Regulation of constitutive and microbial pathogen-induced human macrophage migration inhibitory factor (MIF) gene expression
Abstract
The cytokine macrophage migration inhibitory factor (MIF) is an important regulator of innate immunity, inflammation and oncogenesis. However, four decades after its identification, the molecular mechanism(s) regulating the expression of the MIF gene remain largely unknown. Analyses of human monocytic (THP-1), epithelial (HeLa and A549) and keratinocytic (HaCat) cells transfected with wild-type, truncated and mutated MIF promoter reporter constructs, and electrophoretic mobility shift assay, chromatin immunoprecipitation, and siRNA inhibition indicated that the transcription factors specificity protein (Sp)1 and cAMP response element-binding protein (CREB) are critical positive regulators of constitutive human MIF gene expression. Albeit located in a cytosine guanine dinucleotide island, the MIF gene was found to be hypomethylated, an observation consistent with high baseline transcriptional activity. Moreover, stimulation of THP-1 cells and of peripheral blood mononuclear cells with microbial products up-regulated phosphorylated Sp1 nuclear content, Sp1 DNA-binding activity, MIF promoter activity and MIF mRNA levels in a MEK1/2-, Sp1-dependent manner. Taken together with previous observations of an important role for MIF in pro-inflammatory macrophage responses, these present findings suggest a key role for Sp1 and CREB in transcriptional regulation of MIF gene expression and MIF-dependent host antimicrobial innate immune defense.
Abbreviations:
-
- CRE:
-
cAMP response element
-
- Sp:
-
specificity protein
Introduction
Macrophage migration inhibitory factor (MIF) was discovered 40 years ago as a T cell cytokine that inhibited the random migration of peritoneal exudate cells 1, 2. Although it was one of the first cytokines to be discovered, MIF remained an enigmatic effector molecule for more than two decades. A human MIF cDNA was cloned in the late 1980s 3. Shortly thereafter MIF was rediscovered as a neuroendocrine peptide released in a hormone-like fashion by the anterior pituitary gland and the adrenal cortex after exposure to the endotoxin (LPS) of Gram-negative bacteria, corticotropin-releasing hormone, or physiological stress 4–7.
Lately, MIF has emerged as an integral component of innate immune and inflammatory responses (reviewed in 8). Unlike most cytokines, MIF is constitutively expressed by immune (monocytes/macrophages, dendritic cells, T and B lymphocytes and mast cells) and non-immune (endocrine and epithelial cells) cells and is released rapidly after exposure to microbial products, pro-inflammatory cytokines, mitogens or stress 4, 5, 7, 9–11. Directly or indirectly, MIF promotes the expression of numerous proinflammatory molecules, including cytokines, adhesion molecules, matrix metalloproteinases, nitric oxide and products of the arachidonic pathway 8, 12. A rather unique feature of MIF is the fact that it is induced by glucocorticoids and functions as a counter-regulator of the immunosuppressive and anti-inflammatory effects of glucocorticoids by overriding glucocorticoid-induced MAPK phosphatase 1 (MKP-1) expression and inhibition of cytokine production 6, 13. Therefore, MIF plays an important role in promoting pro-inflammatory responses of innate immune cells and the host antimicrobial defense response.
In recent years, MIF has been shown to promote innate immune responses in response to Gram-negative bacteria by up-regulating the expression of TLR4, the signal transducing molecule of the LPS receptor complex, thus facilitating the sensing of endotoxin-containing bacteria 14, 15. MIF also sustains pro-inflammatory macrophage responses by inhibiting p53-mediated apoptosis induced by LPS 16. Finally, MIF has been shown to inhibit AP-1 activity by binding to its co-activator JAB1/CSN5, to reduce pro-oxidative stress-induced apoptosis and to induce a rapid and transient activation of ERK1/2 17–20. Consistent with its pro-inflammatory properties and role in innate immunity, MIF has been implicated in the pathogenesis of acute and chronic infectious, inflammatory and autoimmune diseases, such as septic shock, asthma, acute respiratory distress syndrome, rheumatoid arthritis, glomerulonephritis and inflammatory bowel diseases (reviewed in 8).
Beside inflammation and innate immunity, MIF has also been implicated in cell proliferation and oncogenesis. Indeed, MIF was shown to induce proliferation of mouse fibroblasts via sustained activation of ERK1/2 MAPK 17 and to inhibit p53 or retinoblastoma/E2F tumor suppressor pathways 21–23. Recently, MIF was reported to be necessary for optimal expression of cyclin D1, an important regulator of G1 phase progression, in a Rho GTPase/MAPK manner 24. Together with the fact that MIF is overexpressed in tumors and that MIF-deficient fibroblasts are resistant to p53 and H-ras mediated cell transformation, these data suggest an important role for MIF in the control of cell growth and oncogenesis (reviewed in 25).
Yet, more than 15 years after the cloning of a human MIF cDNA, the transcriptional regulation of MIF gene remains largely unknown. To fill this gap, we determined which cis-acting regulatory elements and trans-acting transcription factors are implicated in the regulation of MIF gene expression in several cell types at baseline and after stimulation of innate immune cells (i.e. monocytes/macrophages and PBMC) with microbial pathogens.
Results
Identification of cis-acting regulatory elements critical for human MIF promoter activity
To localize DNA sequences regulating MIF promoter activity, deletion constructs of the human MIF gene ranging from −2802 to +129 were cloned into a luciferase reporter vector and transiently transfected into THP-1 cells. Constructs −2802/+129, −2688/+129, −1720/+129 and −1072/+129 drove strong luciferase activity, whereas construct +44 drove background luciferase activity (Fig. 1A). To localize the upstream boundary of the MIF minimal promoter, four additional promoter constructs (−858/+129, −508/+129, −157/+129 and −81/+129) were generated by PCR. Deletion of the MIF promoter from −858 bp to −157 bp did not change luciferase activity, that was slightly but statistically significantly reduced in construct −81/+129. 3′-end deletions until position +47 either of the −508/+129 construct or of the –157/+129 construct did not change promoter activity (Fig. 1B), indicating that the +47/+129 region was not critical for optimal transcriptional activity of the MIF promoter. Altogether, these data suggested that important cis-acting regulatory elements controlling the transcription of the MIF gene were localized between −81 and +47 bp. We therefore focused our attention on the analysis of the proximal region of the MIF promoter, even though regulatory elements in a more distal section of the promoter might also modulate MIF expression.
