IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells
Abstract
We have previously shown that human monocyte-derived dendritic cells (DC) express indoleamine 2,3-dioxygenase (IDO), as well as several other enzymes of the kynurenine pathway at the mRNA level upon maturation. The tolerogenic mechanisms of this pathway remain unclear. Here we show that LPS-treated DC metabolize tryptophan as far as quinolinate. We found that IDO contributes to LPS and TNF-α + poly(I:C)-induced DC maturation since IDO inhibition using two different inhibitors impairs DC maturation. IDO knock-down using short-hairpin RNA also led to diminished LPS-induced maturation. In line with these results, the tryptophan-derived catabolites 3-hydroxyanthranilic acid and 3-hydroxykynurenine increased maturation of LPS-treated DC. Concerning the molecular mechanisms of this effect, IDO acts as an intermediate pathway in LPS-induced production of reactive oxygen species and NF-κB activation, two processes that lead to DC maturation. Finally, we show that mature DC expand CD4+CD25high regulatory T cells in an IDO-dependent manner. In conclusion, we show that IDO constitutes an intermediate pathway in DC maturation leading to expansion of CD4+CD25high regulatory T cells.
Abbreviations:
-
- 1-MT:
-
1-methyltryptophan
-
- 3-OHAA:
-
3-hydroxyanthranilic acid
-
- 3-OHK:
-
3-hydroxykynurenine
-
- shRNA:
-
short-hairpin RNA
Introduction
The immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) transforms tryptophan into kynurenine, which is then metabolized into other catabolites through the action of enzymes within the kynurenine pathway 1. IDO is expressed by mature DC among other cell types 2–4. A specific subset of human DC (CD123+CCR6+) can express IDO 5, although the culture conditions are critical for the reproducibility of these results 6, 7. The mechanisms by which IDO mediates its tolerogenic effects remain controversial (for review see 8–10). Tryptophan-derived catabolites can mediate the tolerogenic effects of IDO by inducing apoptosis of activated but not resting T cells 11. However, it is unclear whether human DC can metabolize tryptophan beyond kynurenine and whether tryptophan-derived catabolites can modulate DC biology.
We have previously shown that human mature DC express IDO as well as other enzymes within the kynurenine pathway 4, suggesting that kynurenine is not a terminal metabolite in human DC. In mice, it has recently been shown that DC can metabolize tryptophan as far as quinolinate, one of the terminal catabolites generated by the kynurenine pathway 12. Moreover, the paracrine production of tryptophan-derived catabolites by tolerogenic DC can confer suppressive properties to immunogenic DC 12. Very recently, it has been shown that tryptophan starvation and tryptophan-derived catabolites can transform naive CD4+CD25– T cells into CD4+CD25+Foxp3+ regulatory T cells (Treg) 13. However, the role of DC was not analyzed.
CD4+CD25+ Treg can be expanded by LPS-matured DC 14 and given that IDO expression in DC can be induced by TLR ligands, such as LPS 2 and poly(I:C) 4, we hypothesized that LPS-matured DC can induce proliferation of Treg in an IDO-dependent manner. LPS-induced proliferation of Treg licenses T effector mechanisms, since proliferating Treg transiently lose their regulatory properties 15. When the immune response needs to be suppressed, expanded and resting Treg with increased suppressive capacities on a per-cell basis suppress effector T cells 16. Therefore, the pro-inflammatory molecule LPS induces T effector responses and triggers a feedback regulatory mechanism to control the immune response by expanding Treg.
Here we show that LPS-treated human DC metabolize tryptophan as far as the terminal catabolite quinolinate. Furthermore, we demonstrate that tryptophan catabolism promotes maturation induced by LPS since IDO inhibition with two different molecules and short-hairpin RNA (shRNA) inhibits LPS-induced maturation. In agreement with this, the tryptophan-derived catabolites 3-hydroxyanthranilic acid (3-OHAA) and 3-hydroxykynurenine (3-OHK) increase maturation obtained with LPS. At the mechanistic level, IDO promotes DC maturation by increasing reactive oxygen species (ROS) production and NF-κB activation. Finally, we show that expansion of Treg by LPS-matured DC is dependent on IDO activity. These observations could constitute a new tolerogenic mechanism for IDO.
