Identification of a novel negative role of flagellin in regulating IL-10 production
Abstract
Toll-like receptors (TLR) expressed by cells of the immune system play a central role in the generation of immune responses against pathogens. Following TLR ligation, both pro-inflammatory and anti-inflammatory mediators are produced in order to elicit an immune response that controls the microbial infection while limiting tissue damage. Among these mediators, the pro-inflammatory cytokine IL-12 and the anti-inflammatory cytokine IL-10 are known to play major roles. Here, we show that in vitro or in vivo stimulation with flagellin, the TLR5 ligand, does not result in IL-10 production. Furthermore, flagellin inhibits IL-10 production by other specific TLR ligands at the protein and mRNA levels while increasing IL-12p70 production. Several studies have linked the activation of extracellular signal-regulated kinases with IL-10 induction by TLR. We have observed that LPS-induced extracellular signal-regulated kinase activation was significantly decreased in flagellin-treated macrophages, suggesting that this pathway might play a role in the inhibition of IL-10 production observed in flagellin-treated macrophages. Flagellin-mediated IL-10 inhibition was not observed in cells that do not express TLR5, supporting that this effect is indeed TLR5-dependent. This study provides a new insight into the role of flagellin recognition by TLR5 in shaping the immune response elicited by flagellated microorganisms.
Abbreviation:
-
- PEM:
-
peritoneal elicited macrophages
Introduction
Toll-like receptors (TLR) expressed in cells of the innate immune system represent the first sensors of conserved microbial components and are critical for the generation of productive immune responses against pathogens 1. Macrophages and dendritic cells (DC) display a broad repertoire of TLR through which they can interact with different microbial products either simultaneously, sequentially or in different cellular compartments 2, 3. This interaction results in profound changes in the phenotype and function of these cells many of which are directed towards cytokine production. Cytokines play an important role in shaping and expanding the immune response against pathogens and in terminating it before tissue damage occurs.
Following TLR engagement, several cytokines are produced by macrophages and DC, such as the pro-inflammatory mediator IL-12 4 and the anti-inflammatory cytokine IL-10 5. IL-12 is required for resistance against bacterial and parasitic infections, but persistently elevated levels will result in autoimmunity 4. IL-10 has been shown to play a key role in limiting the magnitude and intensity of inflammatory responses to prevent tissue damage 5. In fact, lethal autoimmunity caused by aberrant inflammatory response to commensal microorganisms has been observed in IL-10-deficient mice 6. Therefore, production of IL-10 and IL-12 in response to TLR ligands determines not only the initiation and amplification but also the duration and termination of the immune response against pathogens 7.
Flagellated microorganisms express several TLR ligands including flagellin, a protein constituent of the bacterial flagella. Flagellin is recognized by TLR5 8, a receptor expressed mainly in epithelial and immune cells 3, 9–11. A particular characteristic of certain flagellated bacteria such as Salmonella and Legionella is their ability to induce inflammation in the colon and lungs. In these organs immune responses are tightly regulated mainly by the production of IL-10 to avoid inflammation caused by commensal microorganisms 12–14. Although flagellin has been shown to be central in the induction of inflammatory responses by flagellated bacteria in these environments 15–17, the underlying mechanism(s) have not been fully elucidated. The recent description of the TLR5–/– mice will help to better define the function of TLR5 in vivo 18, 19.
Here, we show that macrophages treated in vitro with flagellin did not produce the anti-inflammatory cytokine IL-10, but produce the pro-inflammatory mediator IL-12. Our in vitro observation was reproduced in vivo, demonstrating that IL-12 but not IL-10 was produced by flagellin-treated mice. Furthermore, our results show that flagellin negatively influences the production of IL-10 in response to other TLR ligands, while augmenting IL-12p70 production. Our findings have revealed a previously unknown effect of flagellin in negatively regulating IL-10 production. These results provide a potential explanation of how flagellin might shape the inflammatory responses elicited by flagellated pathogens.
Results
Flagellin-stimulated peritoneal elicited macrophages produce IL-12 but not IL-10
Peritoneal elicited macrophages (PEM) were first treated in vitro with increasing concentrations of purified flagellin or with LPS for 24 h. Supernatants were collected and production of IL-12p40/p70 and IL-10 were determined by ELISA. Reminiscent of previous studies 5, LPS-treated PEM produce both the pro-inflammatory cytokine IL-12 as well as the inhibitory cytokine IL-10 in a dose-dependent manner (Fig. 1A). Treatment of PEM with flagellin also resulted in a dose-dependent production of IL-12p40/p70 (Fig. 1B). The magnitude of this response was, however, not as potent as the one induced by LPS (Fig. 1A versus 1B, black squares).
