Blockade of the OX40 ligand prolongs corneal allograft survival
Abstract
Although corneal transplantation is one of the most common tissue transplantations and is known to have a high graft acceptance rate, occasional corneal graft rejection remains a cause of blindness. OX40, a member of the TNF receptor superfamily, is expressed on activated T cells, and transmits a costimulatory signal by binding to OX40 ligand (OX40L) expressed on several cells with antigen-presenting functions. Using a blocking monoclonal antibody (mAb) against murine OX40L, we investigated the role of OX40 in a murine model of corneal transplantation. C3H/He mouse corneas were transplanted to BALB/c mice orthotopically. Administration of anti-OX40L mAb significantly reduced allograft rejection, and increased graft survival rate to 40% at 8 weeks after transplantation, while all corneas were rejected within 5 weeks in control IgG-treated mice. Similar reduced rejection was observed when wild-type donor corneas were transplanted to OX40L-deficient recipients. In vitro study revealed that the anti-OX40L mAb treatment reduced proliferative response and IFN-γ production of draining lymph node cells in response to stimulation with donor alloantigen. These results demonstrate that OX40L blockade is effective for prolongation of corneal allograft survival by inhibiting recipient T cell activation.
Abbreviation:
-
- MST:
-
mean survival time
Introduction
Corneal transplantation is currently one of the most common organ transplantations, and is known as the most successful type of transplantation. Consistent with the success of human corneal grafts, a high spontaneous acceptance rate has been demonstrated in the rodent model of corneal transplantation. The success of corneal graft is at least partly explained by the fact that the cornea is an immune-privileged site 1, 2, and the tissues are sheltered from alloantigen-specific immune responses. However, rejection of corneal allograft still occurs occasionally, resulting in blindness.
Allograft rejection is primarily mediated by T cells, especially by CD4+ T cells. Besides the interaction of T cell receptor (TCR) with an antigen presented by major histocompatibility complex (MHC), several costimulatory signals are required for adequate activation process of T cells. One promising strategy to prevent allograft rejection and facilitate tolerance is blockade of costimulatory signals. Monoclonal antibodies (mAb) and fusion proteins that block costimulatory signals via CD40/CD154 3, 4 and CD28/B7 5, 6 have been investigated and display varying degrees of success in the mouse model of corneal transplantation. A simultaneous blockade of more than one costimulatory pair enhanced corneal allograft survival 7.
OX40 (CD134), a member of the tumor-necrosis factor (TNF) receptor superfamily, is expressed on activated T cells 8, 9 and transmits a costimulatory signal by binding to OX40 ligand (OX40L) expressed on several cells with antigen-presenting functions 9–18. The OX40/OX40L interaction is crucial for the survival of activated T cells and the generation of memory-type cells 17. In rat heart and skin graft models, a combined blockade of OX40/OX40L and CD28/B7 was synergistic in promoting allograft survival 19. Blockade of OX40L with OX40-Ig was effective in prolonging the survival of minor histocompatibility complex-mismatched heart graft 20. A study of skin graft using CD28 and CD154 double-deficient mice, in which costimulatory signals via both CD28/B7 and CD40/CD40L are abolished, has revealed that OX40 costimulation plays a key role in supporting the activation of a subset of CD4+ T cells and CD8+ T cells to alloantigen 21. Although the role of OX40 in the mouse heart graft or GVHD has been also reported 20, 22, 23, the involvement of OX40 in corneal allograft rejection has not been documented. In the present study, we examined the roles of OX40L in the corneal allograft rejection in a mouse model of corneal transplantation, using a blocking anti-OX40L mAb and OX40L-deficient mice.