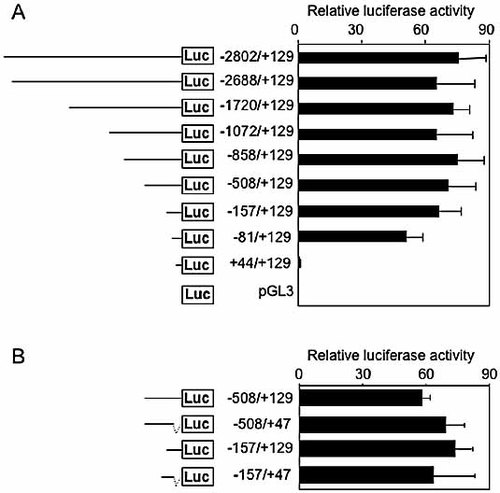
Analyses of human MIF promoter activity in THP-1 cells. (A, B) THP-1 cells were transiently transfected with an empty pGL3 vector or with human MIF promoter deletion constructs –2802/+129, –2688/+129, –1720/+129, –1072/+129, –858/+129, –508/+129, –157/+129, –81/+129 and +44/+129 (A) or –508/+129, –508/+47, –157/+129 and –157/+47 (B), obtained as indicated in Materials and methods. Cells were co-transfected with a Renilla luciferase vector. Results are expressed as the ratio of luciferase activity to Renilla luciferase activity. Data are means ± SD of triplicates from one representative experiment. p = 0.02 and p < 0.005 for –81/+129 and +44/+129 versus –2802/+129, respectively.
Computer-assisted sequence analysis of the proximal 200 bp of human MIF promoter revealed that it did not contain a TATA box, but numerous GC-boxes typically found in proximal promoters of house keeping genes and implicated in the binding of specificity protein (Sp)1 transcription factor to DNA. In line with this observation, two potential Sp1 DNA-binding sites were identified at −64 bp (distal Sp1, Sp1d) and −42 bp (proximal Sp1, Sp1p). Moreover, two c-AMP response element (CRE) at −78 bp (distal CRE, CREd) and at −20 bp (proximal CRE, CREp) and single c-Myb (−153), AML-1a (+1) and AP-4 (+34) sites were identified in the vicinity of the transcriptional start site (Fig. 2A). To investigate the functional significance of these potential DNA-binding sites we performed site-directed mutagenesis within the −157/+129 MIF promoter construct (Fig. 2C). Disruption of c-Myb, AML-1a, AP-4, CREd or Sp1d site did not affect promoter activity. However, disruption of either the Sp1p site or of the CREp site in THP-1 cells reduced promoter activity by 40% (p = 0.01) and 65% (p = 0.005), respectively (Fig. 2B). It also reduced promoter activity by 40 and 25% in HeLa cells, albeit not statistically significantly. It suggested that taken independently the CREp site might be less critical in HeLa cells than in THP-1 cells. Yet, dual disruption of the Sp1p and CREp sites nearly fully abolished promoter activity in both cell types (p = 0.001 in THP-1 and p = 0.01 in HeLa cells), indicating that these two cis-acting regulatory elements are essential for basal MIF promoter activity in monocytic and epithelial cells lines. In agreement with these findings, Sp1 and CRE sites were found to be conserved in human and mouse MIF promoters, whereas the other elements were species specific (Fig. 2C).
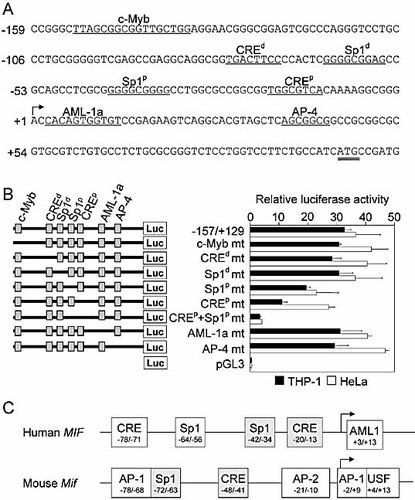
Identification of DNA-binding sites regulating human MIF promoter activity. (A) 5′-Flanking sequence of the human MIF gene and potential DNA-binding sites (underlined) for transcription factors. CREd and Sp1d and CREp and Sp1p designate the distal and proximal CRE sites and Sp1 sites. The ATG start codon is underlined twice. (B) Site-directed mutations of c-Myb, CREd, Sp1d, Sp1p, CREp, Sp1p+CREp, AML-1a and AP-4 and were introduced into the −157/+129 MIF promoter. THP-1 (black bars) and HeLa (white bars) cells were transiently transfected with either an empty pGL3 vector, or wild-type or mutant (mt) promoter constructs together with a Renilla luciferase vector. Data are means ± SD of triplicates from one representative experiment. THP-1 cells: p = 0.01, p = 0.005 and p = 0.001 for Sp1p mt, CREp mt and Sp1p+CREp mt constructs versus wild-type construct. HeLa cells: p = 0.01 for the Sp1p+CREp mt construct versus wild-type construct. (C) Comparison of the proximal promoters of the human and mouse MIF genes. The proximal Sp1 and CRE sites regulating the constitutive human and mouse MIF promoter activity (see Discussion) are highlighted in grey.
Sp1 and CREB positively regulate MIF promoter activity
To identify the transcription factors binding to the Sp1p or CREp regulatory elements, we performed EMSA using nuclear extracts isolated from resting THP-1 cells. Single specific retarded complexes were detected when using either Sp1p (Fig. 3A) or CREp (Fig. 3B) double-stranded oligonucleotides. Complex specificity was demonstrated by dose-dependent competition studies (Fig. 3A and B). The identity of the proteins involved in complex formation was investigated by supershift experiments. The Sp1p complex was supershifted when using anti-Sp1 antibodies, but not when using anti-Sp2, anti-Sp3 or anti-NF-κB p65 antibodies (Fig. 3C). Likewise, the CREp complex was supershifted when using CREB phosphorylated on serine residue 133 (phospho-CREB) antibodies, but not when using anti-Sp1 or anti-NF-κB p65 antibodies (Fig. 3D). These results strongly suggested that the transcription factors Sp1 bound to Sp1p and CREB to CREp regulatory elements, acting as positive regulators of MIF gene expression.
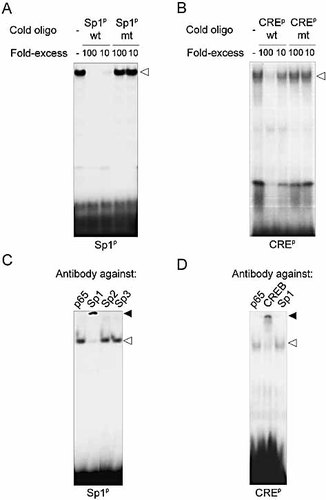
Sp1 and CREB bind to the Sp1p and CREp sites of human MIF promoter. Nuclear extracts of THP-1 cells were incubated with radiolabeled Sp1p (A and C) or CREp (B and D) oligonucleotides. Competition analyses were performed by adding 100-fold or 10-fold excess of unlabeled wild-type (wt) or mutant (mt) Sp1p and CREp oligonucleotides to the reaction mixtures (A and B). Nuclear extracts were preincubated with antibodies directed against NF-κB p65, Sp1, Sp2, Sp3 or phospho-CREB (CREB) before addition of Sp1p or CREp radiolabeled oligonucleotide to the reaction mixture (C and D). Specific and supershifted complexes are marked with open and closed arrows. Results are representative of three independent experiments.