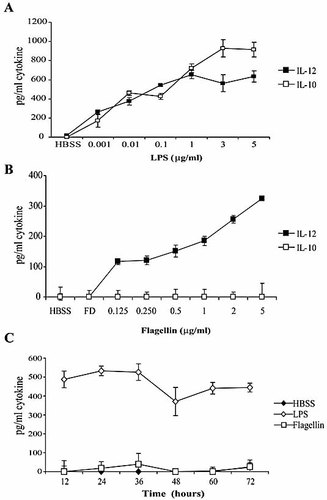
IL-12p40/p70 but not IL-10 is detected in the supernatants of flagellin-stimulated PEM. (A, B) PEM from BALB/c mice (1×105 cells/well) were cultured for 24 h with the indicated concentrations of LPS (A), flagellin (B) or proteinase K-digested flagellin (FD, volume added equivalent to 5 μg/mL flagellin) (B). Supernatants were then collected and IL-12p40/p70 and IL-10 were determined by ELISA. Data represent mean ± SD of triplicate cultures. Shown is a representative experiment of three independent experiments with similar results. (C) PEM were treated with flagellin (5 μg/mL), LPS (1 μg/mL) or with HBSS. Supernatants were collected at the indicated time points and the production of IL-10 was determined by ELISA. Data represent mean ± SD of triplicate cultures. Shown is a representative experiment of two independent experiments with similar results.
IL-10 is commonly produced by macrophages upon stimulation with different TLR ligands, but to our surprise we did not detect any IL-10 production in flagellin-stimulated PEM (Fig. 1B, open squares). The lack of IL-10 production was not the result of delayed production of this cytokine, since kinetic studies showed that even after 72 h of treatment IL-10 was still not detected in the supernatants of flagellin-treated PEM (Fig. 1C, open squares). In contrast, when PEM were stimulated with LPS, IL-10 was detected at early time points and its levels remained elevated throughout the duration of the incubation period (Fig. 1C, open diamonds).
One concern in the interpretation of the results described above is the possibility that flagellin preparations could be contaminated with LPS. However several controls have ruled out this possibility. First, using the Limulus amebocyte lysate test, no LPS contamination was detected in our flagellin preparation. Second, digestion of the flagellin preparation with proteinase K resulted in loss of its ability to induce IL-12 production in PEM (Fig. 1B, FD; Fig. 2A), indicating that this effect is induced by a protein and not by other non-proteinaceous potential contaminants in the preparation.
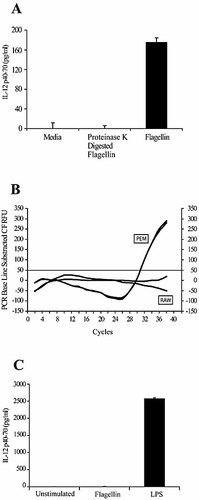
IL-12p40/p70 production by flagellin-stimulated macrophages is not caused by endotoxin contamination. (A) Proteinase K-digested flagellin does not induce IL-12 production by PEM. Peritoneal macrophages were cultured in media alone or in the presence of flagellin (5 μg/mL) digested or not with proteinase K. Supernatants were collected after 24 h and IL-12p40/p70 was quantified by ELISA. Shown is one of at least three experiments made. (B, C) RAW 264.7 cells do not express detectable TLR5 mRNA levels and do not respond to flagellin stimulation. (B) TLR5 mRNA levels from PEM or RAW 264.7 cells were determined by quantitative real-time RT-PCR. GAPDH mRNA levels were similar among the samples compared (data not shown). Shown is a representative experiment of two independent experiments with similar results. (C) RAW 264.7 wild-type cells were cultured for 24 h with flagellin (5 μg/mL), LPS (1 μg/mL) or media alone. Supernatants were collected and the levels of IL-12p40/p70 were measured by ELISA. Data represent means ± SD of triplicate cultures from a representative experiment of three with similar results.
Finally, given the previous demonstration that elicitation of inflammatory effects by flagellin requires binding to TLR5 8, 18, we treated the macrophage cell line RAW 264.7, which lacks TLR5 20 (Fig. 2B, RAW) but still expresses TLR4, with flagellin or LPS. While treatment with LPS resulted in IL-12 production by these cells, treatment with flagellin failed to induce such a response (Fig. 2C), indicating that induction of IL-12 by flagellin requires expression of TLR5 in the target cell (Fig. 2B). The absence of IL-12 production in RAW 264.7 cells also provides additional support to our claim that the flagellin preparation used in our experiments was LPS-free, since some LPS contamination might have otherwise resulted in IL-12 production by RAW 264.7 cells. To conclude, these data show that the effects induced by flagellin upon macrophages are not due to LPS contamination and require expression of TLR5 in the responding cells.
IL-12 but not IL-10 is detected in the serum of flagellin-treated mice
To confirm the above results in vitro, we assessed the production of IL-10 and IL-12 in mice that received a single intravenous injection of purified flagellin or LPS. As shown in Fig. 3A, similar levels of IL-12p40/p70 were detected in the serum of LPS- or flagellin-treated mice. However, a completely divergent outcome in IL-10 production was observed following treatment with either TLR ligand. While significant levels of IL-10 were detected in the serum of LPS-treated mice, this cytokine could not be detected in the serum of flagellin-treated mice (Fig. 3B).
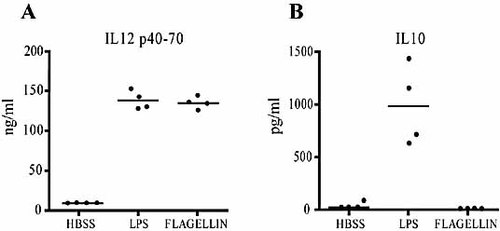
Serum levels of IL-12p40/p70 and IL-10 in mice treated with LPS or flagellin. BALB/c mice were injected intravenously with either flagellin (10 μg), LPS (10 μg) or an equal volume of HBSS (0.1 mL). Blood was collected 1.5 h later and serum levels of IL-12p40/p70 (A) or IL-10 (B) were determined by ELISA. Shown is a representative experiment with four animals per group of two independent experiments with similar results. Each dot represents an animal and the line indicates the group's mean.