Results
LPS-matured human DC catabolize tryptophan as far as quinolinate
First, we confirmed that DC treated with IFN-γ, LPS and poly(I:C) produce the tryptophan-derived catabolite kynurenine and that the IDO inhibitor 1-methyltryptophan (1-MT) can impair kynurenine production (Fig. 1A). IDO expression was up-regulated in LPS-treated DC in approximately 90% of cells (Fig. 1B).We next determined whether human mature DC can catabolize tryptophan beyond kynurenine. We found that about 90% of LPS-treated DC produce quinolinate (Fig. 1B), one of the terminal catabolites of tryptophan 1. Thus, human mature monocyte-derived DC produce kynurenine and continue catabolizing this molecule as far as quinolinate, indicating that they also produce other intermediate catabolites of the kynurenine pathway such, as 3-OHAA and 3-OHK.
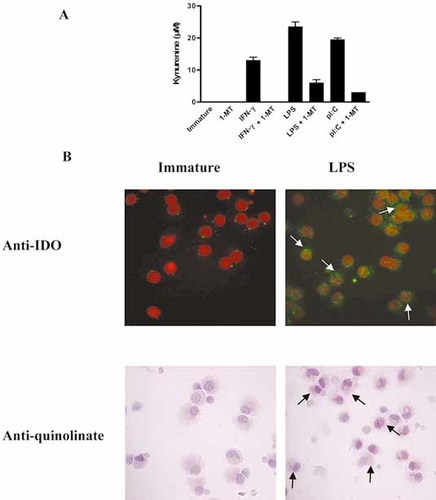
The vast majority of human mature DC express IDO and metabolize tryptophan beyond kynurenine as far as quinolinate. (A) DC were treated for 48 h with LPS, IFN-γ or poly(I:C) with or without a 3-h prior incubation with the IDO-specific inhibitor 1-MT. Kynurenine production was quantified in the culture supernatant. (B) IDO expression (green staining) was analyzed by immunohistology. Nuclei were stained with TOPRO-3 (red). Green spots in immature DC represent background staining. Positive cells are shown with white arrows. (C) Quinolinate production by DC was studied using a specific antibody (red staining). No positive cells were found in immature DCs. Black arrows indicate positive cytoplasmic staining in the LPS condition. One experiment representative of three is shown.
IDO promotes DC maturation through the generation of tryptophan catabolites
We speculated that tryptophan catabolites could modulate DC biology in an autocrine manner. DC were treated with LPS in the presence or absence of 1-MT or the tryptophan catabolites 3-OHAA and 3-OHK, and the DC phenotype was studied (Fig. 2A, B). IDO inhibition with 1-MT in LPS-treated cells led to about 35% inhibition in the expression of CD80, as compared to LPS alone. 3-OHK but not 3-OHAA increased LPS-induced up-regulation of CD80. CD86 expression was reduced by 65% when IDO was inhibited during maturation. 3-OHK further increased the expression of this costimulatory molecule, as compared to LPS alone. With 1-MT, MHC class II molecules were inhibited by 30%, but no effect of tryptophan catabolites was observed. The percentage of CD83+ DC was reduced by 65% when DC were treated with LPS + 1-MT vs. LPS alone, while 3-OHAA increased the percentage of CD83+ cells when added to LPS-treated DC.
