C3H mice were rejected after grafting (mean survival time = 16 days, n=8), suggesting that antigen-specific tolerance was established in the animals with mixed chimerism. Heart grafts from chimeric mice that survived for 160–180 days were collected and analyzed using H&E staining. As shown in Fig. 5C, heart grafts in control mice showed signs of rejection as early as 7 days after transplantation, whereas there was no sign of tissue damage in chimeric mice from day 7 until day 180.
Discussion
Success of BM transplantation relies on effective inhibition of recipient immune responses and prevention of GVHD. In this study, we have demonstrated that DN-Treg can inhibit NK cell-mediated donor BM graft rejection in sub-lethally irradiated parent-to-F1 and fully MHC-mismatched transplant models. Furthermore, we have found that DN-Treg-mediated NK cell suppression occurs through a PFN/FasL-dependent mechanism, both in vivo and in vitro. Importantly, donor DN-Treg could promote mixed chimerism without inducing GVHD. These results suggest a potential approach to control innate immune responses, which occur after BM transplantation, with the use of DN-Treg.
Although recipient T cells play a major role in rejection of allogeneic grafts, NK cell-mediated allograft rejection has been well discussed 4–7. Furthermore, NK-mediated BM rejection typically occurs at an earlier stage 12. The role of NK cells in BM graft rejection was well addressed in the hybrid resistance model, in which both irradiated F1 hybrid and T/B cell-deficient RAG–/– F1 mice rejected parental BM cells by NK cells 10–14. Conversely, NK cell-deficient F1 mice accepted parental BM grafts. Hence, parent-to-F1 hybrid resistance provides a reliable model for us to study the possibility of NK cell suppression by DN-Treg in vivo. Our data demonstrate that adoptive transfer of donor DN-Treg can inhibit NK cell-mediated parental BM rejection and promote survival of early donor-derived progenitors (Fig. 1). Furthermore, a similar effect of DN-Treg was found in a fully MHC-mismatched model (B6 to BALB/c, Fig. 3A). Interestingly, B6 DN-Treg do not only help B6 BM, but also third-party C3H BM survival in BALB/c recipients (Fig. 3A). This result also suggested that DN-Treg could be used in a broad range for treatment.
Previous studies have demonstrated that early mixed chimerism may not always persist over the long term, particularly with regimens that involve a combination of immunosuppressive techniques 34, 35. Hence, tolerance in mixed chimerism must be more carefully assessed in the setting of transplantation, owing to the possibility that chimerism may be dissociated with tolerance 36–38. The results of DN-Treg-mediated NK cell suppression was intriguing, prompting us to further study whether adoptive transfer of DN-Treg could promote engraftment of allogeneic BM cells. We detected donor-derived lymphocytes (H-2kb+ or GFP+) in recipients after 180 days (Fig. 4A). Furthermore, donor-derived CD3+ cells, CD19+ cells and CD11b+ cells were identified in chimeric mice after 100 days (Fig. 4C). This result correlated with the CFU results obtained 7 days after BM transplantation (Fig. 3A). Furthermore, the chimeric mice accepted donor heart and skin grafts but rejected third-party C3H skin grafts (Fig. 5A, B). Hence, suppression of NK cells by adoptive transfer of DN-Treg provides a long-term benefit by achieving stable mixed chimerism and donor-specific tolerance.
Following the first reports that described its use in a murine model 39, 40, mixed chimerism was developed successfully in porcine and non-human primate models using immunosuppressive regimens 41–48. More recently, mixed chimerism has produced clinical benefit in a number of transplant patients 49–54. The risk of GVHD, which is associated with donor T cells, is a major obstacle in BM transplantation. Depletion of donor T cells before BM transplantation reduces the risk of GVHD, but it also limits the success of mixed chimerism. Several studies have tried to solve this dilemma. It has been shown that CD4+ Treg from the donor or recipient can control GVHD after BM transplantation 21, 55–58. In previous studies, we have found that donor DN-Treg could suppress donor CD8+ T cells, which caused GVHD in a single MHC-mismatched model 32. Others also reported that anti-host DN-Treg could suppress the function of CD8+ T cells and prevent autoimmune diseases 29.