To confirm that Sp1 and CREB were involved in the regulation of MIF gene expression, we first transfected HeLa cells with siRNA duplexes to reduce Sp1 and CREB expression. Twenty-four hours later, HeLa cells were transfected with the −157/+129 MIF promoter construct and cultured for an additional 24 h. Cell extracts were then collected for measurements of Sp1 and CREB levels and MIF promoter activity. Sp1 and CREB siRNA-transfected cells exhibited strongly reduced levels of Sp1 and CREB (Fig. 4A). Impaired Sp1 and CREB expression was associated with a twofold reduction of MIF promoter activity (Fig. 4B). Dual transfection with Sp1 and CREB siRNA increased the inhibitory effect on MIF promoter activity compared to single transfection, although not in a statistically significant manner. We next transiently transfected Sp1-deficient Sf9 insect epithelial cells with increasing amounts of an expression vector encoding for Sp1 together with −157/+129 MIF promoter construct. As shown in Fig. 4C, introduction of Sp1 in Sf9 cells increased MIF promoter activity in a dose-dependent manner. Lastly, we incubated THP-1 cells with increasing concentrations of mithramycin A, an antibiotic known to bind to GC-rich DNA regions and therefore interfering with Sp1 binding to DNA. As anticipated, mithramycin A strongly inhibited MIF promoter activity (Fig. 4D) and MIF mRNA expression (Fig. 4E) in THP-1 cells. Altogether, these studies confirmed that Sp1 and CREB regulate constitutive MIF expression.
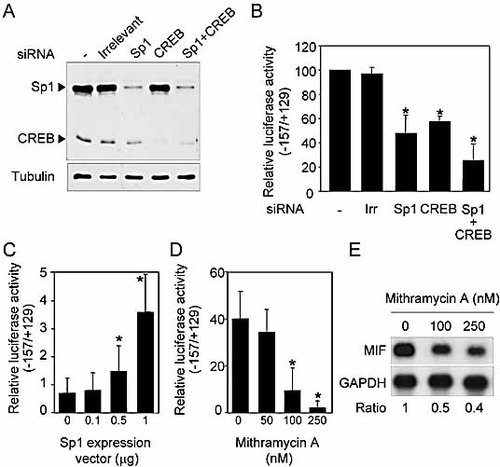
Sp1 and CREB regulate human MIF promoter activity. (A, B) HeLa cells were transfected either with siRNA directed against human Sp1 and CREB given either alone or in combination or with nonspecific siRNA (Irrelevant/Irr). Twenty-four hours later, cells were transfected with −157/+129 promoter construct together with a Renilla luciferase vector and incubated for an additional 24 h. Expressions of Sp1, CREB and tubulin were analyzed by Western blotting (A) and promoter activity assessed by measurement of luciferase activity (B). The activity obtained in cells not transfected with siRNA was set at 100%. Data are means ± SD (n = 6). (C) Sf9 cells were transfected with −157/+129 promoter construct together with a Sp1 expression vector. Data are means ± SD (n = 6). (D) −157/+129 promoter activity in THP-1 cells incubated for 16 h with mithramycin A (mean ± SD; n = 3). (E) THP-1 cells were incubated for 16 h with increasing concentrations of mithramycin A. MIF and GAPDH mRNA expression was analyzed by Northern blotting. *p <0.05.
Sp1 and CREB regulate basal MIF gene expression in several cell types
To determine whether the same cis- and trans-acting elements also regulated the expression of the MIF gene in other cell types, we studied A549 airway epithelial cells and in HaCat keratinocytes. Abundant MIF mRNA (HeLa>A549>HaCat>THP-1 cells) was detected by Northern blotting in epithelial cells and keratinocytes (Fig. 5A). Comparable levels of luciferase activity were measured in THP-1, HeLa and A549 cells transfected with the −157/+129 MIF promoter construct, which was slightly lower in HaCat keratinocytes (Fig. 5B). Like in THP-1 and in HeLa cells, dual disruption of Sp1p and CREp DNA-binding sites also nearly completely abolished MIF promoter activity in A549 and HaCat cells (Fig. 5B). We next performed EMSA to confirm that nuclear proteins also bind to Sp1p and CREp sites. As shown in Fig. 5C and D, specific retarted complexes were detected when using Sp1p and CREp oligonucleotides. The intensity of the retarded Sp1p (HeLa>A549=THP-1 >HaCat) and CREp (HaCat=THP-1>HeLa>A549) complexes varied among the cell lines. Altogether, these data suggested that Sp1- and CRE-DNA-binding regulate constitutive transcriptional activity of MIF in a fairly broad range of cells, including myeloid cells, epithelial cells and keratinocytes.
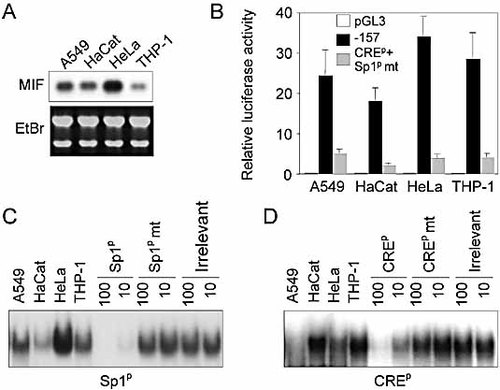
Sp1p and CREp DNA-binding sites regulate basal transcription of the MIF gene in several human cell types. (A) Northern blot analysis of MIF mRNA expression in THP-1 monocytic cells, HeLa cervix epithelial cells, HaCat keratinocytes and A549 airway epithelial cells. (B) Cells were transiently transfected with the empty basic pGL3 vector (white bars), the −157/+129 promoter construct (black bars) or the −157/+129 promoter construct with mutations of the Sp1p and the CREp sites (grey bars) together with a Renilla luciferase vector. Data are means ± SD of at least six determinations from two to five independent experiments. p <0.001 for Sp1p+CREp mt construct versus wild-type −157/+129 construct in all cell types. (C, D) Nuclear extracts were analyzed by EMSA using radiolabeled Sp1p (C) or CREp (D) oligonucleotides. Competition analyses were performed by adding 100-fold or 10-fold excess of unlabeled wild-type or mutant (mt) oligonucleotides to the reaction mixture. Results are representative of three independent experiments.