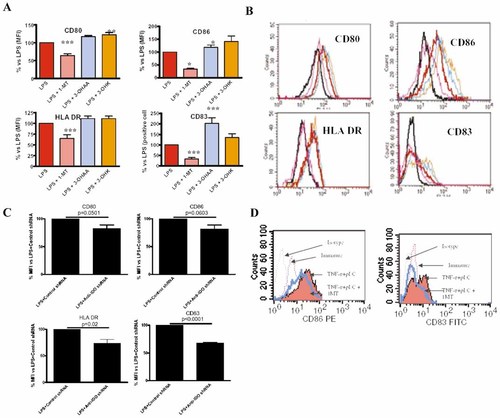
IDO promotes LPS-induced DC maturation. (A, B) Immature or LPS-matured DC (LPS added at time 0) were incubated in the presence or absence of 1-MT. Twenty-four hours later, the tryptophan catabolites 3-OHAA or 3-OHK were added and the cells cultured for a further 24 h. Cell viability was not affected by these treatments, as assessed by propidium iodide staining (not shown). (A) For CD80, CD86 and HLA-DR, the percentage of median fluorescence intensity ± SD as compared to the LPS condition (100%) is represented. The percentage of positive cells is shown for the CD83 marker. The means ± SD of nine different experiments are shown for LPS + 1-MT condition, and of four experiments for LPS + 3-OHAA and LPS + 3-OHK; *p<0.05, **p<0.01, ***p<0.001 as compared to LPS. (B) One experiment representative of nine is shown for the LPS + 1-MT condition, and one representative of four for LPS + 3-OHAA and LPS + 3-OHK. Treatments are represented by the same colors as in (A). The black line represents non-treated DC (immature). Tryptophan catabolites alone did not modify expression of CD80, CD86, CD83 or MHC class II (not shown). (C) Phenotypic maturation upon treatment with LPS and transfection of DC with plasmids coding for control shRNA or anti-human IDO shRNA. Means ± SD are shown; n=3; p value is shown for each marker. (D) Determination of phenotypic maturation of DC treated with TNF-α + poly(I:C) in the presence or absence of 1-MT. One experiment representative of three is shown. Similar results were obtained with poly(I:C) without TNF-α.
In two of three experiments, addition of tryptophan catabolites increased expression of maturation markers by cells treated with LPS + 1-MT to the levels observed with LPS alone, whereas no effect was observed with tryptophan catabolites alone (Supporting Information Fig. 1). Tryptophan catabolites did not result in DC toxicity as analyzed by TOPRO-3 staining (Supporting Information Fig. 2). Our results with tryptophan catabolites do not exclude the possibility that tryptophan starvation could also play a role in DC maturation, as recently described for anergic T cells 17.
A recent matter of debate is weather IDO inhibition with 1-MT impairs DC maturation by inhibiting IDO activity 1 or, alternatively, by off-target effects 2. We therefore aimed to knock-down IDO using plasmidic DNA coding for anti-human IDO. Anti-IDO shRNA decreased kynurenine production by 20%. Importantly, even with this suboptimal inhibition we were able to impair DC maturation by knocking down IDO (Fig. 2C). However, CD80 and CD86 were not significantly inhibited when using anti-IDO shRNA. We think that these molecules are not significantly inhibited because we obtained only a 20% inhibition of IDO enzymatic activity. This result suggests a dose-dependent effect. Unfortunately, we did not succeed in increasing the percentage of IDO inhibition due to difficulty in transfecting DC. However, we observed that pharmacologic inhibition of IDO by 1-MT has a dose-dependent effect (Supporting Information Fig. 3). Results similar to those obtained with 1-MT and shRNA were observed with the recently described IDO inhibitor methyl-thiohydantoin-tryptophan 18 (Supporting Information Fig. 4). Poly(I:C)-induced maturation could also be significantly prevented by inhibiting IDO (Fig. 2D).
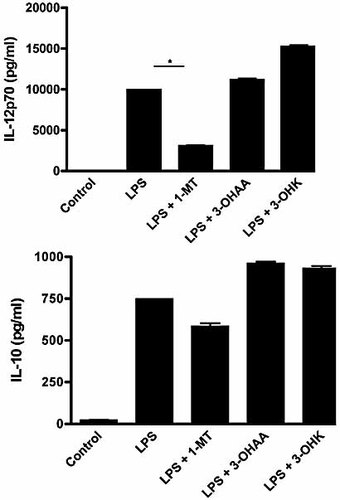
Cytokine production in the culture supernatant. Supernatants from experiments performed in Fig. 2 were analyzed by ELISA to quantify IL-12p70 and IL-10 production; *p<0.05. Tryptophan catabolites alone, without LPS, did not modify cytokines levels (not shown).