Results
Expression of OX40 during corneal allograft rejection
To examine the possible role of OX40 in corneal allograft rejection, we first investigated mRNA expression of OX40 in transplanted cornea and cell-surface expression of OX40 on lymphocytes collected from periorbital lymph nodes (neck lymph nodes) of the recipient mice. Previous reports showed that all C3H/He mouse corneas transplanted to BALB/c mice were rejected 5, 7. Therefore, we used BALB/c mice orthotopically transplanted with allogeneic C3H/He mouse corneas as the experimental group, and BALB/c mice transplanted with syngeneic BALB/c mouse corneas as the control group. Three weeks after corneal transplantation, transplanted corneas and the periorbital lymph node cells were collected from each group of mice (n = 3 in each group). Messenger RNA were extracted from those corneas, and expression of OX40 was analyzed by RT-PCR. As shown in Fig. 1A, OX40 was expressed in allogeneic cornea-grafted mice but not in syngeneic cornea-grafted mice. Since CD4+ T cells are pivotal for developing corneal transplantation rejection 24–26, OX40 expression on CD4+ T cells among the lymph node cells was analyzed (Fig. 1B and C). Compatible with the results of OX40 mRNA expression in the cornea, although 12.8% of CD4+ T cells of syngeneic cornea grafted mice expressed OX40, the expression was significantly increased to 16% in CD4+ T cells of allogeneic cornea grafted mice, even though there was almost equal number of CD4+ T cells in allogeneic (33.0%) and syngeneic (29.0%) mice. These data suggested that costimulation via OX40 is involved in corneal transplant rejection process. However, due to the small difference in OX40 expression between syngeneic and allogeneic cornea grafted mice, ligation by OX40L may take place at locations other than lymph nodes.
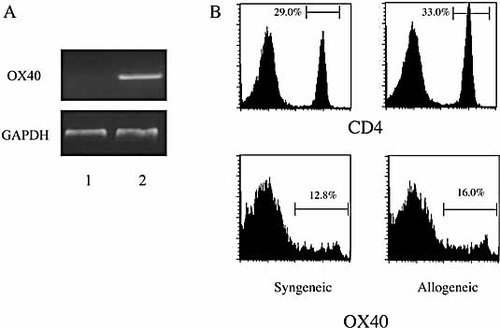
Expression of OX40 in mouse cornea and in draining lymph nodes. Three weeks after allogeneic or syngeneic corneal transplantation, grafted corneas and draining lymph nodes were removed and expression of OX40 was analyzed by RT-PCR and FACS, respectively. (A) OX40 mRNA is expressed in grafted corneas of allogeneic recipients (Lane 2) but not in those of syngeneic recipients (Lane 1). (B) CD4+ cells in draining lymph nodes of mice grafted allogeneic corneas are almost equal to that of mice grafted syngeneic corneas (33 and 29%, respectively), whereas OX40 expression is higher in the CD4+ cells of allogeneic recipients (16%) than in syngeneic recipients (12.8%). The histograms are representative of three mice in each group.
Effect of anti-OX40L mAb treatment on corneal allograft survival
To examine the role of OX40 in corneal allograft rejection, BALB/c mice transplanted with C3H/He mouse corneas were administered with anti-mouse OX40L mAb or control rat IgG for 3 weeks after transplantation. Rejection was evaluated by the clinical score as described in the Materials and methods. All corneal grafts were rejected within 5 weeks (mean survival time (MST) = 2.4 ± 0.3 weeks) in the recipients given control IgG (Fig. 2A), and the opacity scores in these mice were higher than grade 2 from 2 weeks after transplantation (Fig. 2B). Administration of anti-OX40L mAb significantly improved the survival of corneal allografts, and the survival rate was maintained at 40% even at 8 weeks after transplantation (MST = 5.2 ± 0.9 weeks). Opacity score of the mice given anti-OX40L mAb was significantly suppressed as compared with mice treated with rat IgG, and average score of the anti-OX40L-treated group was below grade 2 throughout the observation period. These data indicated that the blockade of OX40L inhibited corneal graft rejection and promoted graft acceptance. Stereomicroscopic observations showed that control IgG treated mouse cornea was vascularized and had severe opacity (Fig. 2D), while anti-OX40L mAb treated mouse cornea was clear (Fig. 2E). In addition, cell infiltration and vascularization were observed pathologically in control IgG treated mouse cornea (Fig. 2F), but these were not observed in anti-OX40L mAb-treated mouse cornea (Fig. 2G).