In this study, we found that, although adoptive transfer of donor CD4+ or CD8+ T cells has benefits for donor BM survival (Fig. 3C), either CD4+ or CD8+ T cells induce GVHD (Fig. 3D–F). The mice that received CD4+ or CD8+ T cells developed body weight loss, mortality and liver damage. In contrast, adoptive transfer of up to 107 donor DN-Treg did not induce GVHD in a fully MHC-mismatched model (Fig. 3D–F). Hence, donor DN-Treg may provide an alternative tool to promote allogeneic engraftment in clinical BM transplantation.
Treg have the ability to suppress conventional CD4+ and CD8+ T cells as well as to help regulate APC function. The suppressive function of CD4+ Treg has previously been shown to be mediated by IL-10, CTLA-4 and/or membrane-bound TGF-β 59. In addition, recent studies have suggested that chemokine metabolism 60, indirect tryptophan metabolism 61, FasL 62 and GZM 63 could also be involved in the function of Treg. In CD4+ Treg-mediated syngenic NK cell suppression, TGF-β provides a major suppressive mechanism 20, 64, 65. Similarly, the mechanism of DN-Treg-mediated immune suppression differs in different models. Alloreactive DN-Treg suppress CD8+ T cells through Fas-FasL interactions 22, 23, 29. However, DN-Treg can also suppress CD4+ T cells through a Fas-independent mechanism involving the inhibition of IL-2 production in CD4+ T cells 66.
Interestingly, our recent study showed that xenoantigen-activated DN-Treg were found to target syngeneic B and T cells by a PFN/GZM-dependent mechanism, both in vivo and in vitro 26. The different mechanism by which DN-Treg attack target cells might result from the activation pattern of DN-Treg by different antigen or from the molecular engagement of DN-Treg with target cells. DN-Treg are quite different from conventional T cells, lacking detectable levels of CD4, CD8, CD28, ICOS, 4-1BB, OX40 and CD40L 22.
In the current study, we have found that, although less efficient, PFN–/– DN-Treg were still effective in helping donor progenitors to survive in recipients compared to DN-Treg from wild-type B6 or FasL-mutant gld mice (Fig. 2A). Consistent with the CFU results (Fig. 2A), in vitro killing assay demonstrated that B6 and gld DN-Treg can mediate direct killing of BALB/c NK cells (Fig. 2B), whereas PFN–/– DN-Treg had a reduced killing capacity to NK cells (Fig. 2B), implying that PFN may have a major role in DN-Treg-mediated NK cell suppression, although a Fas-FasL contribution can not be excluded (Fig. 2A, B). It remains unknown whether DN-Treg directly eliminate NK cells or indirectly change other factors involved in NK cell activity and function in vivo.
Classically, PFN is thought to form pores on target cell plasma membrane, which allows diffusion of cytolytic molecules (GZM A and GZM B) into target cells. Furthermore, PFN helps release of GZM in target cells and subsequently induces target cell death. We have found that DN-Treg express high levels of GZM A and GZM B (Fig. 2C), which might explain why DN-Treg more efficiently suppress NK cell function compared with CD8+ T cells in the CFU assay (Fig. 2A). However, in this study, it is still unknown whether CD4+ and CD8+ T cells utilize a similar mechanism or different mechanisms to suppress NK cells in vitro and in vivo.
In summary, our study has demonstrated an effective way to control anti-donor NK cell function after allogeneic BM transplantation using adoptive transfer of donor-derived DN-Treg. This may provide an alternative method that promotes BM engraftment in clinical transplantation.
Materials and methods
Animals
B6 (H-2b), BALB/c (H-2d), B6 PFN–/–, FasL-deficient B6Tnfsfgld (gld), and B6 GFP mice were purchased from Jackson Laboratories (Bar Harbor, ME) and Charles River Laboratories (Wilmington, MA). The animals were maintained in the animal facility at the University of Western Ontario using approved protocols and procedures.
