PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis
Abstract
To compare the roles of programmed death 1 ligand 1 (PD-L1) and PD-L2 in regulating immunity to infection, we investigated responses of mice lacking PD-L1 or PD-L2 to infection with Leishmania mexicana. PD-L1–/– and PD-L2–/– mice exhibited distinct disease outcomes following infection with L. mexicana. In comparison to susceptible WT mice, PD-L1–/– mice showed resistance to L. mexicana, as demonstrated by reduced growth of cutaneous lesions and parasite burden. In contrast, PD-L2–/– mice developed exacerbated disease with increased parasite burden. Host resistance to L. mexicana is partly associated with the development of a Th1 response and down-regulation of the Th2 response. Both PD-L1–/– and PD-L2–/– mice produced levels of IFN-γ similar to WT mice. However, the development of IL-4-producing cells was reduced in PD-L1–/– mice, demonstrating a role for PD-L1 in regulating Th cell differentiation. This inadequate Th2 response may explain the increased resistance of PD-L1–/– mice. Although no alterations in Th1/Th2 skewing were observed in PD-L2–/– mice, PD-L2–/– mice exhibited a marked increase in L. mexicana-specific antibody production. Increased Leishmania-specific IgG production may suppress the healing response through FcγR ligation on macrophages. Taken together, our results demonstrate that PD-L1 and PD-L2 have distinct roles in regulating the immune response to L. mexicana.
Abbreviations:
-
- PD-1:
-
programmed death 1
-
- PD-L1/2:
-
PD-1 ligand 1/2
-
- SHP-1/2:
-
Src homology 2 domain-containing phosphatase 1/2
Introduction
Pathways in the B7:CD28 family have key roles in regulating T cell activation and tolerance. These pathways not only provide critical positive second signals that augment and sustain T cell responses, but also contribute key negative second signals that inhibit T cell responses 1–3. The programmed death 1 (PD-1):PD-1 ligand 1 (PD-L1; B7-H1)/PD-L2 (B7-DC) pathway is one of the newer pathways in the B7:CD28 family 4–8. PD-1 is only known to bind PD-L1 and PD-L2, and the two PD-1 ligands do not bind to other CD28 family members. The PD-1 receptor is expressed more broadly than CD28 and up-regulated on B cells as well as T cells upon activation. PD-1 provides negative signals by recruiting protein tyrosine phosphatases, Src homology 2 domain-containing phosphatase 1 (SHP-1) and SHP-2, following engagement 9. The functional significance of PD-1 inhibitory signals is demonstrated by the phenotype of PD-1-deficient (–/–) mice. PD-1–/– mice on the C57BL/6 background develop a lupus-like disease, while PD-1–/– mice on the BALB/c background develop a fatal cardiomyopathy associated with anti-cardiac autoantibodies 10, 11. These results suggest that PD-1 may inhibit T and/or B cell activation and is important in regulating peripheral tolerance.
The discovery of two ligands for PD-1 has led to comparative analyses of these ligands. PD-L1 and PD-L2 exhibit distinct patterns of expression, with PD-L1 being expressed more broadly than PD-L2 in mice 12, 13. PD-L1 is expressed on resting and up-regulated on activated B, T, myeloid and dendritic cells (DC). PD-L1 also is expressed in non-hematopoietic cells including vascular endothelial cells and in nonlymphoid organs including heart, lung, pancreas, muscle and placenta. The expression of PD-L1 within nonlymphoid tissues suggests that PD-L1 may regulate inflammatory responses in tissues and/or self-reactive T or B cells in the target organs. In contrast, PD-L2 is inducibly expressed only on macrophages and DC. The stimuli that up-regulate PD-L1 and PD-L2 also differ. On macrophages, PD-L1 expression is stimulated by IFN-γ, whereas PD-L2 expression is up-regulated by IL-4 14. On DC, IFN-γ is a potent stimulus for PD-L1 expression and IL-4 is a strong inducer of PD-L2, but these differences are not absolute. IFN-γ also can up-regulate PD-L2 and IL-4 can up-regulate PD-L1 on DC. These expression studies suggest that PD-L1 may have a preferential role in regulating Th1 responses, whereas PD-L2 may regulate Th2 responses. Thus, the relative functions of PD-L1 and PD-L2 may depend on the tissue microenvironment and cytokine milieu.