IL-10 mRNA dynamics in macrophages treated with flagellin or LPS
To better understand the mechanisms(s) by which flagellin-stimulated PEM, but not LPS-stimulated PEM, are unable to produce IL-10 protein, we next determined the kinetics of IL-10 mRNA in cells treated with either TLR ligand. In response to LPS stimulation, a rapid increase in IL-10 mRNA was observed by 3 h, followed by a peak response at 6 h. This induction is followed by a progressive decline and, after 12 h of exposure to LPS, the IL-10 mRNA levels were back to baseline (Fig. 4A, open squares). In sharp contrast, the magnitude and kinetics of IL-10 mRNA were significantly different in flagellin-treated PEM. After 3 h of incubation, induction of IL-10 mRNA was detected by RT-PCR. This initial response was followed by a rapid decline and by 6 h, IL-10 mRNA levels in flagellin-treated PEM were equivalent to the levels in untreated cells (Fig. 4A, black squares).
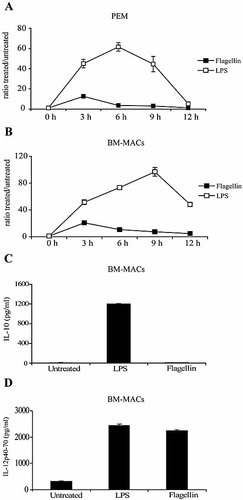
IL-10 mRNA dynamics in flagellin-stimulated macrophages. PEM (A) or BM-derived macrophages (B) were cultured in complete media and stimulated with either LPS (1 μg/mL) or flagellin (5 μg/mL). Cells were then harvested in TriZol at the indicated time points. RNA was extracted from these cells and IL-10 mRNA relative to GAPDH mRNA was determined by quantitative RT-PCR. Data show the ratio expressed in arbitrary units of treated cells versus untreated cells obtained at each of the indicated time points. Shown is a representative experiment of three independent experiments with similar results. (C, D) BM-derived macrophages were cultured for 24 h with LPS (1 μg/mL) or flagellin (5 μg/mL). Supernatants were collected and the levels of IL-10 and IL-12p40/p70 quantified by ELISA. Data represent means ± SD of triplicate cultures. Shown is a representative experiment of two independent experiments with similar results.
In order to expand the above observations further, we generated bone marrow (BM)-derived macrophages and exposed them to either LPS or flagellin. As shown in Fig. 4B, a similar pattern of IL-10 mRNA expression was observed in flagellin-treated BM-derived macrophages. Furthermore, reminiscent of our findings with PEM (Fig. 1B), IL-12p40/p70 but not IL-10 was detected in the supernatants of BM-derived macrophages that were stimulated with flagellin (Fig. 4C, D). In summary, unlike macrophages treated with LPS, flagellin-treated macrophages display a short-lived expression of IL-10 mRNA that seems to be not sufficient enough to result in IL-10 protein production.
Flagellin inhibits IL-10 production induced by specific TLR ligands
During an ongoing infection, flagellin and other TLR ligands expressed in flagellated bacteria are likely to be recognized either simultaneously or sequentially by cells of the immune system displaying TLR. In order to find whether flagellin could influence the production of IL-10 in response to other TLR ligands, we treated PEM with flagellin and the TLR4 ligand (LPS) either simultaneously (Fig. 5A–C) or sequentially (Fig. 5D–F).
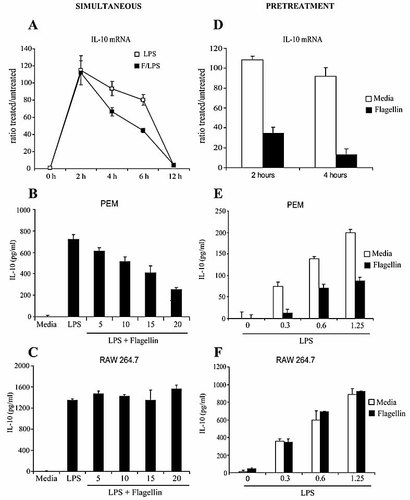
Flagellin inhibits LPS-induced IL-10 protein and mRNA. (A) PEM were stimulated with 1 μg/mL LPS alone (open squares) or LPS (1 μg/mL) plus 20 μg/mL flagellin (F/LPS, black squares). Cells were then harvested at the indicated time points. IL-10 mRNA levels were determined as in Fig. 4. (B, C) PEM or RAW 264.7 cells were stimulated with LPS alone (1 μg/mL) or LPS plus increasing concentrations of flagellin for 24 h. Supernatants were then collected and IL-10 production determined by ELISA. (D) PEM were treated with 5 μg/mL flagellin (black bars) or with media (white bars) for 3 h, followed by LPS (1 μg/mL). Cells were harvested at the indicated time points after the addition of LPS. (E, F) PEM or RAW 264.7 cells were cultured with flagellin (5 μg/mL) or media for 3 h, followed by LPS (1 μg/mL). Supernatants were collected 24 h after LPS addition and the levels of IL-10 were determined by ELISA. Shown is a representative experiment of two (A–C), four (D) or three (E, F) independent experiments.
