Direct manipulation of activator protein-1 controls thymocyte proliferation in vitro
Abstract
B cell activating transcription factor (BATF) belongs to the activator protein-1 (AP-1) superfamily of basic leucine zipper transcription factors and forms heterodimers with Jun that possess minimal transcriptional activity. Mice carrying a p56lckHA-BATF transgene were created to observe the effects of constitutive expression of this well-characterized AP-1 inhibitor on T cell proliferation. Consistent with the role of AP-1 in promoting the proliferation of many cell types, BATF-transgenic thymocytes proliferate poorly in vitro when stimulated with anti-CD3ϵ and anti-CD28 antibodies or with Concanavalin A. However, when BATF-transgenic thymocytes were stimulated using a standard treatment of PMA and ionomycin, proliferation is normal. The responsiveness to PMA and ionomycin can be attributed to the dramatic disappearance of the hemagglutinin antigen (HA)-tagged BATF protein which is a PKC-dependent process caused by the down-regulation of the p56lck proximal promoter coupled with the rapid turnover of the HA-BATF protein. These studies describe conditions of T cell stimulation that negatively influence transcription of the widely used p56lck proximal promoter expression cassette. In addition, the unique circumstances of this regulation were exploited to demonstrate that inhibition of AP-1 activity by BATF exerts a direct, and reversible, effect on T cell proliferation in vitro.
Abbreviations:
-
- AP-1:
-
activator protein-1
-
- BATF:
-
B cell activating transcription factor
-
- bZIP:
-
basic leucine zipper
-
- DN:
-
CD4–CD8– double-negative
-
- DP:
-
CD4+CD8+ double-positive
-
- EMSA:
-
electrophoretic mobility shift assay
-
- ERK:
-
extracellular regulated kinase
-
- HA:
-
hemagglutinin antigen
-
- mTOR:
-
mammalian target of rapamycin
-
- P + I:
-
PMA plus ionomycin
-
- SP:
-
CD4+ or CD8+ single-positive
Introduction
T cells are vital to the immune response. Hematopoietic stem cells migrate from the bone marrow to the thymus where they proceed through multiple stages of expansion and rounds of selection to produce mature T cells that exit the thymus and populate the peripheral immune system. Along their path to maturity, the tightly regulated activity of numerous transcription factors, including nuclear factor of activated T cells, GATA-binding protein 3, erythroblastosis virus E26 oncogene homolog 1 and activator protein-1 (AP-1), control their fate 1, 2.
AP-1 is composed of dimers of the Fos, Jun, and activating transcription factor families of basic leucine zipper (bZIP) proteins that preferentially bind AP-1 DNA sites 3. The DNA binding and transcriptional activities of AP-1 are strictly controlled during thymocyte development. AP-1 activity is present in double-negative (TCR–CD4–CD8–) (DN) thymocytes, but is virtually absent in double-positive (TCR+CD4+CD8+) (DP) cells 4–7. AP-1 activity is restored in single-positive (TCR+CD4+ or TCR+CD8+) (SP) thymocytes and is induced further following T cell stimulation 4–7.
A complete picture of the role of AP-1 in T cell development has been difficult to obtain. Since AP-1 is a collective term referring to homodimers and heterodimers of several bZIP protein families, functional compensation has limited the usefulness of gene disruption approaches in defining how individual AP-1 complexes contribute to T cell development 8–10. Studies using dominant-negative bZIP proteins that should, in theory, restrict the activities of multiple AP-1 dimers have yielded disparate results. Transgenic expression of FosB2, a truncated, transcriptionally inactive variant of FosB, reduced the number of DP thymocytes and increased levels of CD4+ thymocytes in older mice 11. Using the p56lck proximal promoter to drive thymus-specific expression of TAM-67, a c-Jun truncation mutant, a 50% reduction in thymic cellularity was observed due to decreases in both DP and SP cells 12. Most recently, thymic over-expression of B cell activating transcription factor (BATF), a naturally occurring Jun dimerization partner that lacks a transcription activation domain, generated mice having normal numbers and percentages of the major T cell subsets 13. However, these mice show defects in cytokine gene expression and a dramatic deficiency in Vα14i NKT cells 13.