Whether PD-L1 and PD-L2 have similar or distinct functions is under active investigation. Initial reports suggested that PD-L1 and PD-L2 inhibit T cell proliferation and cytokine production, while others suggested that these molecules are costimulatory, enhancing proliferation and effector functions. The phenotype of PD-L1–/– mice 15 demonstrates that PD-L1 has a critical negative regulatory role in vivo. PD-L1 inhibits CD4+ and CD8+ IFN-γ production in vivo. PD-L1–/– mice on the 129Sv/Jae background develop greatly exacerbated experimental autoimmune encephalomyelitis, a Th1 cell-mediated autoimmune disease of the central nervous system. These results indicate roles for PD-L1 in inhibiting Th1 responses and regulating T cell tolerance.
To compare the in vivo functions of PD-L1 and PD-L2, we have generated mice lacking PD-L1 or PD-L2. In this report, we compare the responses of 129Sv/Jae PD-L1–/– and PD-L2–/– mice to infection with Leishmania mexicana. The immune response to the intracellular protozoan L. mexicana provides a sensitive probe of Th differentiation during the immune response to infection 16–19. Most strains of mice are susceptible to L. mexicana. Critical to L. mexicana pathogenesis is the balance of Th1 and Th2 responses. IFN-γ production activates macrophage leishmanicidal activity, while IL-4 counter-regulates the protective effects of Th1 cytokines on macrophages. Therefore, host resistance is associated with the development of a Th1-dominated response with very low levels of Th2 cytokines, while susceptibility is associated with a vigorous Th2 response. In addition, the humoral response is important in the pathogenesis of L. mexicana. Leishmania-specific antibodies may facilitate parasite entry into macrophages 20. Opsonized L. mexicana amastigotes also promote IL-10 production by macrophages. Both FcγR and circulating IgG are needed for chronic disease, suggesting that ligation of FcγR on macrophages by Leishmania-specific IgG triggers IL-10 production. IL-10 can act to inhibit nitric oxide synthesis, IL-12 and antigen presentation, and suppresses protective immunity 21, 22.
Our studies demonstrate that PD-L1 and PD-L2 distinctly regulate the immune response during infection with L. mexicana. PD-L1–/– mice are markedly resistant to disease and develop smaller lesions and lower parasite burdens than susceptible WT controls. In contrast, PD-L2–/– mice exhibit larger lesions and greater parasite burdens than susceptible WT controls. The disease resistance observed in L. mexicana-infected PD-L1–/– mice is associated with impaired Th2 cytokine production and an intact Th1 response. PD-L2–/– mice develop a Th1/Th2 response similar to WT, but produce elevated levels of parasite-specific IgM and IgG2a, which may suppress protective immunity through several mechanisms. Thus, PD-L1 and PD-L2 have unique roles in regulating immune responses in vivo and host immunity following infection with L. mexicana.
Results
Generation of PD-L2–/– mice
To compare the essential functions of PD-L1 and PD-L2 in vivo, we generated PD-L2–/– mice by gene targeting (Fig. 1). The targeting vector replaced the IgV exon of PD-L2 (Fig. 1A) with the hygromycin drug resistance gene. Germ-line-transmitting chimeric mice generated from targeted 129Sv ES cells were bred with 129Sv mice and progeny heterozygous for the PD-L2 mutation were interbred. Genotyping of progeny resulting from an intercross of two heterozygotes yielded mice carrying the PD-L2 mutation with the expected frequency (Fig. 1B). We confirmed the lack of PD-L2 protein expression in PD-L2–/– mice by examining PD-L2 expression on DC from WT and PD-L2–/– mice (Fig. 1C). PD-L2–/– mice were grossly normal. CD4+, CD8+, CD19+, CD11c+, or CD11b+ cell numbers were comparable in spleen, thymus, and lymph nodes (LN) of WT and PD-L2–/– mice, and there were no spontaneous changes in CD62L, CD44, CD45Rb, and CD25 expression on T cells (data not shown). Frequencies of CD4+CD25+CD45Rblo regulatory T cells in PD-L2–/– mice were similar to WT mice. PD-L1 expression was comparable in WT and PD-L2–/– mice (data not shown).