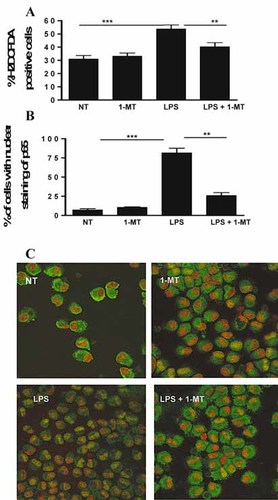
IDO promotes LPS-induced maturation by increasing ROS production and NF-κB activation. (A) ROS production was studied as described in the Materials and methods. The means ± SD of eight experiments are shown on the graphs; **p<0.01; ***p<0.001. (B) Anti-NF-κB (p65) staining was performed on cytospun cells. One-hundred cells in each condition were counted and the percentage of cells with nuclear staining of p65 is represented on the graph. The percentage is a mean of three experiments; **p<0.01, ***p<0.001. (C) Anti-NF-κB (p65) staining is shown in green and nucleus staining (TOPRO-3) in red. One experiment representative of three is shown.
In terms of cytokine production, IDO inhibition also led to a significantly reduced expression of IL-12p70, while 3-OHK but not 3-OHAA increased LPS-induced synthesis of this cytokine (Fig. 3). IL-10 production was reduced by 1-MT, but to a lesser extent than IL-12 (Fig. 3). Both tryptophan catabolites increased this cytokine as compared to LPS. Taken together, these observations suggest that upon TLR stimulation, IDO promotes DC maturation.
IDO promotes DC maturation by increasing ROS production and NF-κB activation
Tryptophan catabolites generated through the kynurenine pathway, such as 3-OHAA and 3-OHK, can increase ROS production in several cell types 19, 20. Oxidative stress induces DC maturation 21, 22, and antioxidant molecules can inhibit this differentiation process 21. We thus hypothesized that this could be a mechanism by which IDO mediates LPS-induced DC maturation. To study this, we measured oxidative status in DC (Fig. 4A). As already described 22, LPS increased ROS production. This phenomenon was inhibited by co-treatment with 1-MT. Similar results were obtained with the dihydroethidium probe that specifically recognizes O (Supporting Information Fig. 5). These results show that IDO activity partially mediates LPS-induced ROS production by DC.
(Supporting Information Fig. 5). These results show that IDO activity partially mediates LPS-induced ROS production by DC.
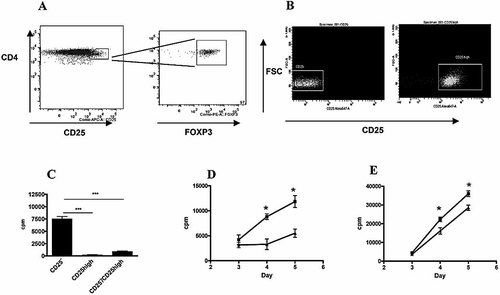
Mature DC expand Treg in an IDO-dependent manner. (A) CD4+CD25high T cells express FOXP3. (B) A typical cell sorting of CD4+CD25– and CD4+CD25high T cells purified from human peripheral blood. In this experiment, 99% purity for the former and 99.9% for the latter were reached. Purities were always superior to 98%. (C) CD4+CD25– T cells proliferated when co-cultured with allogeneic immature DC. In contrast, CD4+CD25high Treg were unresponsive to this stimulus and were able to inhibit proliferation of CD4+CD25– T cells by 90% at a 1:1 ratio. (D) CD4+CD25high Treg proliferated in the presence of allogeneic mature DC (LPS-treated) and 100 U/mL IL-2 (triangles). This proliferation was inhibited by 50% when DC were matured in the presence of 1-MT (squares). (E) CD4+CD25– effector T cells were stimulated in the same conditions as Treg. Slight differences in their proliferation were noted when the stimulatory DC were matured with LPS (triangles) or LPS + 1-MT (squares). Spontaneous proliferation of T cells cultured without DC was subtracted in all cases. One experiment representative of three is shown; *p<0.05, ***p<0.001.
The role of IDO in NF-κB activation was also studied (Fig. 4B, C). As expected, LPS led to nuclear translocation of NF-κB in 82 ± 10% of cells. Interestingly, IDO inhibition with 1-MT dramatically inhibited nuclear translocation of p65 (only 26 ± 6.5% of cells showed a nuclear staining), showing that LPS-induced NF-κB translocation is dependent on IDO activity. Similar results were obtained with the IDO inhibitor methyl-thiohydantoin-tryptophan (Supporting Information Fig. 6). Hence, IDO mediates LPS-induced DC maturation by increasing ROS production and NF-κB activation.