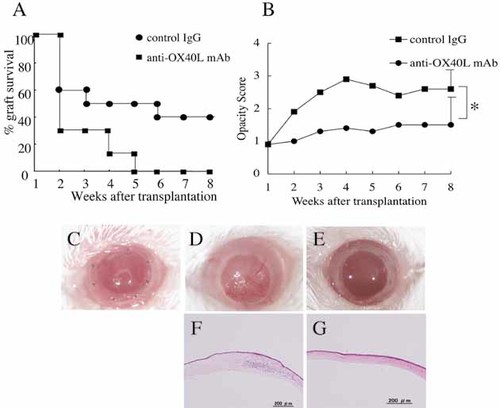
Administration of anti-OX40L mAb prolongs corneal allograft survival. C3H/He mouse corneas were transplanted orthotopically to BALB/c recipients. Mice were given 300 µg of anti-OX40L mAb or control IgG every other day from the day of transplantation to 3 weeks after graft. Each group consists of ten mice in two experiments. Significant differences in both graft survival rate (A) and opacity score (B) were observed between the recipients treated with anti-OX40L mAb and those treated with control IgG. Forty percent of grafts survived at 8 weeks after transplantation in the anti-OX40L mAb-treated recipients, whereas all grafts were rejected within 5 weeks in the control IgG-treated recipients. Mean survival time (MST) was 2.4 ± 0.3 weeks in the control IgG-treated group and 5.2 ± 0.9 weeks in the anti-OX40L-treated group. Significant differences were observed in score of 8 weeks after transplantation; *, p <0.05. (C–G) Stereomicroscopic and histological observations of the transplanted corneas soon after transplantation (C), rejected cornea of control IgG-treated recipients at 8 weeks after transplantation (D and F), and surviving cornea from anti-OX40L mAb-treated recipients at 8 weeks after transplantation (E and G).
Effect of anti-OX40L mAb treatment on allo-reactive T cells
Since corneal allograft rejection was significantly inhibited in the anti-OX40L mAb-treated recipient, we examined recipient T cell responses to donor antigen. Three weeks after C3H/He cornea transplantation, anti-OX40L mAb- or control IgG-treated BALB/c mice were sacrificed and single-cell suspensions of neck lymph nodes were prepared as responder cells for MLR (n = 3 in each group). Irradiated spleen cells from C3H/He mice were used as stimulator cells. As shown in Fig. 3, the proliferative response to donor alloantigen was almost completely suppressed in the anti-OX40L-treated recipients as compared to the control Ig-treated recipients. Furthermore, although addition of exogenous IL-2 in part restored the suppressed T cell proliferative response in the anti-OX40L-treated recipients, the response was still significantly less than that in the control mice. These results indicated that the OX40L blockade rendered recipient T cells hyporesponsive to donor alloantigen.
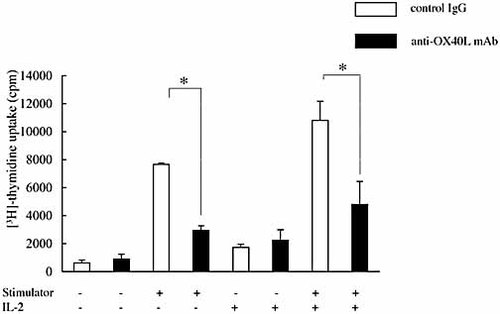
Anti-OX40L mAb treatment induces recipient T cell hyporesponsiveness to donor alloantigen. C3H/He mouse corneas were grafted to BALB/c mice, and the recipients were treated with anti-OX40L mAb or control IgG. Mice were sacrificed 3 weeks after corneal transplantation. Single-cell suspension of the neck lymph nodes was used as the responder for mixed lymphocyte reaction. Responder cells (2.5 × 105) were cultured with irradiated C3H/He mouse spleen cells (2.5 × 105) as the stimulator with or without IL-2 (200 U/mL) in 96-well round-bottom microtiter plates. After 5 days, proliferation was assessed by [3H]thymidine incorporation. Data are presented as the mean ± SD of triplicate samples. Lymph node cells from the anti-OX40L mAb-treated mice showed a significantly reduced proliferative response to donor alloantigen as compared with those from the control IgG-treated mice. This reduction was not reversed by the addition of recombinant IL-2. The experiment represents the data from three pooled mice. The experiment was repeated three times with similar results; *, p <0.01.
Effect of anti-OX40L mAb treatment on IFN-γ production by donor-reactive T cells
We also examined IFN-γ and IL-10 production by draining lymph node cells from anti-OX40L- or control IgG-treated recipients (n = 3 in each group) in response to the donor antigen. As shown in Fig. 4, secretion of IFN-γ was mostly abolished in the anti-OX40L mAb-treated recipients as compared to the control IgG-treated recipients, which was not restored by exogenous IL-2. IL-10 was not detectable in any samples (data not shown).