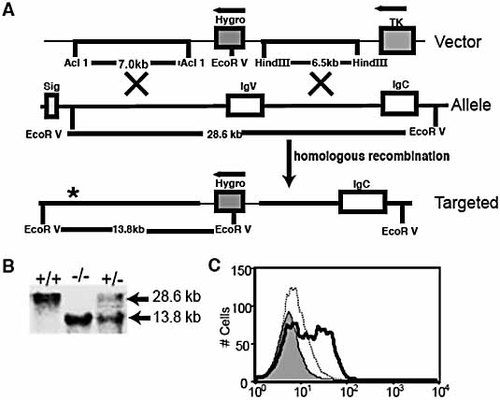
Generation of PD-L2–/– mice. (A) The PD-L2 targeting vector is shown in the top panel, with the hygromycin cassette replacing the IgV region. The middle panel shows the genomic organization of the PD-L2 gene (not to scale). Exons are open boxes. Homologous recombination is represented in the lower panel. * indicates the position of the probe. (B) Genomic DNA from PD-L2+/+, PD-L2+/–, and PD-L2–/– mice were digested with Eco RV and subjected to Southern blot analysis. The 28.6-kb fragment represents the WT allele and a 13.8-kb fragment represents the mutant allele. (C) DC from WT and PD-L2–/– mice were stained for PD-L2 expression. WT (thick), PD-L2–/– (shaded), IgG2a isotype (dotted).
Distinct outcomes of L. mexicana infection in PD-L1–/– and PD-L2–/– mice
WT 129Sv mice are susceptible and develop progressive, non-healing cutaneous lesions following s.c. inoculation of L. mexicana amastigotes. These lesions can increase to a diameter of 1 cm over 9–12 wk. Lesion size reflects the degree of parasite replication. Immunohistochemical analyses revealed that both PD-L1 and PD-L2 are expressed in cutaneous lesions in WT mice (data not shown). To evaluate the roles of PD-L1 and PD-L2 in regulating the response to L. mexicana, we infected mice lacking PD-L1 or PD-L2 with L. mexicana.
PD-L2–/– mice exhibited statistically significant larger lesions (12 ± 1 mm) than WT mice (9.0 ± 0.5 mm) (p <0.01) (Fig. 2A). In marked contrast, PD-L1–/– mice developed smaller lesions than WT mice (WT, 9 ± 0.5 mm vs. PD-L1–/–, 5.0 ± 0.5 mm, p <0.01). At 9 wk, both WT and PD-L2–/– mice exhibited inflammatory infiltrates and multiple areas of necrosis in the subcutaneous tissue, while inflammation was substantially reduced in PD-L1–/– mice with fewer parasitized macrophages and an absence of tissue damage (data not shown). Thus, the development of lesions is distinct in mice lacking PD-L1 or PD-L2: Lesions are exacerbated in PD-L2–/– mice but reduced in PD-L1–/– mice.
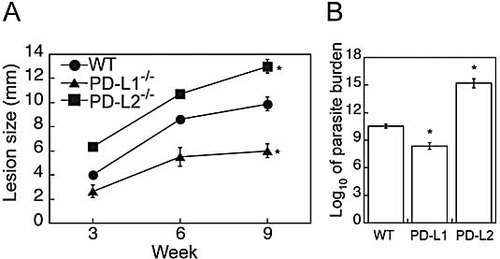
The outcome of L. mexicana infection is distinct in PD-L1–/– and PD-L2–/– mice. (A) Mean lesion diameter in WT (•), PD-L1–/– (▴), and PD-L2–/– (▪) mice was monitored for 9 wk. (B) Parasite burdens were quantified in lesions by limiting dilution assays at 9 wk after infection. Limiting dilution assays were performed on individual mice. Data are expressed as the mean log titer of four to five mice ± SEM. * denotes p <0.01 as compared to WT.