Consistent with the well-established role of AP-1 in cell proliferation 1, 3, 10, transgenic thymocytes expressing the AP-1 inhibitor BATF proliferate poorly when stimulated in vitro with anti-CD3ϵ and anti-CD28 Ab 13. Here we show that the proliferation of BATF-expressing thymocytes in vitro is impaired when stimulated with the lectin Concanavalin A (Con A), but not when the cells are exposed to a phorbol ester (PMA) and calcium ionophore (ionomycin) (P + I). Interestingly, treatment of BATF-transgenic thymocytes with these standard pharmacological stimulators of T cell signaling caused a PKC-dependent loss of BATF protein which is due, in large part, to the transcriptional down-regulation of the p56lck proximal promoter. In contrast, a BATF transgene driven by the T cell-specific CD2 promoter increases expression following treatment with P + I, and under these circumstances, T cell proliferation remains impaired.
These studies are the first to describe conditions of T cell stimulation that negatively influence transcription of the widely used p56lck proximal promoter expression cassette. The ability to exploit these unique experimental circumstances to eliminate transgenic BATF expression in thymocytes has enabled us to demonstrate conclusively that BATF, a Jun dimerizaton partner and inhibitor of AP-1 activity, exerts a direct, and reversible, effect on T cell proliferation.
Results
Thymocytes expressing the p56lckHA-BATF transgene show impaired proliferation in response to specific mitogenic stimuli
The role of the AP-1 transcription complex in cell proliferation is well established 1, 3, 10. Previously, our laboratory demonstrated that T cell proliferation in response to treatment with anti-CD3ϵ or anti-CD3ϵ plus anti-CD28 Ab was decreased by over 50% in transgenic mice expressing a hemagglutinin (HA)-tagged version of the AP-1 inhibitor BATF from a thymocyte-specific transgene (p56lckHA-BATF) 13. To test whether BATF expression inhibits the proliferative response of T cells to other mitogenic stimuli, thymocytes from transgenic and non-transgenic (WT) mice were cultured in the presence of Con A or P + I. After 48 h, [3H]thymidine was added to the cultures for an additional 18 h. Proliferation was measured by the incorporation of label into DNA. While the treatment of transgenic thymocytes with Con A produced the expected ∼50% decrease in cell proliferation compared to the control, treatment of transgenic and non-transgenic thymocytes with P + I resulted in nearly equivalent levels of cell proliferation (Fig. 1A).
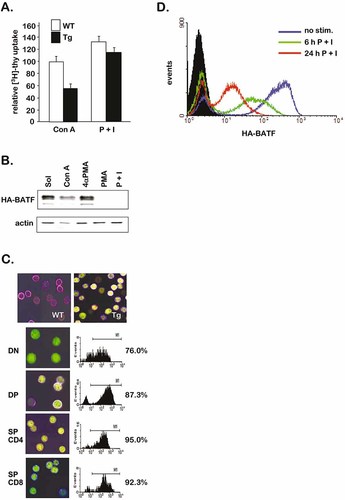
HA-BATF protein is lost following P + I stimulation of p56lckHA-BATF-transgenic (Tg) thymocytes. (A) Thymocytes from Tg and non-Tg (WT) mice were stimulated in vitro with Con A or P + I for 48 h and labeled with [3H]thymidine for 18 h. Incorporation of [3H]thymidine into DNA was quantified by scintillation counting and used as an indicator of cell proliferation. Four mice were used per group. Results were averaged and presented relative to proliferation in the WT Con A-treated group (set to 100). Bars indicate SEM. The experiment was performed a total of four times with similar results. (B) Immunoblot analysis using an anti-HA mAb to detect HA-BATF protein 24 h after stimulation of Tg thymocytes with vehicle (Sol), Con A, 4αPMA, PMA, or P + I. The membrane was stripped and reprobed with an Ab against cellular actin as a control. (C) Tg HA-BATF protein is present in all major T cell subsets in the thymus. WT and Tg thymocytes were stained with anti-CD4-PE (red), anti-CD8-PE-Cy5 (blue), and anti-HA-FITC (green). Images of non-sorted (top panels) and sorted DN, DP and SP CD4 and SP CD8 populations (lower panels) were captured using confocal microscopy. The percentage of HA-positive cells (M1) in each sorted population is indicated on the right. (D) P + I treatment of Tg thymocytes results in a progressive loss of HA-BATF protein. Total thymocytes resting (no stim.) or treated with P + I for 6 h or 24 h were stained with anti-HA-FITC and analyzed by FCM. For each distribution, the mean fluorescent intensity was calculated as 253.2 (no stim.), 62.2 (6 h), and 18.0 (24 h). The experiment was performed twice with similar results.