IDO-dependent DC maturation is needed to expand Treg
LPS-matured DC have been described to expand CD4+CD25+ Treg 14. We speculated that one of the physiological roles of IDO-induced maturation when DC are activated through TLR4 could be the expansion of Treg. To study this, we purified CD4+CD25high Treg from peripheral blood. CD4+CD25high cells from human peripheral blood have already been described as Treg 23. As expected, we confirmed that these cells were FOXP3+ (Fig. 5A). Purity of the CD4+CD25– and CD4+CD25high populations was always >98% (Fig. 5B). CD4+CD25high cells displayed suppressive activity in that they inhibited the proliferation of CD4+CD25– T cells by 90% when stimulated with allogeneic DC (Fig. 5C). Thus, CD4+CD25high cells used in this study can be considered unambiguously as Treg. In agreement with this, CD4+CD25high Treg showed marginal proliferation when stimulated with allogeneic immature DC, even in the presence of IL-2 (Supporting Information Fig. 7).
However, when CD4+CD25high Treg were stimulated with LPS-matured DC and IL-2, significant proliferation was observed (Fig. 5D). Interestingly, IDO inhibition with 1-MT during the maturation phase of DC with LPS led to a 50% decrease of Treg proliferation (Fig. 5D). We thus concluded that IDO-dependent DC maturation is important to expand Treg. Of expanded Treg, 80% were FOXP3+, showing that proliferation was not due to contamination by effector cells, which, upon a 5-day MLR, were FOXP3+ at 40% (Supporting Information Fig. 8). In clear contrast to Treg, only a slight decrease in proliferation of CD4+CD25– effector T cells was observed when the stimulator DC had previously been treated with LPS + 1-MT as compared to LPS alone (Fig. 5E).
Discussion
We have previously shown that human mature DC express IDO as well as other enzymes of the kynurenine pathway at the mRNA level 4, suggesting that kynurenine is not a terminal metabolite in human DC. In the present work, we show that human monocyte-derived DC matured with LPS metabolize tryptophan as far as quinolinate. In recent years, several investigators have described tolerogenic properties of DC expressing IDO (for reviews see 8–10). However, less attention has been paid to the expression of other enzymes of the kynurenine pathway by DC 24. Very recently, Fallarino et al. 12 have shown that murine DC can metabolize tryptophan as far as quinolinate. Moreover, the paracrine production of tryptophan-derived catabolites by tolerogenic DC can confer suppressive properties to immunogenic DC 12.
Since tryptophan-derived catabolites such as 3-OHAA, 3-OHK and quinolinic acid can modulate NF-κB activation 25, 26 and ROS production 19, 20, we speculated that these molecules could modulate DC maturation. In agreement with this hypothesis, IDO inhibition with 1-MT led to reduced DC maturation when stimulated with LPS or poly(I:C). Our results strongly suggest that IDO promotes LPS-induced DC maturation by inducing activation of NF-κB and ROS production. Consistent with our results, IFN-induced genes can mediate LPS-induced DC maturation 27, 28. Tryptophan catabolites alone did not lead to DC maturation (Supporting Information Fig. 1). In agreement with this, although IFN-γ induced IDO activity (Fig. 1A), it was not able to increase phenotypic maturation (Supporting Information Fig. 9). Thus, the effects of IDO on DC can depend on the presence or absence of TLR signaling. In a similar manner, CD40 and BCR signals need TLR signaling to activate B cells 29.
Since 1-MT can inhibit the transport of L-tryptophan into the cell 30, reduced expression of CD80, CD86, CD83 and HLA-DR in the presence of LPS and 1-MT could be simply due to a block of tryptophan uptake and therefore a general reduction in protein synthesis. However, this possibility was discarded since heme oxygenase-1 protein levels were increased in the LPS + 1-MT condition as compared to LPS alone (Supporting Information Fig. 10). In addition, in two of three experiments tryptophan catabolites restored maturation of DC treated with LPS + 1-MT to levels similar to those observed with LPS alone (Supporting Information Fig. 1). Since the effects of 1-MT were confirmed with anti-IDO shRNA and another IDO inhibitor, off-target effects of 1-MT seem improbable. Moreover, the fact that tryptophan catabolites had an opposite effect to IDO inhibition further supports the hypothesis that tryptophan catabolism plays a role in modulating DC biology. Endotoxin contamination of tryptophan catabolites is unlikely since 3-OHAA and 3-OHK alone had no effect (Supporting Information Fig. 1). Moreover, in our system, 1 µg/mL LPS is a saturating dose, since no further increase in maturation markers was observed at 2 µg/mL (Supporting Information Fig. 11).