Antibodies
DN-Treg were characterized with fluorescent-conjugated monoclonal antibodies that specifically recognize TCRαβ, CD3, CD4, CD8, CD25, CD28, NK1.1, DX5 (CD49b) and TCRγδ (eBioscience, San Diego, CA). To detect donor-derived lymphocytes, anti-mouse H-2kb/Db (clone 28-8-6) and H-2kd/Dd were used (BD Biosciences, Missassauga, Canada). Data were acquired and analyzed on a Cytomics FC500 flow cytometer (Beckman Coulter, Missassauga, Canada). CD4 depletion antibody (YTS191.1) and CD8 depletion antibody (YTS169.4) were developed from ATCC cell lines. NK cell depletion antibody, anti-Asialo GM1, was purchased from Cedarlane (Burlington, Canada).
Isolation of DN-Treg, CD4+ T cells and CD8+ T cells
CD4+ T cells and CD8+ T cells were purified by MACS beads directly from spleen cells. To purify DN-Treg, spleen and lymph node cells, obtained from B6 or gld or PFN–/– mice, were treated with anti-DX5, anti-CD4 and anti-CD8 MACS beads (Miltenyi Biotec, Auburn, CA) to deplete CD4+, CD8+ and NKT cells. The remaining cells were added to anti-CD90 (Thy-1)-coated MACS beads (Miltenyi Biotec) to purify CD4–D8– T cells. The purity of naive DN-Treg was monitored by flow cytometry and TCRγδ−CD3+CD4–CD8–NK1.1– cells were >92% pure. Purified B6 DN-Treg were stimulated with anti-CD3 (2 μg/mL) in RPMI 1640 supplemented with 10% FCS, penicillin (100 U/mL), streptomycin (100 μg/mL), glutamin (2 mM), sodium pyruvate (1 mM), HEPES (10 mM), β-mercaptoethanol (0.5 mM), and 50 IU/mL IL-2. Twenty-four hours later, the activated DN-Treg were i.v. transferred into recipient mice with BM cells.
BM transplantation
BM cells were obtained by flushing the femurs and tibia of sex-matched B6 mice with cold (4°C) PBS containing 1% FBS. T cells were depleted by anti-CD90 (Thy-1)-MACS beads (Miltenyi Biotec). The absence of T cells was confirmed prior to the transfer of BM cells, by staining with anti-CD3, anti-CD4, anti-CD8 and flow cytometry analysis. TCD BM cells were i.v. injected into sub-lethally irradiated (6.5 Gy, 70 cGy/min, Cobalt-60) CB6F1 (H-2b × d) or BALB/c mice. The recipient mice were housed in a pathogen-free barrier. Some B6 donor BM cells were co-transplanted with different doses of DN-Treg. The control group received either CD4+ or CD8+ T cells, or BM cells alone.
CFU assay
Recipient spleen cells were collected 7 days after transplantation to detect early progenitor cells in a CFU assay, which has been used to detect donor-derived progenitor cells in early stages of immune reconstitution 20, 21, 67. Briefly, the spleen cells were re-suspended at 2×105–5×105/mL in colony-forming Iscove's medium (MethoCult; StemCell Technologies, Vancouver, Canada) in the presence of 30% FCS, 0.86% methylcellulose, 5 ng/mL IL-3, 10 ng/mL GM-CSF and antibiotics for 5 days. Any colony consisting of over 50 cells was counted and the number of colonies was averaged from triplicate wells. Then all CFU cells were collected and washed to detect donor-derived GFP fluorescence using flow cytometry.
Real-time PCR
B6 DN-Treg and CD8+ T cells were activated by either BALB/c APC or anti-CD3 for 3–4 days in the presence of IL-2 (50 IU/mL). Total RNA was extracted with a spin column according to the manufacturer's protocol (QIAGEN, Mississauga, Canada). cDNA pools were synthesized with the StrataScript First Strand Synthesis System according to the manufacturer's protocol (Stratagene, La Jolla, CA). Primers were designed using Primer Express, primer designing software from Applied Biosystems (Streetsville, Canada): GZM A: TGTGCTGGCGCTTTGATTG and GAACTTAGATCTCTTTCCCA; GZM B: CGATCAAGGATCAGCAGCC and CTGGGTCTTCTCCTGTTCT; PFN: GAAGACCTATCAGGACCAGTACAACTT and CAAGG TGGAGTGGAGGTTTTTG; FasL: GAAGGAACTGGCAGAACTCCG and CCCTGTTAATG GGCCACACT; β-actin: CCAGCCTTCCTTCCTGGGTA and CTAGAAGCATTTGCGGTGCA. The gene sequences were obtained from the www.ncbi.nlm.nih.gov database.