Parasite burdens in cutaneous lesions were enumerated by limiting dilution assays at 9 wk. PD-L2–/– mice exhibited a significant 105-fold increase in parasite burden compared to WT mice (WT 10.5 ± 0.5, PD-L2 15 ± 1 log of dilution factor, p <0.01) (Fig. 2B). The resistance to disease observed in PD-L1–/– mice was reflected in a 100-fold reduction in parasite burden (10.5 ± 0.5 vs. 8 ± 0.5 log fold dilution, WT vs. PD-L1, p <0.01 as compared to WT and PD-L2–/– mice). Together, our results show that PD-L1 and PD-L2 have opposing effects on the ability of the host to control infection.
PD-L1, but not PD-L2, influences the development of Th2 responses to L. mexicana
To begin to investigate the basis for the distinct outcomes of L. mexicana infection in PD-L1–/– and PD-L2–/– mice, we compared Th cell responses following infection. The outcome of disease during L. mexicana infection reflects the complex interplay between Th1 and Th2 cytokines. IFN-γ is required for macrophage activation and intracellular elimination of parasites, while IL-4 inhibits macrophage activation and protective host immunity. To evaluate cytokine production, we restimulated equal numbers of inguinal LN cells at 9 wk post infection with L. mexicana freeze-thaw antigen for 4 days to expand the parasite-specific effector population. To analyze Th1- and Th2-associated cytokine production, we performed intracellular cytokine staining for IL-4 and IFN-γ on the expanded populations. PD-L1–/– mice exhibited a statistically significant decrease in the percentage of CD4+ IL-4+ cells as compared to WT (2.05% vs. 0.69%, p <0.05) (Fig. 3A). No difference was observed in the percentage of CD4+ IL-4+ cells in the PD-L2–/– mice. We also did not observe any differences in the percentage of CD4+ IFN-γ+ or IFN-γ+ IL-4+ cells among the groups (Fig. 3B). PD-L1–/– mice exhibited a statistically significant increase in the IFN-γ/IL-4 ratio, as a result of a decreased IL-4+ population, as compared to WT. The IFN-γ/IL-4 ratios were comparable in WT and PD-L2–/– mice (Fig. 3C). Levels of IL-4 and IL-10 as detected by ELISA were also reduced in the PD-L1–/– but not the PD-L2–/– group, compared to WT (Fig. 4 and data not shown). IFN-γ levels as detected by ELISA were similar among the groups. These results suggest that the ameliorated disease course in PD-L1–/– mice may be a result of a decreased Th2 response. However, the exacerbated disease in PD-L2–/– mice was not due to an enhanced Th2 response.
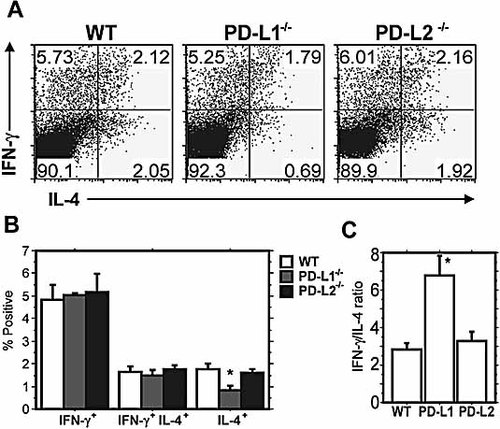
CD4+ IL-4+ cells are decreased in the absence of PD-L1. Intracellular cytokine staining for IFN-γ and IL-4 was performed on CD4+ cells on day 4 following restimulation with L. mexicana antigen. (A) Representative flow cytometry plots gated on CD4+ cells are shown. (B) Percentages for IFN-γ+, IFN-γ+ IL-4+, and IL-4+ cells were calculated. (C) The IFN-γ/IL-4 ratio was calculated. PD-L1–/– mice, but not PD-L2–/– mice, developed a significant increase in the IFN-γ/IL-4 ratio. These results are representative of three experiments with three to five mice per group. Data are means ± SEM. * p <0.05 as compared to WT.