Sp1 and CREB bind to native MIF promoter
To verify that Sp1 and CREB bound to the bona fide MIF promoter in vivo, we performed chromatin immunoprecipitation (ChIP) assays. Chromatin isolated from THP-1 cells was incubated with antibodies directed against Sp1, CREB or NF-κB p65 (negative control). Immunoprecipitated DNA was then amplified by PCR using oligonucleotides surrounding the MIF promoter region containing Sp1p and CREp sites. As shown in Fig. 6A and quantified in Fig. 6B, a fragment was amplified using genomic DNA, input chromatin and DNA immunoprecipitated with antibodies directed against Sp1 or CREB, but not when using antibodies directed against NF-κB p65. Similar bindings of Sp1 and CREB to the MIF promoter were detected when using chromatin isolated from PBMC (Fig. 6B) and HeLa cells (data not shown). Therefore, ChIP assays indicated that the transcription factors Sp1 and CREB bound to cognate regulatory elements in the proximal part of the MIF promoter of THP-1 and HeLa cell lines and of primary cells as well.
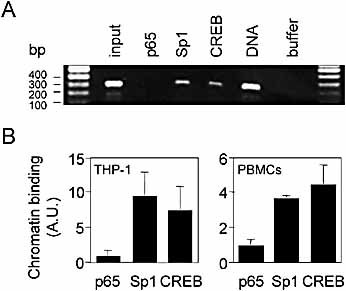
Binding of Sp1 and CREB to bona fide MIF promoter. (A) Chromatin immunoprecipitation of unstimulated THP-1 cells was performed using antibodies directed against Sp1, CREB and NF-κB p65. DNA purified from the immunoprecipitated material was amplified by PCR using primers MIFS7 and MIFAS1 (Table 1) adjacent to the Sp1p and CREp DNA-binding sites. As controls, PCR reactions were also performed with chromatin used as input for the immunoprecipitation (input), buffer and genomic DNA (DNA). (B) Quantification of p65, Sp1 and CREB band intensities in THP-1 cells (means ± SD of four independent experiments) and PBMC (means ± SD of two independent experiments) expressed as ratio of p65, Sp1 or CREB over input. p <0.05 for Sp1 or CREB versus p65 (THP-1 cells and PBMC). A.U., arbitrary units.
DNA methylation does not alter MIF gene expression
DNA methylation is a key epigenetic mechanism of gene silencing. Methylation of cytosine residues by DNA methyltransferases within CpG sites in the human genome may inhibit gene transcription by interfering with the binding of regulatory factors to DNA. Computer analysis of the MIF promoter identified a CpG island of approximately 1.2-kb length starting 300 bp upstream of the transcriptional start site (Fig. 7A). Thirty-four CpG sites were detected within the proximal MIF promoter (ranging from −300 to +1 bp). Several of the CpG sites were part of the binding sites of transcription factors, which included the Sp1p and CREp sites. We therefore hypothesized that variation of MIF expression in THP-1, HeLa, HaCat and A549 cells might be influenced by differences in DNA methylation. To test this hypothesis, we sequenced sodium bisulfite-treated genomic DNA. Indeed, nonmethylated cytosines are converted to uracils in bisulfite-treated single stranded DNA, whereas 5-methyl-cytosine residues are protected from this conversion. Upon sequencing of PCR-amplified bisulfite-treated DNA, conservation of cytosine implied that the corresponding residue was methylated. A 491-bp fragment of bisulfite-treated genomic DNA, which included the 34 CpG sites of the proximal MIF promoter, was amplified by PCR, cloned and sequenced. As shown in Fig. 7B, only 2 of the 34 cytosine residues within the identified CpG sites were found to be methylated. The 2 methylated cytosines, located at position 9 and 19, were observed in one of five sequences obtained from HaCat keratinocytes and from THP-1 monocytic cells. To confirm this observation in primary cells, we analyzed the methylation status of the MIF promoter in PBMC isolated from three healthy subjects. Only 2 methylated CpG sites, located at position 24 and 30, were detected in 2 of 12 sequences (Fig. 7B), confirming that methylation of CpG sites within the proximal MIF promoter is a rare event.
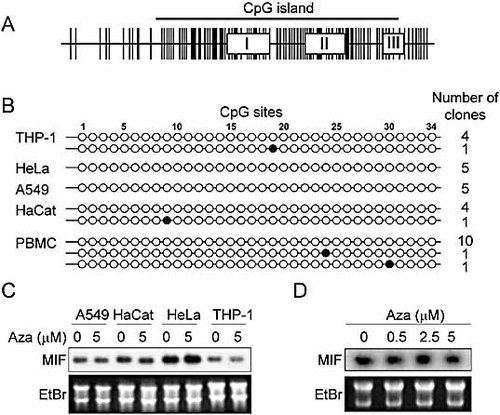
Epigenetic modification of MIF gene expression: no evidence for DNA methylation of MIF CpG island. (A) In silico analysis of CpG sites (vertical lines) within a 4-kb human genomic DNA fragment containing the MIF gene (exons are depicted by boxes) identified a CpG island of about 1.2 kb. (B) Methylation status of the 34 CpG sites of the proximal human MIF promoter (ranging from −300 to +1 bp) in THP-1, HeLa, A549 and HaCat cells and in PBMC isolated from three healthy subjects. Unmethylated and methylated cytosines are represented by white and black circles, respectively. The total number of individual clones analyzed is on the right side of the panel. (C, D) Northern blot analyses of MIF mRNA levels in THP-1, HeLa, A549 and HaCat cells (C) and BM-derived macrophages (D) cultured for 24 h with 5-aza-2′-deoxycitidine (Aza).
Given that the CpG island extends beyond the proximal MIF promoter to encompass the entire MIF gene (Fig. 7A), methylation of CpG sites may occur outside the MIF promoter and nonetheless affect MIF expression. To explore this possibility, cell lines were cultured for 24 h with 5-aza-2′-deoxycitidine, a DNA methyltransferase inhibitor. As shown in Fig. 7C, treatment of A549, HaCat, HeLa, or THP-1 cells with 5-aza-2′-deoxycitidine did not alter MIF mRNA expression. Similar results were obtained with primary bone marrow-derived macrophages (Fig. 7D). These observations argued strongly against a role for DNA methylation as an epigenetic mechanism affecting MIF gene expression in myeloid, epithelial or keratinocytic cells.