To establish the levels of BATF protein expressed from the p56lckHA-BATF transgene in thymocytes treated for 24 h with Con A, PMA, or P + I, an anti-HA immunoblot was performed. Groups in which the transgenic thymocytes were treated with vehicle only or with a non-functional PMA analog, 4αPMA, served as controls. As shown in Fig. 1B, while BATF protein expression was maintained in the presence of Con A or 4αPMA, the HA-tagged BATF protein was not detected in the cultures treated with P + I or with PMA alone. Similar results were obtained when this experiment was repeated with thymocytes isolated from two additional lines of independently derived p56lckHA-BATF mice (data not shown). These findings indicate that a mitogenic signaling pathway stimulated by PMA (± ionomycin), but not other mitogenic treatments, triggers the disappearance of the HA-BATF protein. Additionally, these data suggest that as the level of BATF protein is reduced in these cells, the inhibitory effect of BATF on AP-1 activity is removed, and the proliferative response of thymocytes is restored.
To demonstrate that the p56lckHA-BATF transgene is expressed, as expected, in all major T cell subsets in the thymus, thymocytes were isolated and stained with fluorescently conjugated Ab to CD4 and CD8. The surface-stained cells were fixed, permeabilized and stained using a fluorescently conjugated anti-HA mAb to detect the HA epitope tag on BATF. DN, DP and SP cells were isolated by FACS, and the percentage of cells in each population staining positive for HA-BATF was determined. As shown in Fig. 1C, all T cell subsets express the HA-BATF transgene.
To utilize fluorescent detection to monitor the treatment-induced disappearance of the HA-BATF protein over time, thymocytes were rested for 24 h or stimulated with P + I for 6 h and 24 h, stained with the anti-HA mAb as described above, and analyzed by FCM. A comparison of the mean fluorescent intensities of each experimental group shows that by 6 h, intracellular HA staining is reduced to 20% of the untreated control and decreases to 5% of the control by 24 h (Fig. 1D). The residual staining at 24 h is likely due to detection of the HA epitope, which is predicted to be a more stable peptide than the full-length HA-BATF fusion protein detected by immunoblotting (Fig. 1B).
Regulation of p56lck transgene expression in primary thymocytes occurs at the level of transcript accumulation
To distinguish whether the loss of HA-BATF protein following P + I treatment is associated with a reduction in p56lck transgene transcripts, or with a change affecting HA-BATF protein translation or stability, total RNA was isolated from control thymocytes and from thymocytes treated with P + I for 24 h and was subjected to RNA blot analysis. Results show that HA-BATF mRNA decreased significantly after P + I treatment, but not after treatment with Con A (Fig. 2A). Since the BATF protein has a short half-life and is turned over with similar kinetics as other AP-1 proteins (14, 15, C. Deppmann and E. Taparowsky, unpublished data), these results suggest that P + I stimulation functions primarily at the level of BATF mRNA accumulation to produce the reduction in HA-BATF protein observed in our experiments.