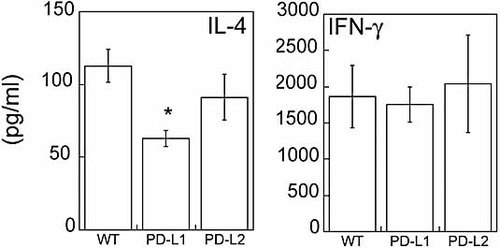
Th2 cytokines are reduced in PD-L1–/– infected mice as compared to WT. Draining LN cells (4 × 106) were restimulated with 40 µg/mL L. mexicana antigen. Levels of IL-4 and IFN-γ were determined on day 4 by ELISA. Data are means ± SEM of four to five mice per group. * p = 0.01 as compared to WT.
PD-L2–/– but not PD-L1–/– mice exhibit enhanced levels of L. mexicana-specific IgM and IgG2a
The expression of PD-1 on B cells prompted us to examine whether elimination of PD-L1 or PD-L2 alters L. mexicana-specific antibody responses. At 2 wk after infection, levels of parasite-specific IgM were enhanced in the PD-L2–/– group as compared to WT (p <0.05) (Fig. 5A). No difference in parasite-specific IgM was observed between WT and PD-L1–/– groups at 2 wk. At 9 wk post infection, PD-L1–/– mice showed a slight decrease in parasite-specific IgG1 and IgG2a, but this was not statistically significant (Fig. 5B; p = 0.13 and p = 0.12). In contrast, PD-L2–/– mice exhibited 3–5-fold higher levels of parasite-specific IgG2a and total IgG2a as compared to WT (p <0.05) (Fig. 5B, C). This elevated IgG2a in the PD-L2–/– mice may contribute to the exacerbated disease observed in these mice. Interestingly, IgG2a has been reported to have the highest binding affinity to macrophages and FcγR, while IgG1 has been reported to be a poor interactor 23, 24. The increased Leishmania-specific IgG2a in PD-L2–/– mice may lead to increased binding of opsonized parasites to FcγR and suppress protective immune responses. No differences were observed in total IgE among the groups (data not shown).
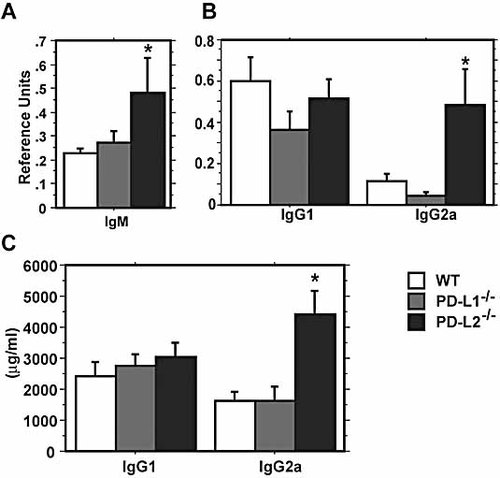
Ig responses during L. mexicana infection are enhanced in PD-L2–/– mice. (A) At wk 2 post infection, sera were analyzed for parasite-specific IgM by ELISA. PD-L2–/– but not PD-L1–/– mice exhibited a statistically significant increase in parasite-specific IgM. (B) Sera at wk 9 post infection were analyzed for parasite-specific IgG1 and IgG2a by ELISA. PD-L2–/– mice exhibited a significant increase in L. mexicana-specific IgG2a as compared to WT. (C) Total IgG1 and IgG2a levels were determined at 9 wk post infection. No differences were seen in total IgG1 among the groups. Total IgG2a was elevated in PD-L2–/– mice, but not PD-L1–/– mice, as compared to WT. All data are means ± SEM of 6–15 mice per group. * denotes p ⩽0.05 as compared to WT.