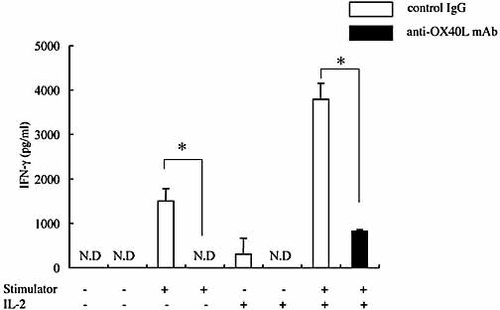
Anti-OX40L mAb treatment impairs recipient T cell secretion of IFN-γ in response to donor alloantigen stimulation. As in Fig. 3, lymph nodes cells from the recipients treated with anti-OX40L mAb or control IgG were stimulated with irradiated C3H/He mouse spleen cells with or without IL-2. Supernatants of the cultures were collected 48 h later, and analyzed for IFN-γ contents by ELISA. Data are presented as the mean ± SD of triplicate samples. IFN-γ production was significantly reduced in the anti-OX40L-treated group as compared with the control IgG-treated group. This suppression was observed even in the presence of recombinant IL-2. The experiment represents the data from three pooled mice. The experiment was repeated once with similar results; *, p <0.01, ND, not detectable.
Corneal transplantation using OX40L-deficient mice
To further confirm the involvement of OX40, we used OX40L-deficient BALB/c mice as recipients. When C3H/He mouse corneas were transplanted to WT BALB/c mice, all corneas were rejected within 5 weeks (MST = 2.9± 0.3), whereas 42% of the C3H/He mouse corneas transplanted to OX40L-deficient BALB/c mice were viable even at 8 weeks after transplantation (MST = 5.9 ± 0.6) (Fig. 5A). OX40L-deficient BALB/c mice significantly prolonged corneal allograft survival (p <0.05). Furthermore, opacity score of the corneas transplanted to OX40L-deficient mice was significantly (p <0.05) lower than that of the corneas transplanted to WT mice throughout the observation period (Fig. 5B). These results were consistent with those of using anti-OX40 mAb (Fig. 2).
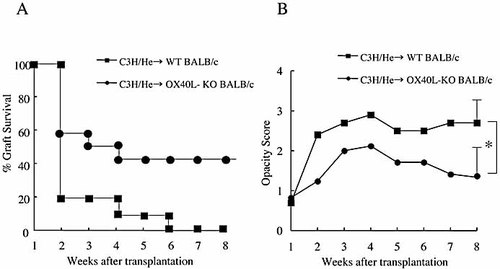
Corneal allograft survival using OX40L-deficient mice as recipients. C3H/He mouse corneas were transplanted to WT or OX40L-deficient BALB/c mice (A and B). Each group consisted of ten to twelve mice in two experiments. (A) The graft survival rate was 42% in the OX40L-deficient mice at 8 weeks after transplantation, whereas all the grafts were rejected within 6 weeks in WT mice. Significant differences were observed. (B) Opacity score of the corneas transplanted to OX40L-deficient mice was lower than that of the corneas transplanted to WT mice throughout the observation period. Significant differences were observed in score of 8 weeks after transplantation; *, p <0.05.
Discussion
OX40 and OX40L are members of the TNFR and TNF superfamilies, respectively, and have been recognized as efficient T cell costimulatory molecules 27–29. Blockade of the OX40/OX40L interaction has been shown to prevent experimental autoimmune diseases and transplant rejection 13, 19, 21–23, 30–34. In the present study, we have demonstrated that blockade of OX40L delays orthotopic corneal allograft rejection.
OX40 is expressed on activated T cells, primarily CD4+ T cells, although expression on CD8+ T cells has been also found to a lesser degree 35, 36. Ligation with OX40L further activated T cells expressing OX40 27, and CD4+ T cells of OX40L-deficient mice exhibited impaired effector function and reduced memory formation 37. In the present study, mice transplanted with allogeneic corneas showed up-regulated OX40 mRNA expression in the grafted corneas and increased surface OX40 expression on CD4+ T cell population in periorbital lymph nodes as compared with mice transplanted with syngeneic corneas (Fig. 1). These results confirmed that T cells activated in corneal transplant rejection process were expressing OX40.