As shown in Fig. 5A, no differences in IL-10 mRNA levels were observed in peritoneal macrophages after 2 h of exposure to either LPS alone (white squares) or LPS in the presence of flagellin (F/LPS: black squares). However, unlike stimulation with LPS alone, IL-10 mRNA levels declined rapidly by 4 and 6 h of simultaneous engagement of TLR4 and TLR5. Flagellin-induced IL-10 inhibition is also reflected at the protein level. As displayed in Fig. 5B, a dose-dependent inhibition of IL-10 production was observed when PEM were treated with LPS (1 μg/mL) in the presence of increasing concentrations of flagellin. Inhibition of IL-10 was not observed when the TLR5-deficient cell line RAW 264.7 was treated with LPS and flagellin, indicating that flagellin-induced IL-10 inhibition in response to LPS requires TLR5 expression (Fig. 5C).
A more profound inhibition of IL-10 mRNA expression was observed when PEM were exposed to flagellin (5 μg/mL) prior to LPS stimulation (Fig. 5D). In addition, decreased levels of IL-10 protein were observed in PEM pre-treated with flagellin (5 μg/mL) and then stimulated with increasing concentrations of LPS (Fig. 5E). In the pre-treatment setting, flagellin at a dose of 5 μg/mL, which has minimal inhibitory effect when used simultaneously with LPS (Fig. 5B), displayed stronger IL-10-inhibitory properties. Conversely, this inhibitory effect of flagellin on IL-10 production was not observed when TLR5-deficient RAW 264.7 cells were pre-treated with flagellin (Fig. 5F).
To determine whether a similar inhibitory effect on IL-10 production occurs when PEM are exposed to TLR ligands other than LPS, we treated PEM with flagellin followed by stimulation with either zymosan (TLR2/6 ligand) or CpG (TLR9 ligand). As shown in Fig. 6A, treatment of PEM with increasing concentrations of zymosan results in IL-10 production in a dose-dependent manner (open bars). However, when these cells were treated with flagellin and then zymosan, a dramatic decrease in IL-10 production was observed (Fig. 6A, black bars). In contrast, such an inhibitory effect on IL-10 production was not observed when PEM were treated with flagellin and CpG (Fig. 6B). To summarize, our results have revealed a previously unknown effect of flagellin in the regulation of IL-10 production in response to specific TLR ligands.
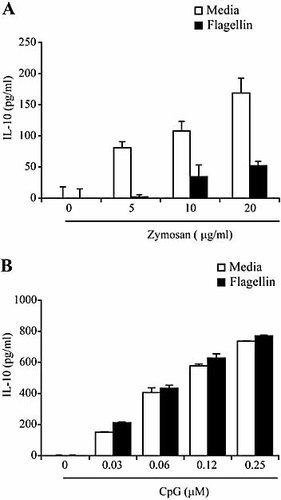
IL-10 production by macrophages treated with flagellin and other TLR ligands. PEM were cultured in media alone (white bars) or with 5 μg/mL of flagellin (black bars) for 3 h, followed by stimulation with either zymosan (A) or CpG (B). Supernatants were collected 24 h later and the production of IL-10 protein was determined by ELISA. Data represent means ± SD of triplicate cultures. Shown is a representative experiment of two independent experiments with similar results.
Flagellin treatment does not affect the stability of LPS-induced IL-10 mRNA
In order to determine whether suppression of LPS-induced IL-10 by flagellin was caused by an increase in IL-10 mRNA degradation, we treated macrophages with actinomycin D, an inhibitor of mRNA synthesis, and followed the changes induced by flagellin in the stability of LPS-induced IL-10 mRNA. Fig. 7A shows that actinomycin D suppresses mRNA synthesis. For this control experiment, cells were treated with LPS plus actinomycin D or LPS plus media for 2 h. Cells were then harvested and the levels of IL-10 mRNA were measured by RT-PCR. Actinomycin D (5 μg/mL) completely blocked the synthesis of new mRNA (LPS+ActD).
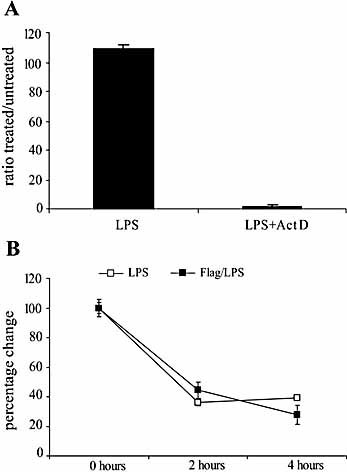
Inhibition of LPS-induced IL-10 production by flagellin is not caused by a decrease in IL-10 mRNA stability. (A) PEM were cultured with LPS (1 μg/mL) plus actinomycin D (5 μg/mL) or vehicle. After 2 h of treatment the cells were harvested and the IL-10 mRNA levels relative to GAPDH were determined by real-time RT-PCR. (B) Actinomycin D or vehicle was added to the media 2 h after LPS treatment of PEM that had been cultured with or without flagellin. Cells were harvested at the indicated times after actinomycin D addition and the IL-10 mRNA levels relative to GAPDH mRNA were determined by real-time RT-PCR. Shown is a representative experiment of two independent experiments with similar results.