Agaugue et al. 31 have recently published similar observations regarding the effect of 1-MT on LPS-induced DC maturation. However, their interpretation of these results is clearly different from ours. Agaugue et al. 31 claim that 1-MT can interfere with TLR signaling in DC independently of IDO activity. Although this is a very interesting and well-conducted study, we think that the evidence they present is, to a certain extent, indirect and could be interpreted differently. For example, the authors found that addition of kynurenine or a large excess of tryptophan (2.5 mM) did not counteract or mimic, respectively, the effect of 1-MT on DC function. We believe that the lack of biological effects when adding tryptophan might simply mean that tryptophan starvation is not implicated in the effect, but the role of tryptophan catabolites cannot be discarded. Furthermore the authors cannot affirm that in the conditions studied, tryptophan effectively competed with 1-MT (used at 1 mM), because the affinity of 1-MT for IDO can be five- to tenfold higher than that of tryptophan 32, 33. Furthermore, the effects of kynurenine have been shown to be largely improved by adding other tryptophan-derived catabolites 11. Thus, a negative result by adding kynurenine alone does not exclude the effect of tryptophan catabolites. Here we unambiguously show, using an shRNA approach, that IDO does indeed increase human DC maturation upon LPS treatment.
In the present study we also show that one of the physiological roles of IDO-induced maturation when DC are activated through TLR-4 could be the expansion of Treg. It is well known that LPS-matured DC expand CD4+CD25+ Treg 14, 34, but the role of IDO in this process is described here for the first time. It is interesting to note that minor differences in the proliferation of CD4+CD25- effector T cells were observed when the stimulator DC had previously been treated with LPS or LPS + 1-MT (Fig. 5E). This observation can be explained by the fact that 1-MT inhibits maturation of LPS-treated DC and thus diminishes the stimulatory capacity of DCs, but at the same time IDO inhibition (prevention of tryptophan starvation and kynurenine production) increases proliferation of effector T cells. Our interpretation of these observations is that Treg may be less susceptible to the inhibitory activity of IDO as compared to effector T cells. Therefore, Treg proliferation could not be inhibited by IDO activity, in contrast to that of effector T cells.
The study of a possible differential susceptibility between effector and Treg to inhibition of proliferation by IDO is the subject of further studies. In agreement with this hypothesis, it is known that different T cell subsets can show a differential susceptibility to apoptosis induced by IDO 35. Furthermore, IDO can lead to induction of T cell activation-induced cell death 36. Interestingly, Treg are substantially less sensitive to T cell activation-induced cell death than effector T cells 37. Therefore, IDO might not induce apoptosis in Treg. Moreover, although IDO activity can inhibit T effector proliferation, this is not true in some settings 7, 38. Indeed, little is known about the susceptibility of T cells to IDO regulation. Overall, our results show that IDO promotes maturation of LPS-treated DC and thereby favors expansion of CD4+CD25high Treg. These observations could constitute a new tolerogenic mechanism for IDO.
Materials and methods
Generation of human monocyte-derived DC
Human immature DC were generated as previously described 39. Briefly, monocytes were enriched by elutriation (>85% CD14+) and cultured for 6 days in medium supplemented with IL-4 (100 U/mL; AbCys, Paris, France) and GM-CSF (500 IU/mL; AbCys). Next, immature DC were harvested and cultured (106 cells/mL) in plates coated with poly(2-hydroxyethyl methacrylate) (Sigma, St. Louis, MO) to prevent cell adhesion. Maturation was induced by a 48-h incubation with 50 µg/mL poly(I:C) (Sigma, St. Quentin Fallavier, France) and 10 ng/mL TNF-α (AbCys), 1 µg/mL LPS (Sigma) or in the presence of 1000 IU/mL IFN-γ (AbCys) with or without a 3-h prior incubation with the IDO-specific inhibitor 1-MT 32 at 500 µM (Sigma). Methyl-thiohydantoin-tryptophan 18 (Calbiochem, Nottingham, UK) was diluted in DMSO and used at a final concentration of 100 µM. DC were cultured in the presence or absence of the tryptophan catabolites 3-OHAA or 3-OHK (Sigma) at 20 µM for the last 24 h of culture.