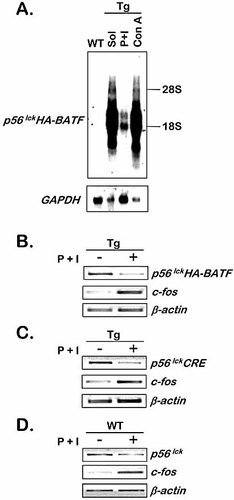
Loss of p56lck transgene transcripts in P + I-treated thymocytes. (A) Thymocytes from non-transgenic (WT) and transgenic (Tg) mice were stimulated for 24 h as indicated, and total RNA analyzed for p56lckHA-BATF mRNA. The migration of 18S and 28S rRNA is indicated to the right of the blot. The membrane was stripped and reprobed for GAPDH mRNA as a control. (B–D) Semi-quantitative RT-PCR showing a decrease in the expression of two unrelated transgenes driven by the same p56lck proximal promoter expression cassette and the endogenous p56lck gene following stimulation of thymocytes with P + I. For each RNA sample, levels of endogenous c-fos and β-actin transcripts were monitored as controls.
It has not been reported previously that the p56lck proximal promoter is subject to regulation in thymocytes treated with P + I. To investigate whether the regulation we have observed for the p56lckHA-BATF transgene is observed for another cDNA expressed from the same p56lck proximal promoter expression cassette, p56lckCre mice were analyzed 16. p56lckCre mice express the bacterial Cre recombinase, which is used routinely for generating conditional gene knockouts in the T cell lineage 16.
Thymocytes from these mice and from p56lckHA-BATF mice were cultured with or without P + I, and after 24 h, total protein and RNA were isolated and analyzed by RT-PCR. As observed with HA-BATF (Fig. 2B), the level of Cre mRNA also is reduced following treatment with P + I (Fig. 2C). However, in contrast to HA-BATF, the decrease in p56lckCre transcription was not reflected by a significant loss of Cre protein by 24 h (data not shown), most likely because the Cre protein is more stable than BATF. c-fos mRNA was monitored in parallel to detect P + I-stimulated gene expression 6 and β-actin mRNA served as a control for sample integrity. Similar results with two distinct transgenes suggested that the endogenous mouse p56lck gene, whose transcription in the thymus is regulated primarily by DNA elements referred to as the proximal promoter 17, would be repressed transcriptionally by P + I. As shown in Fig. 2D, mRNA prepared from C57BL/6 thymocytes treated with P + I for 24 h displayed decreased levels of p56lck mRNA, consistent with the hypothesis that the target of this regulatory phenomenon is the p56lck proximal promoter.
Time-course studies on the P + I-stimulated decrease in HA-BATF protein expression reveal a dependence on signaling through PKC
A time-course experiment was performed to establish when HA-BATF levels begin to decrease following P + I treatment. Total thymocytes were isolated from p56lckHA-BATF-transgenic mice and were stimulated with P + I or vehicle alone. Protein was isolated from the cultures after 2, 4, 6, 8, 24 and 48 h and analyzed by anti-HA immunoblot for transgenic HA-BATF. As described previously 18, the BATF protein resolved by SDS-PAGE migrates as several distinct mobilities including a fast migrating hypophosphorylated form and at least two slower migrating hyperphosphorylated forms. After only 2 h in the presence of P + I, the faster mobility is no longer detected by immunoblot (Fig. 3A). The slower mobilities show a decrease in expression by 4 h of treatment, and by 24 h, no HA-BATF is detected (Fig. 3A). The observation that the initially synthesized, hypophosphorylated form of BATF is the first to disappear, is consistent with the finding that P + I treatment negatively impacts transcription of the p56lckHA-BATF transgene.