Microbial products up-regulate MIF gene expression
The data presented above indicated that Sp1 and CREB are critical regulators of constitutive expression of the MIF gene, resulting in the accumulation of preformed intracellular pools of the MIF cytokine that is rapidly released upon exposure to microbial products 4, 5, 9. Given that MIF promotes pro-inflammatory responses of innate immune cells and plays a critical role in host innate immune defenses against a broad range of microbial pathogens 4–6, 10, 11, 14–17, 26, 27, we examined whether MIF gene expression was affected by exposure of THP-1 cells to Gram-negative (Escherichia coli and Neisseria meningitidis) and Gram-positive (Streptococcus pneumoniae) bacteria and to microbial products (LPS).
As shown in Fig. 8A–C, stimulation of THP-1 cells with E. coli resulted in a 2- to 3-fold increase in the MIF promoter activity (−1720/+129, −858/+129 and −157/+129 constructs) and 1.6-fold up-regulation of MIF mRNA levels that peaked 2 to 4 h after stimulation and returned to baseline levels within 8 h. Disruption of the Sp1p site, but not of the CREp site, reduced E. coli- induced up-regulation of the −157/+129 MIF promoter activity from 3.1-fold to 1.7-fold, while disruption of both Sp1p and CREp sites fully abrogated promoter induction (Fig. 8D). These findings indicated that the presence of the CREp site was also required to mediate optimal transcriptional activity. Similar increases of Sp1- and CRE-dependent induction of MIF promoter activity and of MIF mRNA expression were observed when THP-1 cells were stimulated with LPS, N. meningitidis and S. pneumoniae (data not shown). Mithramycin A inhibited E. coli-mediated induction of MIF promoter activity in a dose-dependent manner (Fig. 8E), confirming the critical role played by Sp1 DNA-binding activity in MIF expression after exposure to microbial products. We next performed time-course analyses of nuclear protein binding to the Sp1p and CREp sites. Within 1 h of exposure of THP-1 cells to E. coli, the DNA-binding activity to the Sp1p site increased 11-fold and returned to baseline levels within 4 h, while DNA-binding activity to the CREp site remained unchanged over the same period (Fig. 8F). Similar increases of DNA-binding activity to the Sp1p site were observed in THP-1 cells stimulated for 4 h with LPS (9-fold), E. coli (12-fold), N. meningitidis (12-fold) and S. pneumoniae (7-fold) (data not shown). To test whether the increased DNA-binding activity to the Sp1p site was coupled with an increased recruitment of Sp1 to the proximal MIF promoter in vivo, we next performed ChIP assays. No significant increase of Sp1 binding was detected using chromatin isolated from THP-1 cells stimulated with E. coli (Fig. 8G). However, it is now recognized that stimulus-induced phosphorylation of Sp1, mediated by MEK1/2 or p38 28–32, may increase its transactivation activity with or without any changes in its DNA-binding activity. We therefore examined whether MEK1/2, p38 and phosphorylated Sp1 were involved in E. coli-stimulated THP-1 cells. Western blot analyses showed that both phosphorylated and non-phosphorylated Sp1 nuclear contents increased in E. coli-stimulated THP-1 cells, whereas the amount of CREB remained unchanged (Fig. 8H). We next studied the effect of U0126, a specific inhibitor of MEK1/2, and SB203580, a specific inhibitor of p38, on the response of THP-1 cells to E. coli. MIF promoter activity (Fig. 8I), DNA-binding to the Sp1p site (Fig. 8J) and MIF mRNA expression (Fig. 8K) were all inhibited by U0126, but not by SB203580. As expected, mithramycin A also inhibited MIF mRNA up-regulation in E. coli-stimulated THP-1 cells (Fig. 8K). Altogether, these results strongly argue in favor of a central role of the MEK1/2 signaling pathway in the phosphorylation of Sp1 and MIF expression upon exposure to E. coli. Recapitulating the results obtained in THP-1 cells, stimulation of PBMC with E. coli increased total and phosphorylated Sp1 nuclear levels (Fig. 9A), DNA-binding activity to the Sp1p site (Fig. 9B) and MIF mRNA expression (Fig. 9C).
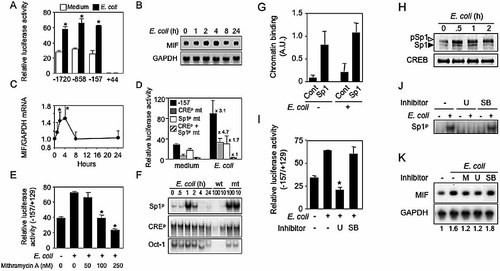
Up-regulation of MIF promoter activity and MIF mRNA expression in THP-1 cells stimulated with E. coli is MEK1/2- and Sp1-dependent. (A) Activity of MIF promoter constructs *p <0.005. (B, C) Northern blot analysis (B) and quantification (n = 3; *p = 0.03) (C) of MIF mRNA expression (D, E) Activity of wild-type and mutated −157/+129 MIF promoter constructs, * p < 0.001. Numbers (D) indicate fold-increase over baseline of luciferase activity. (F) DNA-binding activity analyzed by EMSA as reported in Fig. 3. Results are representative of five experiments. (G) ChIP analysis of THP-1 cells stimulated for 1 h with E. coli (n = 5). (H) Western blot analysis of nuclear phosphorylated (pSp1) and non-phosphorylated (Sp1) Sp1 and CREB. Results are representative of two independent experiments. (I-K) −157/+129 MIF promoter activity (n = 3; *p < 0.001) (I), DNA-binding activity to the Sp1p site (J) and MIF mRNA expression (K) in THP-1 cells pre-incubated for 2 h with mithramycin A (M, 250 nM), U0126 (U) or SB203580 (SB) prior to stimulation with E. coli for 16 (I), 1 (J) or 2 h (K).
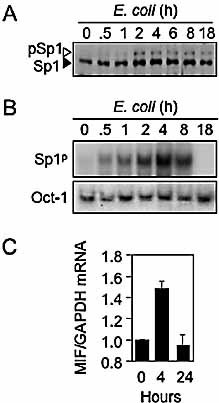
E. coli induces MIF expression in PBMC. Sp1 nuclear content, DNA-binding activity to the Sp1p site and MIF mRNA expression (means ± SD of three independent experiments) were analyzed by Western blot (A), EMSA (B) and Northern blot (C), as described in Fig. 8. Results are representative of three independent experiments.