Real-time quantitative PCR was performed on standardized quantities of cDNA using the Brilliant SYBR Green QPCR Master Mix kit, and amplified DNA products were generated and detected using the M×4000 system (Stratagene). Each PCR amplification condition was set up in triplicate. As endogenous control β-actin amplification was used. The normalized delta threshold cycle value and relative expression levels (2ddCt) were calculated according to the manufacturer's protocol.
Heart and skin transplantation
Intra-abdominal heterotopic B6 (H-2b)-to-BALB/c (H-2d) cardiac transplants were performed in our microsurgery laboratory, in accordance with an approved protocol. Briefly, donor hearts were procured through a butterfly thoracic incision. All major vessels were ligated, except the pulmonary artery and aorta, which was sharply transected. The ascending aorta and pulmonary artery were anastomosed end-to-side to the recipient, by way of the infrarenal abdominal aorta and inferior vena cava. Pulsation of the heart graft was monitored daily. Cessation of beating was defined as the end point of rejection.
To determine donor-specific tolerance, each group of mice received a second graft (skin graft) from donor strain. In brief, a full-thickness (1×1 cm size) donor skin graft was transplanted on the lateral thoracic wall, and the graft was secured with a bandage. Skin grafts were monitored daily after removal of the bandage on day 8. Complete necrosis of the skin graft was defined as rejection. Skin grafts were followed for >100 days. Survival of skin grafts from third-party (C3H) mice was monitored for antigen specificity of the mixed chimerism. Antigen-specific tolerance refers to a mouse that accepts a skin graft from the BM donor B6 strain, but rejects the skin graft from the third-party C3H strain.
GVHD and pathology
Clinical signs of GVHD, including weight loss, diarrhea, ruffled fur and hunched posture, were monitored daily. More than 20% body weight loss or death was considered as termination of GVHD. Tissue samples from major GVHD lesion sites (liver, skin, and intestine) were harvested, stained with H&E, and evaluated by light microscopy for GVHD.
Cytotoxicity assay
The purified DN-Treg from B6, gld and PFN–/– mice were activated by plate-coated anti-CD3 (1 μg/mL) with IL-2 (50 IU/mL) for 3–7 days before being used as effectors. BALB/c NK cells were purified by CD49b+ selection on MACS beads column (Miltenyi Biotec), and were stimulated by IL-2 (1000 IU/mL) for 4–7 days. Any contaminated T cells and macrophages were further depleted by antibody-bonded MACS beads (Miltenyi Biotec) after flow cytometry analysis. We applied the JAM assay to measure DNA fragment and death of target cells 68–70. Activated NK cells were labeled with 10 μCi/mL of [3H]thymidine at 37°C overnight, washed and then used as targets. After co-culture of NK cells with the DN-Treg effector cells at 37°C for 5 h, the cells were harvested and counted in a β-scintillation counter (PerkinElmer). Specific cell lysis was calculated using the following equation: % specific killing = (S–E)/S×100, where E (experimental) is cpm of retained DNA in the presence of effector cells, and S (spontaneous) is cpm of retained DNA in the absence of effector cells.
Statistical analysis
The obtained data were compared using Student's t-test. p values less than 0.05 were considered a significant difference.
Acknowledgements
The authors would like to thank Mrs. Xuyan Huang and Dr. Francis Feng for assistance with cell injection and Ms. Cate Abbott for editing assistance. This study was supported by the Heart and Stroke Foundation of Canada to Z.-X. Zhang, and Fund of Multi-Organ Transplant Program, London Health Sciences Centre, London, Ontario, Canada to Z.-X. Zhang.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