Characterization of DC phenotype, Treg and cytokine production
The surface phenotype of DC was determined using the following antibodies: FITC-conjugated anti-CD80 (MAB104; Beckman-Coulter; diluted 1:30), phycoerythrin-conjugated anti-CD86 (HA5.2B7; Beckman-Coulter; 1:30), anti-CD83 (HB15e; BD Biosciences; 1:30) and anti-HLA-DR (Immu-357; Beckman-Coulter; diluted 1:30). Supernatants were harvested after 48 h and IL-12 p70 and IL-10 were measured using a commercial ELISA kit (BD PharMingen, San Diego, CA). Phycoerythrin-conjugated anti-human FOXP3 was from eBioscience (San Diego, CA).
IDO activity
IDO activity was quantified by measuring kynurenine production in the culture supernatant by colorimetry 40. Briefly, 100 µL of 30% trichloroacetic acid was added to 200 µL of culture supernatant. Next, 125 µL of soluble phase was added to 125 µL of Ehrlich's reagent (100 mg of p-dimethylbenzaldehyde in 5 mL glacial acetic acid) (Sigma) in a microtiter plate. The OD was measured at 492 nm and kynurenine concentration calculated by referral to a kynurenine (Sigma) standard curve.
Transfection of shRNA
Plasmidic DNA coding for anti-human IDO and control shRNA were obtained from Sigma. A mix of four different clones of anti-IDO shRNA, or one clone of control shRNA, were transfected (9 µg plasmid/3×106 DC in 3 mL RPMI medium) using DOTAP reagent (Roche Diagnostics). LPS treatment was performed 4 h after transfection. Analysis of phenotype and kynurenine production in the culture supernatant was performed after 48 h of LPS treatment.
Measurement of cell oxidation states
DC were stimulated for 20 h with LPS, with or without a 3-h prior incubation with 1-MT. The oxidation-sensitive dyes (5-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) or dihydroethidium (Molecular Probes, Montluçon, France) were then added as previously described 41.
Immunocytology
Quinolinate production by DC was studied using a specific antibody 42. This is a rabbit polyclonal antibody that does not cross-react with other tryptophan-derived catabolites 42. Cells were fixed with carbodiimide and then incubated with the primary antibody. Negative controls included non-fixed cells incubated with primary antibody and the detection system. Revelation was performed with an avidin-biotin complex detection kit from Vector Laboratories (Burlingame, CA).
NF-κB nuclear translocation was analyzed by immunofluorescence and images were analyzed by confocal microscopy. An anti-p65 (F-6) monoclonal antibody from Santa Cruz Biotechnology (Santa Cruz, CA) was used at 5 µg/mL. The secondary antibody was an anti-mouse-FITC from Jackson (Suffolk, UK). Cell nuclei were counterstained with TOPRO-3 iodide (Molecular Probes, Eugene, OR) and slides were mounted in ProLong AntiFade reagent (Molecular Probes). Slides were analyzed with a Leica confocal microscope and the Leica TCS NT software.
Mixed leukocyte reaction
DC (2×103) were cultured in triplicate in round-bottom 96-well plates with 104 allogeneic human T cells. CD4+CD25– and CD4+CD25+ T cells were purified from peripheral blood, and sorted using a FACSAria (Becton Dickinson, San Jose, CA). Purity was systematically >98%. Proliferation was determined 5 days later by uptake of [3H]thymidine (Amersham, Orsay, France).
Statistical analysis
Statistical significance was evaluated using a one-way ANOVA test. p<0.05 was considered significant.
Acknowledgements
We are grateful to Maria Cristina Cuturi for critically reading the manuscript and to Vincent Lotteau for sending us his manuscript before publication. This work was financed by the Foundation Progreffe, the Ligue National Contre le Cancer, the Association pour la Recherche sur le Cancer and the INSERM.Conflict of interest: The authors declare no financial or commercial conflict or interest
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