In our next set of experiments, PEM were cultured for 3 h with either flagellin or media before LPS treatment. Actinomycin D was added to the cultures 2 h after LPS treatment and cells were harvested at the indicated time points after actinomycin D treatment. As shown in Fig. 7B, an almost identical rate of IL-10 disappearance was observed, suggesting that pre-treatment with flagellin does not affect IL-10 mRNA stability in response to LPS stimulation.
Flagellin stimulation decreases ERK activation by LPS in macrophages
A general outcome of TLR engagement is the activation of NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways 21. Among the MAPK, several studies have linked the extracellular signal-regulated kinases (ERK) with IL-10 regulation by TLR 22–26. In order to determine whether flagellin stimulation would affect LPS-induced ERK phosphorylation, we stimulated flagellin- or media-treated PEM with LPS. Since significant changes in the LPS-induced IL-10 mRNA levels are detected in flagellin-treated macrophages as early as at 2 h (Fig. 5D), we decided to quantify ERK phosphorylation at earlier time points (30 and 60 min). Interestingly, LPS-induced phosphorylated ERK1/2 levels were considerably reduced in flagellin-treated macrophages as compared to media-treated ones (Fig. 8). These results suggest that decreased ERK activation could play a role in flagellin-mediated IL-10 inhibition, since TLR-induced production of this cytokine has been reported to be dependent on ERK signaling.
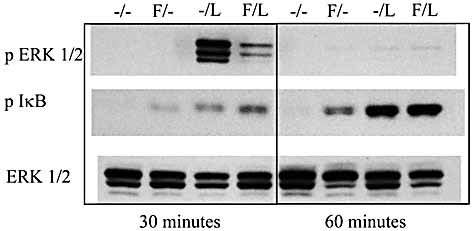
Flagellin treatment decreases ERK activation by LPS in macrophages. PEM were cultured with 5 μg/mL flagellin (F) or just media for 3 h (–) followed by the addition of 1 μg/mL LPS (/L). Cells were harvested 30 min and 1 h after adding LPS. Whole-cell lysates were obtained and subjected to SDS-PAGE immunoblotting with indicated antibodies. Unphosphorylated ERK1/2 was used as loading control. Shown is a representative experiment of two independent experiments with similar results.
The transcription factor NF-κB plays a central role in the induction of cytokine production by TLR. NF-κB is sequestered in the cytoplasm by molecules known as inhibitor of NF-κB (IκB). Phosphorylation of IκB results in its degradation and in the release of NF-κB that now can translocate to the nucleus and activate its target genes 2. Macrophages treated with flagellin or LPS alone show increased IκB phosphorylation, suggesting that treatment with these TLR ligands results in NF-κB activation. As seen in Fig. 8, flagellin treatment in combination with LPS did not result in decreased levels of IκB phosphorylation, suggesting that inhibition of LPS-induced IL-10 by flagellin is not caused through reducing NF-κB activation by LPS.
Flagellin enhances IL-12 production in response to specific TLR ligands
In order to determine whether the inhibitory effect of TLR5 ligation on PEM might affect cytokines other than IL-10, we determined the IL-12p40/IL-12p35 mRNA levels in macrophages treated with flagellin and LPS. First, we observed that when PEM were treated with LPS alone for 2 h (Fig. 9A, –/LPS), they display much higher levels of IL-12p40mRNA relative to PEM stimulated with flagellin alone (Fig. 9A, F/–). Surprisingly, pre-treatment with flagellin increased the level of IL-12p40 in response to LPS stimulation at 2 h treatment (Fig. 9A, black bar). This increase is short-lived since at 4 h, IL-12p40 mRNA levels were back to those observed in PEM treated with LPS alone (Fig. 9A, 4 hours). In addition, an increase in IL-12p35 mRNA levels was observed in PEM treated with flagellin and subsequently stimulated with LPS (Fig. 9B, F/LPS: black bars). However, the response duration was longer relative to IL-12p40 mRNA induction, since enhanced levels of IL-12p35 mRNA were still observed after 4 h of exposure to LPS (Fig. 9B, 4 hours).
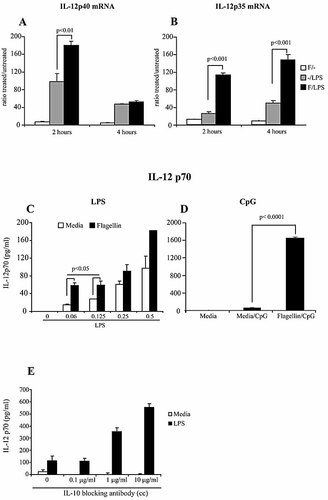
Flagellin-stimulated PEM produce higher levels of IL-12 in response to TLR4 or TLR9 ligands. (A, B) PEM were cultured with 5 μg/mL flagellin (F) for 3 h. Then supernatants were removed and medium alone (–) was added (F/–, white bars). In parallel, PEM were cultured with media alone (–) for 3 h and then LPS was added (–/LPS, gray bar). A third group consists of PEM pre-treated with flagellin (F) for 3 h and then stimulated with LPS (F/LPS, black bar). Cells were harvested at the indicated time points after the addition of LPS and the levels of IL-12p40 (A) or IL-12p35 mRNA (B) were determined as in Fig. 4. Shown is a representative experiment of four independent experiments with similar results. (C, D) PEM were cultured with media alone (white bar) or with 5 μg/mL flagellin (black bar) for 3 h. Then, PEM were treated with increasing concentrations of LPS as indicated (C) or with 0.25 μM CpG (D). Supernatants were collected after 24 h and IL-12p70 levels were determined by ELISA. Data represent mean ± SD of triplicate cultures (p values obtained by Student's t-test analysis). Shown is a representative experiment of three independent experiments with similar results. (E) Macrophages were cultured with LPS (1 μg/mL) plus IL-10-blocking antibody at the indicated concentration. After 24 h of culture the supernatants were harvested, and IL-12p70 production quantified by ELISA. Shown is a representative experiment of two independent experiments with similar results.