Discussion
A rapidly growing body of literature indicates that the cytokine MIF is a critical regulator of innate and acquired immunity, inflammation and oncogenesis 8, 25. Yet, four decades after its identification, the molecular mechanisms regulating MIF gene expression remain unknown. Unlike many other cytokines, MIF mRNA and MIF protein are constitutively expressed by all MIF-containing cells. Here, we show that transcription of MIF in human monocytes/macrophages (THP-1), epithelial (HeLa and A549) and keratinocytic (HaCat) cells is critically dependent upon the binding of Sp1 and CREB transcription factors to proximal Sp1 and CRE regulatory elements located within 50 bp upstream the transcriptional start site of the MIF gene. Interestingly, the proximal Sp1 and CRE sites showed a perfect match with the consensus GC-box (GGGGCGGGG) and CRE half-site (CGTCA), respectively, which was not the case of the distal Sp1 (GGGGCGGAG) and distal CRE (CTTCC) sites. The higher degree of homology of the proximal Sp1 and CRE sites with the consensus sequences most likely accounts for the preferential binding of Sp1 and CREB transcription factors to the proximal DNA-binding sites.
Single mutation of either the proximal Sp1 site or the proximal CRE site reduced MIF promoter activity, while dual mutation of the two sites abolished promoter activity, indicating that Sp1 and CRE cis-acting elements cooperatively regulate constitutive MIF promoter activity. Cooperative involvement of neighboring Sp1 and CRE regulatory elements has previously been involved in the constitutive expression of rat ATP1A1 (formally called Na,K-ATPase α1 subunit) and Creb genes 33, 34, in basal, gastrin and PMA-induced human Chromogranin A (CHGA) expression in gastric carcinoma cells 35, 36 and in prostaglandin-stimulated human ATP1A1 promoter activity in epithelial cells 37. In contrast to a previous report, which suggested a direct physical interaction between Sp1 and CREB to regulate of Na,K-ATPase β1 promoter activity in MDCK cells 37, co-immunoprecipitation experiments did not reveal a similar kind of interaction between Sp1 and CREB in HeLa or THP-1 cells (data not shown). Binding of Sp1 and CREB to closely located Sp1 and CRE sites of the mouse TCR α enhancer has also been shown to play an important role in the formation of the enhanceosome complex involved in developmental regulation of TCR α rearrangement 38. Of note, whereas either Sp1 or CREB have been involved in stimulus-induced expression of various cytokines (such as TNF, IL-2, IL-8, IL-10, MIP-2 and GM-CSF), to the best of our knowledge, MIF is the first example of a cytokine whose basal and stimulus-induced expression depends on combined action of Sp1 and CREB.
Interestingly, while species-specific putative DNA-binding sites have been identified in the human and in the mouse MIF promoters (Fig. 2C), Sp1 and CRE sites have been detected both in human and mouse MIF promoters. The CRE site of the mouse MIF promoter has been implicated in a cAMP-dependent Mif gene activation in anterior pituitary cells 39. One can therefore postulate that common regulatory elements are implicated in constitutive expression of the human and mouse MIF genes. The observation that both Sp1 and CREB are required for optimal transcription of MIF is consistent with the fact that the MIF gene lacks a TATA box. In the absence of a TATA box, Sp1 facilitates the binding of the transcription factor TFIID and assembly of the pre-initiation transcriptional complex needed for RNA polymerase II binding to the promoter 40, 41. Likewise, CREB, CREB-binding protein (CBP) and its paralogue p300 may interact with TFIID and trigger the recruitment of RNA polymerase II complexes 42, 43. One would therefore predict that conditions in which Sp1 and CREB activities are increased would also be associated with up-regulated MIF expression. In agreement with this assumption, microbial stimuli (LPS, peptidoglycan), cytokines (TNF, IL-1), cyclic nucleotides signaling (forskolin), ultraviolet light and hypoxia have been reported to activate CREB and to induce MIF expression 5, 9, 42, 44–46. However, CREB may also exert negative regulatory effects in HIF-1-mediated hypoxic induction of MIF 47. Finally, elevated Sp1 expression and DNA-binding activity have been reported in epithelial tumors 40 and high levels of CREB have been detected in the bone marrow from patients with acute leukemia 48. Overexpression of Sp1 and/or CREB in tumor cells may thus explain why copious amounts of MIF have been detected in cancer cells.
Methylation of the C5 cytosine residues by DNA methyltransferases within CpG sites is a classical epigenetic silencing mechanism. Several studies have been published recently that support a role for DNA methylation in cell type-specific gene silencing. For example, the promoter region of the Arachidonate 15-lipoxygenase gene was methylated in T lymphocytes, but not in monocytes 49 and that of the SERPINB5 gene was methylated in fibroblasts and lymphocytes, but not in mammary epithelium and skin keratinocytes 50, 51. Interestingly, the MIF gene was found to be located within a CpG island and both Sp1 and CRE DNA-binding sites contained CpG sequences 42, 52. Sequencing of the first 34 CpG sites after treatment of DNA from THP-1, HeLa, A549 and HaCat cell lines and human PBMC with bisulfite revealed that CpG methylation of the MIF proximal promoter was a very rare event (4 of 1088 sites were methylated). While most studies have focused on CpG methylation of proximal promoters, recent reports have highlighted the fact that methylation of other gene regions may affect gene expression as well. Methylation of CpG sites located either in the first exon or 1.2 kb upstream of the transcription start site markedly affected expression of the DANJDC15 gene or of the IL8 gene, respectively 50, 53. However, there was no evidence for methylation of CpG sites positioned outside of the MIF promoter. Indeed, MIF mRNA levels of primary BM-derived macrophages, HeLa, A549, HaCat or THP-1 cells did not change upon treatment of cells with the demethylating agent 5-aza-2′-deoxycitidine. Taken together, these data strongly suggest that DNA methylation does not influence MIF gene expression despite its location within a CpG island in the human genome. Beside DNA methylation, chromatin remodeling via histone modifications, such as acetylation, ubiquitination, sumoylation, methylation or phosphorylation is another fundamental epigenetic mechanism regulating gene expression 54. In recent years, chromatin acetylation has been shown to control the expression of several cytokines and immune genes. It would thus be interesting in examining the impact of histone modifications and post-transcriptional regulatory events, such as mRNA stability, translation and protein post-translational localization and secretion, in the control of MIF expression.
A unique feature of MIF is its induction by low dose of glucocorticoids 6, 55. Of note, a negative glucocorticoid-regulatory element (-224 to –216 bp) has been identified in the mouse MIF promoter 56. However, recent studies have also shown that high-dose dexamethasone reduced serum MIF levels and suppressed MIF production by T lymphoblasts and human lung epithelial cells 57. Therefore, it seems that glucocorticoid-induced regulation of MIF production may vary with factors such as species, cell type and type and doses of corticosteroids used. Dissection of the molecular mechanisms underlying the induction or inhibition of MIF expression by glucocorticoids will require further studies.