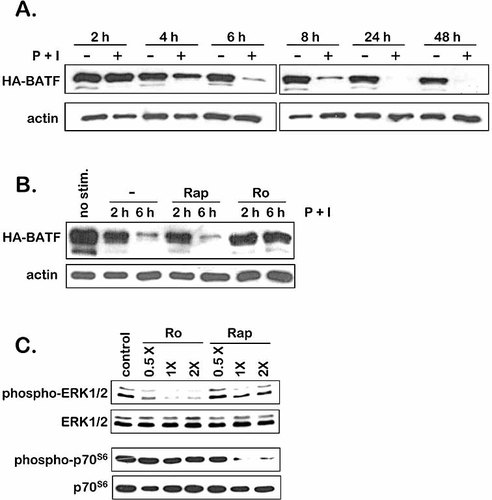
(A) Immunoblots of cell lysates prepared from transgenic thymocytes stimulated with vehicle (–) or P + I (+) for the times indicated. Upper panels have been probed using an anti-HA mAb to detect HA-BATF. The blots were stripped and reprobed for cellular actin as a control. (B) Transgenic thymocytes were left untreated (no stim.) or were treated with vehicle (–), 10 nM rapamycin (Rap) or 0.1 μM Ro320432 (Ro) for 30 min prior to stimulation with P + I for the indicated times. Lysates were prepared and immunoblotted for HA-BATF. The blot was stripped and reprobed for cellular actin as a control. (C) WT thymocytes were treated with vehicle (control), rapamycin (Rap) or Ro320432 (Ro) at the indicated concentrations relative to the standard concentrations used in (B) for 30 min prior to stimulation with P + I for 30 min. Lysates were immunoblotted for phospho-ERK1/2 or phospho-p70S6 kinase, stripped and reprobed for total ERK1/2 and p70S6 kinase as controls.
The treatment of p56lckHA-BATF thymocytes with P + I, or with PMA alone, results in the loss of HA-BATF protein by 24 h (Fig. 1B, 2D). A direct link between the exposure of cells to phorbol ester tumor promoters and the activation of intracellular signaling by PKC is well documented 19, 20. To test whether PKC activity is required for the P + I-induced effect on BATF transgene expression in this system, transgenic thymocytes were treated with P + I in the presence of the PKC inhibitor Ro320432. As a control, cells were treated with P + I in the presence of rapamycin, a downstream inhibitor of the mammalian target of rapamycin (mTOR) pathway which inhibits T cell proliferation in a manner that is independent of PKC 21. As shown in Fig. 3B, the PKC inhibitor totally blocked the loss of HA-BATF while rapamycin had no effect.
To confirm the specificity of these inhibitors, phospho-specific Ab were employed to monitor the activation state of key downstream targets of the PKC and the mTOR pathway in cells treated with different concentration of each inhibitor. As shown in Fig. 3C, Ro320432 blocked the phosphorylation-dependent activation of the extracellular regulated kinases (ERK) 1 and 2, while rapamycin had only a minor influence on ERK1/2 activation. In contrast, rapamycin blocked activation of p70S6 kinase, while the Ro compound had no effect. As expected, both of these inhibitors impaired T cell proliferation (data not shown). We conclude from these studies that the activation of PKC is required for the negative regulation of p56lckHA-BATF transgene expression by P + I.
The P + I-stimulated decrease in BATF alters the cellular profile of AP-1 component expression and activity
Transgenic thymocytes expressing HA-BATF possess a basal level of AP-1 DNA binding activity that increases following stimulation with anti-CD3ϵ and anti-CD28 Ab 13. The AP-1 complexes bound to DNA under these conditions were shown to contain BATF by Ab supershift experiments 13. To examine how P + I treatment impacts the AP-1 DNA binding profile in transgenic thymocytes, nuclear extracts were isolated from transgenic and non-transgenic (WT) thymocytes treated for 6 h with vehicle or with P + I. Protein-DNA complex formation was examined by electrophoretic mobility shift assays (EMSA) using 32P-labeled consensus AP-1 DNA as the probe. As expected, the nuclear extracts from unstimulated cells contain trace AP-1 DNA binding activity, while P + I treatment results in a dramatic induction of protein complexes capable of binding AP-1 DNA (Fig. 4A, left panel). The specificity of AP-1 binding was demonstrated using unlabeled AP-1 DNA as a competitor. The integrity of each protein extract was controlled using 32P-labeled cyclic AMP response element DNA, since cyclic AMP response element binding protein/activating transcription factor complexes show no significant change following treatment with P + I 4 (Fig. 4A, right panel).