The biologically active form of the cytokine IL-12 is a heterodimer composed of IL-12p40 and IL-12p35 proteins (IL-12p70) 4. IL-12p40 has been reported to be produced in excess over IL-12p35 which acts as a limiting factor for IL-12p70 production 27. Therefore, an increase in IL-12p35 might result in elevated IL-12p70 protein production that would not be detected using standard ELISA methods that do not discriminate between IL-12p40 and IL-12p70 production. For this reason, we specifically analyzed IL-12p70 production by PEM pre-treated with flagellin in which we detected an increase in IL-12p35 mRNA levels in response to LPS. As shown in Fig. 9C, when PEM are pre-treated with flagellin and then exposed to increased concentrations of LPS (black bars), they produced higher levels of IL-12p70 relative to PEM treated with LPS alone (open bars). In summary, PEM treated with flagellin and then stimulated with LPS display enhanced expression of both IL-12p40 mRNA (Fig. 9A) and IL-12p35 mRNA (Fig. 9B) as well as increased production of IL-12p70 protein (Fig. 9C).
In order to determine whether flagellin-mediated enhancement of IL-12p70 production occurs only in response to LPS stimulation or could also be observed in response to other TLR ligands, PEM were treated with flagellin in combination with either zymosan (TLR2/6 ligand) or CpG (TLR9 ligand). While treatment with flagellin did not enhance IL-12p70 production in response to zymosan, which remained undetectable (data not shown), a significant increase in IL-12p70 production was observed when PEM were treated with flagellin and CpG (Fig. 9D). These results indicate that ligation of TLR5 in macrophages influences the inflammatory response of these cells to TLR4 and TLR9 ligands (LPS and CpG) resulting in enhanced production of the pro-inflammatory mediator IL-12p70.
IL-10 has been shown to act as a powerful inhibitor of IL-12 production 28, 29. Therefore we analyzed whether blocking IL-10 signaling would enhance IL-12p70 production by LPS-treated PEM. As shown in Fig. 9E, culturing PEM with LPS plus increasing concentrations of IL-10-blocking antibody increased IL-12p70 production in a dose-dependent manner. These data indicate that IL-10 repression by flagellin could be, at least in part, responsible for the increase in IL-12p70 production observed in macrophages treated with flagellin in combination with LPS. Nonetheless, other mechanisms must be also playing a role, since IL-12p70 production was also enhanced in macrophages treated with a combination of flagellin and CpG, even when on those cells IL-10 production was not altered by flagellin stimulation.
Discussion
Most of the TLR ligands induce production of the pro-inflammatory cytokine IL-12 and the anti-inflammatory cytokine IL-10. In this study we found out that flagellin, the TLR5 ligand, does not induce IL-10 protein production either in vitro or in vivo. Therefore, our results unveil a distinctive feature of flagellin relative to other TLR ligands. Furthermore, flagellin negatively influences IL-10 production by macrophages in response to other TLR ligands, while augmenting IL-12p70 production.
Macrophages and DC display a broad repertoire of TLR. This feature enables them to sense different microbial products expressed within the same microorganism either simultaneously or sequentially. While there is evidence of synergy between some TLR to amplify and sustain anti-microbial responses, little is known of whether signaling through a TLR may result in the suppression of a specific cytokine or signaling pathway 7, 30, 31. To our knowledge, this work represents the first evidence that flagellin specifically suppresses IL-10 production by macrophages in response to other TLR ligands.
IL-10 inhibition by flagellin does not affect all TLR ligands and seems to be rather selective. Our results show that flagellin's engagement to the membrane receptor TLR5 decreases IL-10 production in response to ligands whose receptors are also in the cellular membrane such as TLR4 or TLR2/6 32 (Fig. 5, 6A). On the contrary, flagellin does not decrease IL-10 production in response to a ligand (CpG) that binds to an intracellular receptor (TLR9) 33 (Fig. 6B). These findings suggest that some degree of TLR co-localization might be needed for flagellin-mediated IL-10 suppression.
Although TLR have been fundamentally described to work as homodimers 3, the existence of TLR heterodimers has also been reported. TLR2 has been shown to interact with TLR1 or TLR6 to form heterodimers that play an important role in the recognition of bacterial and yeast wall components 34. Recently, TLR5 has been shown to dimerize with TLR4 in flagellin-treated macrophages 35. If TLR5 is able to form heterodimers with other TLR that also localize to the cellular membrane then it is possible that pairing with TLR5 could affect the responses given to these TLR. However, whether such a co-localization and/or dimerization with TLR5 are required for IL-10 inhibition in response to flagellin and other TLR ligands remains undetermined.