Discussion
We have found that PD-L1 and PD-L2 have distinct roles in regulating the immune response to L. mexicana. In the absence of PD-L1, parasite burdens and lesion development are markedly reduced. In contrast, PD-L2–/– mice exhibit more severe disease. The distinct outcomes of L. mexicana infection in PD-L1–/– and PD-L2–/– mice could reflect impaired PD-1 signals in both B cells and T cells. While the Th2 response is decreased in L. mexicana-infected PD-L1–/– mice, no differences in T cell differentiation were observed in PD-L2–/– mice. Instead, PD-L2–/– mice produced increased levels of L. mexicana-specific IgM and IgG2a, which may contribute to the exacerbated disease observed in the PD-L2–/– mice. Thus, PD-L1 and PD-L2 distinctly regulate T and B cell responses to L. mexicana.
The comparable levels of IFN-γ produced by LN cells from L. mexicana-infected WT and PD-L1–/– mice are surprising, since previous studies have shown that blockade or elimination of PD-L1 can increase IFN-γ production by T cells. The reduced number of IL-4+ CD4+ cells in PD-L1–/– mice may be due to a defect in priming cells to become Th2 effectors or a skewing towards the Th1 response. Since PD-L1 is expressed on endothelial cells as well as other non-hematopoietic cells, the levels of IFN-γ produced by in vitro LN cultures may not completely reflect interactions of T cells with PD-L1-expressing cells in vivo. In fact, the reduced Th2 cytokine production by PD-L1–/– LN cultures may indicate elevated IFN-γ levels in vivo and be secondary to in vivo counter-regulation by IFN-γ.
The elevated parasite-specific Ig levels detected in the PD-L2–/– mice suggest that PD-L2 regulates humoral immune responses. The increased parasite-specific IgM levels suggest that T-independent B cell activation can be inhibited by PD-L2, while the enhanced IgG2a levels in PD-L2–/– mice suggest that T-dependent B cell responses can also be controlled by PD-L2. These findings are consistent with in vitro studies showing that PD-1 signaling can inhibit B cell activation. We have observed that lack of PD-L2 leads to increased IgM and IgG2a in a model of allergen-induced airway hyper-reactivity (manuscript under preparation), suggesting that the increased antibody production is not particular to just Leishmania infection.
In summary, our results demonstrate unique roles for PD-L1 and PD-L2 in regulating the in vivo immune response to L. mexicana. CD4+ Th differentiation and B cell responses are distinctly regulated by PD-L1 and PD-L2, leading to opposing outcomes of infection in PD-L1–/– and PD-L2–/– mice.
Materials and methods
Mice
WT, PD-L1–/–, and PD-L2–/– 129S4/SvJae mice were maintained in accordance with the institutional guidelines of Harvard Medical School (Boston, MA). Female 6–8-week-old mice were used for our studies.
PD-L1–/– and PD-L2–/– mice
PD-L1–/– mice were generated as described 15. To generate PD-L2–/– mice, a targeting vector was designed to replace the IgV exon of the PD-L2 gene with the hygromycin drug resistance gene (Fig. 1A). This PD-L2 targeting vector was electroporated into the J1 129S4/SvJae ES cell. Homologous recombinants were identified by Southern blot analysis. The genomic probe used for Southern blotting was external to the genomic DNA used in the targeting vector and was generated by PCR with the following primers (5′ GAATTCTGAAATGAGTGTCCTGACTG 3′, 5′ GAATTCTAACTGTGTTTTCTCTTACA 3′). Eco RV digest yields a 28.6-kb band in the WT and a 13.8-kb band in the targeted allele. Three ES clones with the PD-L2 homologous recombinant event were microinjected into blastocysts and gave rise to germ-line transmission of the PD-L2 mutation. Mice heterozygous for the PD-L2 mutation were interbred. Genotyping of the mice was performed by PCR. PD-L2 primers to detect the IgV region deleted in PD-L2–/– mice were 5′ AAGCTTTAACCCCCGTTACCTTGA 3′ and 5′ CCGCCTGGGACTACAAGTACCTG 3′. Sequences of the hygromycin primers were 5′ AGACCTGCCTGAAACCGAAC 3′, 5′ CAGTCAATGACCGCTGTTAT 3′. PCR products were 223 base pairs (bp) and 350 bp for PD-L2 and hygromycin, respectively.