Although Sp1 DNA-binding activity has been considered to be primarily constitutive, stimulation of immune and non-immune cells with LPS, Staphylococcus aureus, HIV, TGF-β or angiotensin II has been shown to increase Sp1 DNA-binding activity to Sp1 consensus or Sp1 sites of the TNF, IL-10, IL-12p40, CCL22, MMP-1 and tissue inhibitor of MMP-1 (TIMP-1) promoters 28–30, 57–63. MIF mRNA and protein were found to be increased after LPS administration 4 and circulating levels of MIF were elevated in patients with severe sepsis and septic shock 11. The present findings of a marked up-regulation of Sp1 DNA-binding activity, Sp1-dependent increased MIF promoter activity and elevated MIF mRNA expression in THP-1 monocytic cells and in PBMC stimulated with microbial products provide strong evidence supporting a key role for Sp1 in the regulation of MIF expression in innate immune cells and host antimicrobial defense responses.
The induction of IL-10 by LPS in mouse macrophages and that of vascular endothelial growth factor (VEGF) by HGF in human keratinocytes has been shown to be regulated by Sp1 without any changes in nuclear expression or in DNA-binding activity 31, 64, 65. In fact, it is now recognized that stimulus-induced phosphorylation of Sp1 may increase its transactivation activity with or without enhancing its DNA-binding activity. For example, the p38 MAPK signaling pathway has been reported to induce IL-10 via the activation of Sp1 in LPS- and HIV-Tat-stimulated human monocytes/macrophages 28, 30. Similarly, the MEK/ERK MAPK signaling pathway have been implicated in Sp1-dependent induction of either VEGF, TIMP-1 or heme oxygenase-1 transcription by HGF, TGF-β and nerve growth factor (NGF) in keratinocytes, fibrosarcoma cells and PC12 adrenal cells 29, 31, 32. In agreement with these observations, we found that stimulation of THP-1 cells and of PBMC with microbial products up-regulated phosphorylated Sp1 nuclear content, Sp1 DNA-binding activity, MIF promoter activity and MIF mRNA levels in a MEK1/2-, Sp1-dependent manner. The fact that the inhibition of MEK1/2 fully abolished E. coli-induced Sp1 DNA-binding activity and MIF expression emphasizes the concept that phosphorylation of Sp1 plays an important role in mediating MIF gene induction by microbial pathogens.
Interestingly, mutation of the proximal CRE site also markedly reduced MIF promoter activity in response to microbial products. DNA-binding activity to the proximal CRE site did not increase in cells stimulated with microbial products. CREB is activated via the phosphorylation of serine residue 133 in response to various stimuli 42, 45. Previous work has shown that exposure of macrophages to LPS increased CREB-phosphorylation and CREB-dependent gene transcription without concomitant increase of CREB levels 66. Therefore, microbial stimulation may increase MIF promoter activity and promote MIF expression in a CREB-dependent fashion without increasing CREB DNA-binding activity to the proximal CRE site. Interestingly, CREB has recently been characterized as an anti-apoptotic transcription factor in macrophages stimulated with LPS. CREB was shown to promote macrophage survival, at least in part, via the induction of plasminogen activator 2 (PAI-2) which blocks the activation of the pro-apoptotic pathway induced by LPS 66. Reminiscent of this observation, an important mode of action of MIF within the innate immune system is its ability to sustain pro-inflammmatory response by inhibiting LPS-induced, p53-mediated apoptosis in macrophages 16. These observations therefore suggest that MIF belongs, like PAI-2, to the CREB-dependent anti-apoptotic pathway activated by microbial stimulation in the macrophage. It also emphasizes the critical role played by CREB in regulating MIF expression and innate immune responses.
In summary, analyses of the transcriptional regulation of the MIF gene have revealed that the binding of Sp1 and CREB to cis-acting regulatory sequences located in the proximal MIF promoter is essential for constitutive transcription of the MIF gene in several cell types and MIF gene up-regulation in cells of the monocyte/macrophage lineage and PBMC after stimulation with microbial pathogens. Given the critical role played by MIF in promoting pro-inflammatory responses, the identification of transcription factors involved in the regulation of MIF expression is likely to provide important insights into the molecular mechanisms leading to up-regulated expression of MIF that has been shown to play an important role in the pathogenesis of numerous inflammatory, infectious and autoimmune diseases.
Materials and methods
Cells and reagents
Human monocytic THP-1 cells and airway epithelial A549 cells were cultured in RPMI 1640 with Glutamax. Human cervix epithelial HeLa cells and keratinocytic HaCat cells were cultured in DMEM. BM cells isolated from femurs and tibias of mice (under the authorization from the State Veterinary Service, Vaud, Switzerland) were cultured in IMDM containing 2-mercaptoethanol and M-CSF. Spodoptera frugiperda Sf9 cells were cultured in Grace's Insect medium. PBMC were isolated from pools of four to six blood filters (obtained from the Blood Center, Lausanne, Switzerland), purified by Ficoll-Hypaque density gradient (GE Healthcare) and cultured overnight in RPMI 1640 with Glutamax. All media were supplemented with 10% FCS (Seromed) and antibiotics. Where indicated, THP-1 cells, BM-derived macrophages and PBMC were incubated with 5-aza-2′-deoxycitidine, mithramycin A (Sigma), U0126 (10 μM), SB203580 (10 μM) (Calbiochem-Novabiochem), 100 ng/mL Salmonella ultra pure LPS (List Biologicals Laboratories) or 108 CFU/mL heat-inactivated (56°C for 2 h) E. coli O111:B4, N. meningitidis and S. pneumoniae.
Plasmid preparation
A 3-kb fragment corresponding to region −2802/+129 from the human MIF gene was amplified by Expand Long Template PCR System (Roche Diagnostics) from human genomic DNA using MIFS1 and MIFAS1 oligonucleotides (Table 1), cloned into the pGEM®-T Easy vector (Promega) and sequenced. The fragment was subcloned in the BglII site from the pGL3-basic vector (Promega). Constructs −2688/+129, −1720/+129, −1072/+129 and +44/+129 were obtained after digestion of construct −2802/+129 with NsiI-XhoI, PacI-XhoI, PstI-XhoI and SacII-XhoI, respectively, with re-ligation. Constructs −508/+47 and −157/+47 were obtained after digestion of −508/+129 and −157/+129 constructs with SacII-HindIII and re-ligation. Fragments −858/+129, −508/+129, −157/+129 and +81/+129 were amplified by PCR using MIFS2, MIFS3, MIFS7 and MIFS8 together with MIFAS1 oligonucleotide, respectively, purified, cloned in pGEM®-T Easy, sequenced and sub-cloned in pGL3. Potential DNA-binding sites for transcription factors within the −200/+129 region of the MIF promoter were identified by computer analysis. Mutations of DNA-binding-sites were performed by PCR using the QuikChange™ Site Directed Mutagenesis Kit (Stratagene) and sense and antisense mutant oligonucleotides (Table 1).