The mechanism(s) by which flagellin inhibits IL-10 production are not yet elucidated. However, the findings that flagellin-treated macrophages display a transient and short-lived expression of IL-10 mRNA (Fig. 4A, B) along with the decreased levels of IL-10 mRNA observed when macrophages are treated with flagellin and LPS (Fig. 5A, D) suggest that the suppression occurs at the IL-10 gene transcriptional level and/or IL-10 mRNA stability. We did not find differences in the stability of IL-10 mRNA in macrophages treated with LPS alone or with LPS plus flagellin (Fig. 7B), what might indicate that the mechanism of suppression operates at the IL-10 gene transcriptional level.
Recent studies suggest that activation of MAPK might play an important role in regulating TLR-induced cytokine production 36, 37. ERK, a MAPK family member, has been shown to play an important role in the induction of IL-10 by LPS 22 and other TLR ligands 23–25 but not in production of other cytokines such as IL-12 26. Herein, we report that ERK activation by LPS is significantly impaired in flagellin-treated macrophages. These data suggest that flagellin might inhibit IL-10 production through impairing ERK activation by other TLR ligands. However, further experiments will be needed to confirm the role of ERK as well as other signaling pathways in flagellin-mediated IL-10 suppression. An interesting pathway to study would be the PI3K, since this pathway has a negative effect on cytokine induction by TLR and it can be activated by TLR5 38, 39. Furthermore, LPS-induced phosphorylation of IκB was not altered in flagellin-treated macrophages, suggesting that this pathway is not involved in the negative regulation of IL-10 production observed in flagellin-treated macrophages
Interestingly, we have also observed that flagellin enhances the production of IL-12p70 by macrophages in response to other TLR ligands such as LPS or CpG. Given the previous demonstration that IL-10 is a powerful inhibitor of IL-12p70 production 28, 29, one possible explanation for our findings is that flagellin enhances IL-12p70 production in response to other TLR ligands by inhibiting IL-10.
In support of this possibility, stimulation of macrophages with LPS in the presence of IL-10-blocking antibodies results in enhanced production of IL-12p70 (Fig. 9E). However, this mechanism might not be the only explanation for the enhanced IL-12p70 production in flagellin-treated macrophages. First, although treatment of macrophages with flagellin and CpG was able to increase IL-12p70 production (Fig. 9D), it was not accompanied by inhibition of IL-10 (Fig. 6B). Conversely, flagellin treatment resulted in inhibition of IL-10 production in response to the TLR2/6 ligand zymosan (Fig. 6A), but this combination did not influence the levels of IL-12p70 production by macrophages (data not shown). Therefore, the inhibitory effect of flagellin upon IL-10 and its IL-12p70-enhancing effects seem to be independent from each other and likely to be mediated by different mechanisms.
In summary, our findings suggest a potential scenario in which the IL-10-inhibitory properties of flagellin could facilitate the induction of inflammatory responses by flagellated bacteria. This effect could be particularly important in organs such as colon and lungs that favor immune unresponsiveness rather than immune activation due to their production of IL-10.
The central role of flagellin in inducing inflammatory responses against different flagellated bacteria has recently been highlighted 15, 17, 40. These results might indicate that the intrinsic inflammatory properties of other TLR ligands expressed in these bacteria might be regulated by, and/or require flagellin in order to be fully elicited. This is supported by our observation that flagellin inhibits IL-10 mRNA and protein production while it increases the production of IL-12p70 by other TLR ligands. In the absence of flagellin, IL-10 would be produced in response to other TLR ligands such as LPS that is also expressed by flagellated bacteria. In this scenario, it is likely that the inhibitory effects of IL-10 would prevail and lead to minimum or none inflammatory response. Conversely, in the presence of flagellin and its IL-10-inhibitory effects, pro-inflammatory pathways (i.e. IL-12) triggered by flagellin itself or other TLR ligands are unopposed by IL-10 and unleash stronger inflammatory responses.
Interestingly, enhanced inflammation might favor systemic dissemination of flagellated bacteria 41. This concept is supported by recent studies in mice with genetic disruption of TLR5 18. In these mice, it was predicted that if the main effect of flagellin-TLR5 interaction is to induce host's protective inflammatory responses, then the absence of TLR5 would lead to increased susceptibility to infection by flagellated microorganisms. At odds with this prediction, TLR5–/– mice were found to be less susceptible to systemic infection following oral challenge with Salmonella typhimurium 18. Given our results, it is possible that in the absence of flagellin-TLR5 interaction, IL-10 would continue to be produced in response to other TLR ligands. In this case, resistance rather than susceptibility will be the outcome due to the inability of the bacteria to induce inflammation, not as a protective mechanism but as a mechanism to facilitate its systemic dissemination.
In summary, for the first time this study shows flagellin's effect in negatively regulating the production of the anti-inflammatory cytokine IL-10. Furthermore, the observation that flagellin specifically suppresses IL-10 production in response to selected TLR ligands provides a potential explanation to how flagellin might shape the inflammatory responses elicited by flagellated pathogens.
Materials and methods
Mice
Male BALB/c mice (6–8 wk old) were obtained from the National Institutes of Health (Frederick, MD). All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the University of South Florida College of Medicine.