It has been found that CD28/B7 and CD154/CD40 play pivotal roles in costimulating T cell activation and allograft rejection 38, 39. However, corresponding to the subsequent studies that CD28 or CD154 blockade is not always effective in preventing allograft rejection 19, 21, 40–42, blockade of CD28/B7 or CD40/CD154 costimulatory pathway resulted in a limited prolongation of corneal allograft survival 7. These findings indicate that blockade of only one costimulatory pathway is insufficient to protect against allograft rejection. In fact, blocking both the CD28 and CD154 costimulatory pathways has been found to result in stable allograft survival in experiments of skin allograft using CD28 and CD154 double-deficient mice 21. In addition, Demirci et al. 21 demonstrated that markedly prolonged skin allograft survival was achieved in CD28 and CD154 double-deficient mice by treatment with anti-OX40L mAb, although blockade of other costimulatory pathways, such as ICOS/ICOSL and 4-BB/4–1BBL, failed to prolong skin allograft survival. Our present observation of the prolonged corneal allograft survival by OX40L blockade indicates that OX40/OX40L is a critical pathway not only in skin allograft rejection but also in corneal allograft rejection. Without combination with other antibodies, anti-OX40L mAb treatment alone or using OX40L knockout mice as recipients was able to prolong corneal allograft survival. In other organ transplantations such as skin and heart, OX40/OX40L blockade alone had no significant effect on allograft rejection, but OX40/OX40L blockade combined with other costimulatory blockade or donor-specific transfusion prolonged allograft survival 19–21. It is suggested that the OX40/OX40L interaction may be more important in corneal graft rejection than other organ graft rejection.
Our in vitro study indicated that draining lymph node T cells from allogeneic cornea recipients treated with anti-OX40L mAb failed to proliferate and to produce IFN-γ in response to donor alloantigen, and these effects were not restored by exogenous IL-2 (Fig. 3 and 4). Similar results were obtained in a GVHD model treated with anti-OX40L mAb 23. From these results, it is unlikely that the allo-reactive T cells were rendered anergic in the absence of OX40/OX40L costimulation. Since we detected no IL-10 production by T cells from the anti-OX40L mAb-treated recipients, IL-10-mediated suppression is also unlikely. We are thus speculating that anti-OX40L mAb might interfere with priming and/or expansion of the recipient T cells specific for donor alloantigens. This notion is supported by the in vitro findings of Murata et al. 37 demonstrating reduced Th1 and Th2 cytokine production in OX40L-deficient mice and anti-OX40L-treated mice when stimulated with alloantigen.
In our study, OX40L blockade did not inhibit corneal graft rejection completely. Therefore, other costimulatory signals or effector mechanisms are also important in corneal graft rejection. Recent studies revealed that programmed death-1 (PD-1), which is a new member of B7-CD28 superfamily, plays an important role in the survival of corneal allografts43, 44. Hori et al. 44 demonstrated that B7-H1, which is the ligand of PD-1, is constitutively expressed in corneal endothelial cells and stromal cells. This constitutive expression induces apoptosis of effector T cells at the cornea. Watson et al. 43 also provided evidence that B7-H1 is important in corneal graft rejection. They also indicated that another costimulatory molecule inducible costimulatory (ICOS) molecule appears to be less important in corneal allograft rejection.
In summary, our present findings have indicated that administration of anti-OX40L mAb prolonged corneal allograft survival by inhibiting the proliferation and IFN-γ production of alloreactive T cells. Furthermore, corneal allograft survival was prolonged when OX40L-deficient mice were used as recipients. Further investigation into the importance of OX40 costimulation is clearly warranted given its potential therapeutic benefit in promoting successful corneal transplantation.
Materials and methods
Animals
Eight to ten week-old C3H/He (H-2k) and BALB/c (H-2d) mice were purchased from Oriental Yeast (Tokyo, Japan). OX40L-deficient mice BALB/c mice 37, 45 were kindly gifted from Drs. N. Ishii and K. Sugamura (Tohoku University School of Medicine, Sendai, Japan). Animals were treated according to the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. All procedures were performed under sodium pentobarbital anesthesia or ketamine and xylazine mixtures as anesthetics.
Orthotopic corneal transplantation
Orthotopic corneal transplantation was performed as previously described 46. In brief, donor corneal buttons were excised using 2.0-mm Trepan and Vannas scissors and secured to the host graft bed of 2.0-mm diameter by eight interrupted 11–0 nylon (Mani, Tochigi-ken, Japan) suture. Antibiotic ointment was applied and the eyelid was closed until 3 days after surgery. At day 7, corneal sutures were removed in all mice.