HA-BATF-transgenic (Tg) thymocytes show altered AP-1 activity. (A) EMSA using nuclear extracts from unstimulated (no stim.) or P + I-stimulated WT and Tg thymocytes. An AP-1 DNA probe was used for the left panel, and a cyclic AMP response element DNA probe was used for the right panel. The specificity of the detected protein complexes for their target DNA was demonstrated by competition with a 100× molar excess of unlabeled probe DNA. (B) Immunoblot analysis showing the levels of c-Jun, JunB and JunD protein in WT and Tg thymocytes stimulated for 6 h with P + I. The blots were stripped and reprobed for cellular actin and HA-BATF as controls. (C) Immunoblot analysis of c-Jun and JunB levels after 24 h of P + I treatment. The blots were stripped and reprobed for cellular actin and HA-BATF as controls. (D) EMSA using an AP-1 DNA probe and nuclear extracts from WT and Tg thymocytes stimulated for 24 h with P + I.
The AP-1 DNA binding activity in nuclear extracts from p56lckHA-BATF thymocytes treated with P + I for 6 h is not as pronounced as the activity in extracts from WT cells (Fig. 4A, left panel). This suggests that under these stimulation conditions, BATF may influence the efficient induction of other AP-1 family members. Notably, if BATF were to dramatically alter the expression of the Jun family members, overall AP-1 activity would be compromised, since the Jun proteins serve as the key dimerization partners for the Fos proteins and for BATF.
To investigate this possibility, cell extracts from transgenic and non-transgenic (WT) thymocytes, treated for 6 h with P + I, were examined by immunoblot for the expression of c-Jun, JunB and JunD. As shown in Fig. 4B, all three Jun proteins are expressed, although the BATF transgenics show limited induction of c-Jun, enhanced induction of JunB, and no alteration in JunD expression compared to the control. Interestingly, JunB over-expression at the protein and RNA level is a feature of resting BATF-transgenic thymocytes as well (data not shown), indicating that the junB gene is mis-regulated in the presence of BATF. While these data do not provide an obvious explanation for the EMSA result, they confirm that the expression of HA-BATF influences the composition of AP-1 complexes in multiple ways.
Further support for this conclusion was obtained from immunoblots and EMSA performed with thymocyte extracts prepared after P + I treatment for 24 h. Results show that as the HA-BATF protein is lost from the transgenic cells, the changes in Jun family protein expression are reversed (Fig. 4C), and AP-1 DNA binding increases to a level slightly above that observed in WT cells (Fig. 4D).
Cell proliferation in response to P + I requires the loss of HA-BATF
Our results indicate that an increased level of BATF protein expression in thymocytes is associated with a defect in cell proliferation that is reversed following the down-regulation of BATF by P + I. To demonstrate a direct link between BATF protein expression and impaired thymocyte proliferation, we made use of transgenic mice in which the HA-BATF cDNA is expressed using the T cell-specific, human CD2 promoter. We examined the level of HA-BATF protein in CD2-HA-BATF-transgenic thymocytes after 24 h of stimulation with various mitogens. In contrast to the p56lckHA-BATF transgene, the level of HA-BATF expressed from the CD2 transgene increases following P + I stimulation (Fig. 5A).