Isolation of peritoneal elicited macrophages and generation of bone marrow-derived macrophages
BALB/c mice were injected intraperitoneally with 1 mL of thioglycollate (DIFCO Laboratories, Detroit, MI). Four days later, PEM were obtained by peritoneal lavage as previously described 42. BM-derived macrophages were differentiated from BM cells by harvesting the hind legs and flushing the BM with RPMI. Red blood cells were lysed using ACK lysis buffer and the remaining cells extensively washed. Differentiation of BM cells into macrophages was achieved by 5-6 days incubation of the cells in complete medium (20% FBS, 100 μM β-mercaptoethanol in DMEM) supplemented with L-929 cell supernatants as a source of M-CSF 43.
TLR ligands
S. typhimurium flagellin was isolated as previously described 44. Protein purity was verified by Coomassie staining and its identity confirmed by Western blot with an anti-flagellin monoclonal antibody (Igen International, Gaithersburg, MA). Endotoxin removal was accomplished by combination of Detoxi-Gel AffinityPak columns (Pierce, Rockford, IL) and filtration through 100-kD pore size centricon columns (Millipore, Billerica, MA). Endotoxin removal was confirmed using the Limulus amebocyte lysate test (Cambrex, Rutherford, NJ). In those experiments in which flagellin was digested with proteinase K (Sigma-Aldrich, St. Louis, MO) 100 μg/mL proteinase K was used, digestion carried for 4 h at 37°C, and the protease inactivated at 100°C for 1 h. Experiments were also performed with commercially available S. typhimurium flagellin (InVivoGen, San Diego, CA), which yielded identical results. LPS was obtained from Sigma-Aldrich). CpG and zymosan were from InVivoGen and used as indicated in the text.
Real-time RT-PCR analysis
Two million PEM or BM-derived macrophages were plated per well in a 24-well plate. After 2 h, non-adherent cells were washed off with media and attached cells were then treated as indicated. Total RNA was extracted using TriZol reagent (Qiagen, Valencia, CA), and cDNA obtained with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Target mRNA was quantified using MyIQ single-color real-time PCR detection system (Bio-Rad) and iQ SYBR green Supermix (Bio-Rad).
IL-12p35 primers (left oligo ATGGTGAAGACGGCCAGAG, right oligo CAGGTCTTCAATGTGCTGGTT), IL-12p40 primers (left oligo GCAACGTTGGAAAGGAAAGA, right oligo AAAGCCAACCAAGCAGAAGA), IL-10 primers (left oligo CAGGGATCTTAGCTAACGGAAA, right oligo GCTCAGTGAATAAATAGAATGGGAAC), TLR5 primers (left oligo GCATAGCCTGAGCCTGTTTC, right oligo AAGTTCCGGGGAATCTGTTT) and GAPDH primers (left oligo ATGGCCTTCCGTGTTCCTAC, right oligo CAGATGCCTGCTTCACCAC) were used for PCR amplification. Cycling parameters were 3 min 95°C, 15 s 95°C, 30 s 60°C, 40 repetitions, 1 min 95°C. Single-product amplification was confirmed by melting curve analysis and primer efficiency was near to 100% in all the experiments performed. Quantification is expressed in arbitrary units and target mRNA levels were normalized to GAPDH expression. Actinomycin D was purchased from Sigma-Aldrich.
Quantification of cytokines
Mice were treated with flagellin (10 μg/animal), LPS (10 μg/animal) or HBSS (0.1 mL) via tail vein injection. Ninety minutes later animals were sacrificed and blood obtained by heart puncture. Serum was obtained and the levels of IL-12 and IL-10 were determined by ELISA. For in vitro determination of cytokine production, 1×105 PEM were plated by triplicate in 96-well plates and treated for 24 h unless otherwise specified. Supernatants were harvested and kept at –70°C until ELISA for IL-10, IL-12p40/p70 (BD Pharmingen, San Diego, CA) or IL-12p70 (eBioscience, San Diego, CA) was performed following manufacturers’ instructions. Data represent mean ± SD of triplicate cultures. In the experiments in which IL-10 signaling was blocked we used clone JES5-16E3 rat monoclonal antibody (BD Pharmingen). Statistic analysis was performed using Stat view 4.0.
Cell lysates and Western blot
Total cell lysates were prepared in RIPA buffer (150 mM NaCl, 10 mM Tris-HCl pH 7.4, 5 mM EDTA pH 8.0, 0.1% SDS, 1% deoxycholate, 1% Triton X-100) containing protease inhibitor cocktail (Sigma Aldrich) and phosphatase inhibitor cocktail I and II (Sigma Aldrich) and subjected to SDS-PAGE-immunoblot analysis with anti-phospho-p44/42 MAPK (Thr202/Tyr204) polyclonal antibody (Cell Signaling), phospho-IkappaBα (Ser32) (14D4) rabbit monoclonal antibody (Cell Signaling) and anti-MAPK (ERK1+ERK2) monoclonal antibody (Invitrogen).
Acknowledgements
We thank Drs. I. Borrello, A. Beg and K. Wright for helpful discussions and review of the manuscript. This work was supported by PHS grants CA87583 (E.M.S.) and CA100850 (E.M.S.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