Evaluation and scoring of orthotopic corneal transplantation
Orthotopic grafts were observed with slit lamp microscopy at weekly intervals, and judgment of graft survival was performed according to a previously established scoring system 46: 0, clear graft; 1+, minimal superficial nonstromal opacity; 2+, minimal deep stromal opacity; 3+, moderate deep stromal opacity; 4+, intense deep stromal opacity; and 5+, maximum stromal opacity. Grafts with opacity scores of 2+ or greater were considered as rejected.
Antibody treatment
Blocking anti-mouse OX40L (RM134L) mAb was generated in our laboratory as described previously 10. Control rat IgG was purchased from Sigma-Aldrich (St. Louis, MO). Recipients were injected intraperitoneally with 300 µg of anti-mouse OX40L mAb or control rat IgG every other day from the day of transplantation for 3 weeks.
RT-PCR
Total RNA was extracted from a pool of three corneal isografts or allografts, using ISOGEN RNA isolation system (Wako, Osaka, Japan) according to the manufacturer's protocol. Complementary DNA was synthesized at 42°C for 2 h in the presence of SuperScript II RNaseH– Reverse Transcriptase and Random Primers (Invitrogen, Carlsbad, CA) in 20-µL reaction volumes. PCR was conducted in a 50 µL reaction mixture using 0.25 µg of cDNA as template. The PCR mixture consisted of 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl, 0.2 mM dNTP, and 2.5 units of Taq polymerase (TaKaRa, Shiga, Japan). The sequences of sense and antisense primers and expected product sizes were as follows: GAPDH (357 bp); 5′-gaggggccatccacagtcttc and 3′-catcaccatcttccaggagcg, OX40 (303 bp); 5′-tcaagcagaattgcacacct and 3′-ctgtggtggattggacagtg. The amplification cycle involved denaturation at 95ºC for 1 min, annealing at 55ºC for 1 min and extension at 72ºC for 1 min. and was repeated 35 times in a TaKaRa PCR Thermal Cycler 480. Of each PCR product, 5 µL was electrophoresed in a 4% agarose gel containing ethidium bromide in Tris-borate-EDTA buffer and photographed.
Flow cytometry
Anti-CD4-FITC (eBioscience, San Diego, CA) and anti-OX40-PE (eBioscience) were used for immunofluorescent staining. FITC- or PE-labeled rat IgG2a (eBioscience) was used as an isotype control. Single-cell suspension of lymphocytes was prepared from neck lymph nodes. Cells were first incubated with FcBlock (BD PharMingen) to block nonspecific binding before the staining with anti-CD4-FITC and anti-OX40-PE. Stained cells were analyzed on using FACScalibur (Nippon Becton Dickinson Company, Tokyo, Japan) using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Mixed lymphocyte reaction and cytokine production
Three weeks after corneal transplantation, responder lymphocytes obtained from periorbital lymph nodes (neck lymph nodes) of the recipient BALB/c mice (2.5 × 105/well) were cultured with irradiated (30 Gy) stimulator C3H/He spleen cells (2.5 × 105/well) in 96-well U-bottom culture plates containing 0.2 mL of RPMI 1640 (Sigma Aldrich) with 10 mM HEPES, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Invitrogen Life Technologies, Carlsbad, CA), 1 × 10–5 M 2-mercaptoethanol (Sigma Aldrich), and 10% FCS. For cytokine assay, supernatants were collected after 48 h and analyzed for IFN-γ and IL-10 with the ELISA Ready-SET-Go kit (eBioscience). For proliferation assay, cells were cultured for 120 h, and pulsed with 0.5 µCi [3H]thymidine for the last 8 h. Radioactivity was assessed by liquid scintillation spectrometry, and the data are expressed as cpm.
Statistical analysis
MST of the grafts was analyzed by Mann-Whitney test. Results of other experiments were evaluated by Student's t-test. Differences were considered statistically significant at p <0.05.
Acknowledgements
The authors are grateful to Dr. Naoyuki Yamakawa for excellent technical assistance in this study. This work was supported by Grant-in-Aid 16591769 for Scientific Research from the Japan Society for the Promotion of Science.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflict of interest.