|
Oligonucleotide |
5′→ 3′ sequence |
|
|---|---|---|
|
For probe synthesis |
||
|
MIF |
sense |
CACGCTCGCAGTCTCTC |
|
|
antisense |
GAGGCTCAAAGAACAGC |
|
For promoter constructa) |
||
|
MIFS1 |
|
AAGATCTCCAGGTAACATGGCATACTT |
|
MIFS2 |
|
AAGATCTTGTCCTCTTCCTGCTATG |
|
MIFS4 |
|
AAGATCTTTCCCTGGATGGTGATTC |
|
MIFS7 |
|
AAGATCTGGGCTTAGCGGCGGTTGC |
|
MIFS8 |
|
AAGATCTCGGTGACTTCCCCACTCG |
|
MIFAS1 |
|
AAGATCTGGCACGTTGGTGTTTACGAT |
|
For mutagenesis and EMSAb) |
||
|
c-Myb |
|
CCGGGCTTAGCGGCGGTTGCTGGAGGAACG |
|
CREp |
|
GCGGTGGCGTCACAAAAGGCGG |
|
Sp1p |
|
GCCTCGCGGGGGCGGGGCCTGGCG |
|
AML-1a |
|
GACCACAGTGGTGTCCGAGAAGTC |
|
AP-4 |
|
CACGTAGCTCAGCGGCGGCCGCGGCG |
|
c-Myb mt |
|
GGGCTTAGCGGCGTAGACTGGAGGAACGGG |
|
CREd mt |
|
GAGGCAGGCGGAATGTTCCCCACTCGGGGC |
|
CREp mt |
|
GCCGGCGGTGGATACACAAAAGGCGGGAC |
|
Sp1d mt |
|
GACTTCCCCACTCGGTAGAGAGCCGCAGCCCT |
|
Sp1p mt |
|
CAGCCTCGCGGGTAGAGGGCCTGGCGCC |
|
AML-1a mt |
|
GCGGGACCACAGTGACGTCCGAGAAGTC |
|
AP-4 mt |
|
GCACGTAGCTAGATGGCGGCCGCGGCGC |
|
CRE cs |
|
AGAGATTGCCTGACGTCAGAGAGCTAG |
|
Oct-1 cs |
|
GGTCGAATGCAAATCACTAGACGT |
|
For PCR amplification of bisulfited DNA |
||
|
MetMIF1 |
sense |
TGTGGTTTAAAGATAGGAGGTATAGG |
|
|
antisense |
TAATAACAAAAAAACCAAAAAACCC |
- a) BglII restriction sites are underlined.
- b) Base substitutions are underlined. p, proximal; d, distal; mt, mutant; cs, consensus.
Transfection
Cells were incubated in 24-well culture plates and transiently transfected when reaching 30% confluency with 600 ng of a luciferase reporter vector together with 60 ng of a Renilla luciferase control vector using the Fugene 6™ transfection reagent (Roche Diagnostics). Co-transfection with an Sp1 expression construct was performed using the amount of plasmid specified in Figure 4. Total DNA quantity was kept constant by adding empty expression vector. Cells were cultured for 24 h (mammalian cells) or 48 h (Sf9 cells). Luciferase and Renilla luciferase activities were measured using the Dual-LuciferaseTM Reporter Assay System (Promega). Results were expressed as relative luciferase activity (the ratio of luciferase to Renilla luciferase activity). siRNA directed against human Sp1 (Hs_SP1_1_HP) and CREB (Hs_CREB1_5_HP) and a control siRNA (GTATCTCCGGCTTCCATCGTT) were obtained from Qiagen. siRNA transfection was performed using the HiPerFect transfection reagent (Qiagen). Irrelevant, control, siRNA did not influence –157/+129 MIF promoter activity in HeLa cells (i.e. 98% activity in control siRNA treated cells when compared to untreated cells in eight independent experiments). Each transfection experiment was performed at least twice and results of one representative experiment performed in triplicate are shown. Unpaired Student's t-test was used for statistical analysis. The p values <0.05 were considered to be statistically significant.
RNA preparation and analysis
MIF mRNA expression was assessed by Northern blotting 67. The MIF probe was obtained by PCR amplification of human monocyte cDNA (primers are listed in Table 1).
EMSA and supershift
Nuclear extracts were prepared and analyzed as previously described by EMSA 68. Supershift analyses were performed using 06–519 (Upstate), sc-59X, –643X, –644X or –109X (Santa Cruz Biotechnology) antiserum specific for CREB, Sp1, Sp2, Sp3 or NF-κB p65, respectively.
Chromatin immunoprecipitation (ChIP) assay
ChIP analysis was performed using the ChIP assay kit (Upstate) according to the manufacturer's recommendations. IP was performed for 18 h at 4°C using anti-CREB, anti-Sp1 (07–645 from Upstate), or anti-NF-κB p65 antisera. Immunoprecipitated DNA was eluted from the histone-DNA complexes and amplified by PCR using MIFS7 and MIFAS1 primers (Table 1).
Bisulfite methylation assay
Genomic DNA was isolated from post-confluent cells and from PBMC. DNA (2 µg) was digested with EcoRI for 1 h at 37°C, purified by phenol:chloroform:isoamyl alcohol extraction and precipitated. DNA was denaturated 30 min at 42°C in 0.3 M NaOH. After adjustment to 3.3 M sodium bisulfite and 0.5 µM hydroquinone, reactions were incubated for 12–15 h at 55°C in the dark. Genomic DNA was purified, desulfonated, precipitated and resuspended in 10 mM Tris/1 mM EDTA pH 7.5. Two out of fifty microlitters were used for PCR amplification using MetMIF1 primers (Table 1). The amplified products were cloned in the pGEM®-T vector (Promega) and sequenced.
Western blot analyses
Nuclear extracts were analyzed by SDS-PAGE and Western blotting using anti-Sp1 and anti-CREB (07–645 and 06–519, Upstate) antisera and HRP-conjugated immunopure goat anti-rabbit IgG antibody (31460, Pierce). Signals were revealed using the ECL Western blotting analyses system (GE Healthcare).
Acknowledgements
We are grateful to Dr. A. Regamey for providing the HaCat cell line and Amar Abderrahmani for providing the Sp1 expression vector. This work was supported by a grant from the Leenaards Foundation to TR and grants from the Swiss National Science Foundation (32–49129.96 and 3100–066972.01), the Bristol-Myers Squibb Foundation, the Leenaards Foundation and the Santos-Suarez Foundation for Medical Research to TC. TC is a recipient of a career award from the Leenaards Foundation.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no conflict of interest.