To confirm the absence of PD-L2 expression in PD-L2–/– mice, we evaluated PD-L2 protein expression on DC from WT and PD-L2–/– mice. DC were expanded in vivo in WT and PD-L2–/– mice with 20 µg Flt-3L Ig (Bioexpress, West Lebanon, NH) every other day for 10 days. DC were isolated from spleens using MACS CD11c positive selection (Miltenyi, Auburn, CA) and cultured overnight in 1000 U/mL IL-4 (Pharmingen, San Diego, CA) to up-regulate PD-L2 (eBioscience, San Diego, CA). DC were co-stained with anti-CD11c (HL3; Pharmingen) and anti-PD-L2 mAb (TY25; eBioscience) (Fig. 1C).
Infection
L. mexicana parasites (MYNC/BZ62/M379) were maintained in the rumps of BALB/c mice. Amastigotes were isolated from animal reservoirs as described 16. Mice were infected with 5 × 106 L. mexicana amastigotes by subcutaneous injection into the lower back. Lesion diameter was measured for up to 9 wk 16. Parasite burdens were quantified by limiting dilution assay 25. Briefly, lesions were dissociated and serial tenfold dilutions were performed. Plates were cultured for 5 days at 26°C to allow the promastigotes to grow. The dilution at which parasites were undetectable was recorded. Data are expressed as the mean log titer ± SEM.
Serum ELISA
Mice were bled on wk 2 and 9 after infection. Sera were analyzed for total and Leishmania-specific IgM, IgG1, and IgG2a, and total IgE by ELISA 16. L. mexicana-specific IgM, IgG1, and IgG2a were measured by ELISA by coating with L. mexicana freeze-thaw antigens. Sera isotypes were detected with alkaline phosphatase-conjugated anti-mouse IgG1, anti-mouse IgG2a, and anti-mouse IgM antibodies (Southern Biotech, Birmingham, AL). The limits of detection for IgG1, IgG2a, and IgE were 10 ng/mL, 10 ng/mL, and 50 ng/mL respectively. A reference sample was run in conjunction with all test samples and used to calculate the level of parasite-specific sera in reference units.
In vitro cell preparations
Inguinal LN were collected 9 wk post infection. LN cells (4 × 106) were cultured with 40 µg/mL L. mexicana freeze-thaw antigen 16. On day 4 of restimulation, levels of IFN-γ and IL-4 were determined by ELISA using the following antibody pairs: IL-4 (BVD4–1D11, B11-3); IFN-γ (AN-18, P4-6A2). The limits of detection were 20 pg/mL for both IL-4 and IFN-γ. Intracellular cytokine staining was performed using the Cytoperm/Cytofix kit (Pharmingen) as per the manufacturer's directions. Cells were surface stained with anti-CD4-PerCP-Cy5.5 antibody (RM4-5). Intracellular cytokine staining was performed with anti-IFN-γ-allophycocyanin (XMG1.2) and anti-IL-4-PE antibodies (BVD4–1D11).
Statistical significance
Student's unpaired t-test was used to determine the statistical significance of the individual values obtained.
Acknowledgements
The authors wish to thank Cherry Kingsley for her helpful discussions. We also thank Baolin Chang, Tatyana Chernova and John Burgess for their technical support. This work was supported by grants from the Juvenile Diabetes Foundation (R.J.M.G.), National Institutes of Health (R.J.M.G., A.S., G.J.F., A.H.S.), and the Wellcome Trust and Leukemia and Lymphoma Society (Y.E.L.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