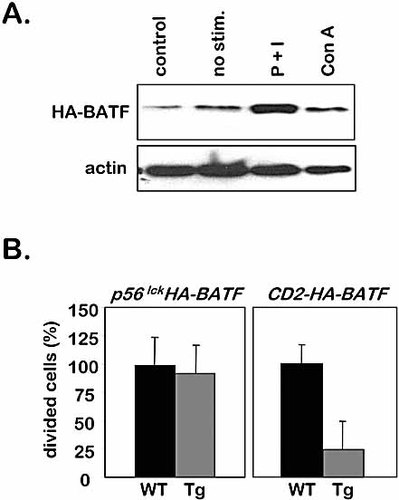
CD2-HA-BATF-transgenic (Tg) thymocytes proliferate poorly when stimulated with P + I. (A) Immunoblot detecting levels of HA-BATF in cell lysates from untreated CD2-HA-BATF thymocytes (control) or CD2-HA-BATF thymocytes treated for 24 h with vehicle only (no stim.), P + I or Con A. The blot was stripped and reprobed for cellular actin as a control. (B) CFSE proliferation assay using WT and Tg thymocytes isolated from p56lckHA-BATF (left panel) and CD2-HA-BATF (right panel) mice. Thymocytes were loaded with CFSE dye, treated with P + I for 72 h, and proliferation assessed using FCM to measure dilution of the CFSE dye. Unstimulated controls were used to determine CFSE staining efficiency (data not shown). The experiment was performed in triplicate and the results averaged. The proliferation of Tg thymocytes is expressed relative to the proliferation of WT cells, which is set at 100%. Bars indicate SEM.
This provided an opportunity to test whether the loss of HA-BATF is necessary for proliferation in response to P + I. Thymocytes isolated from p56lckHA-BATF and CD2-HA-BATF-transgenic and non-transgenic (WT) mice were loaded with CFSE dye. Following stimulation with P + I for 72 h, cell division in the cultures was quantified by FCM as a function of CFSE dye dilution (Fig. 5B). For the P + I-treated p56lckHA-BATF thymocytes, the number of cells undergoing division was similar to the control (set at 100%). However, for the CD2-HA-BATF mice, where treatment with P + I increases the level of HA-BATF, cell division was reduced to 20% of the control. This is compelling evidence to support the role of BATF as a potent AP-1 modulator that restricts the proliferation of thymocytes in response to a variety of mitogenic stimuli.
Discussion
BATF is a member of the AP-1 superfamily of bZIP transcription factors 22, 23. BATF functions as a heterodimer with the Jun proteins to generate complexes that display little to no transcriptional activity when bound to AP-1 target genes 18, 23, 24. BATF is expressed in hematopoietic cells and tissues 23, and we hypothesize that BATF is a critical modulator of AP-1 transcriptional events during the growth and differentiation of various blood cell lineages 23–26. p56lckHA-BATF mice express BATF during all stages of thymic T cell development and were created to test our hypothesis. In previous studies, we demonstrated that BATF-transgenic thymocytes show reduced levels of AP-1 target gene activation in vivo 24, but show normal development of all T cell subsets, with the exception of Vα14i NKT cells, which are dramatically under-represented in these animals 13.
In efforts to exploit BATF to learn more about the role of AP-1 activity in T cell proliferation, transgenic thymocytes were challenged with several mitogenic stimuli. As we anticipated, BATF expression was associated with a reduced capacity to proliferate when cells were stimulated with Con A or with anti-CD3ϵ and anti-CD28 Ab. On the other hand, when the cells were treated with P + I – the standard combination of pharmacological stimulants used to activate T cells – the BATF Tg cells proliferated as well as WT cells.
Here we show that this response is due to a PKC-dependent decrease in the accumulation of transcripts from the p56lckHA-BATF transgene. This phenomenon also was noted using a different transgene (p56lckCre), as well as the endogenous mouse p56lck gene, demonstrating that this regulation targets the thymus-specific p56lck proximal promoter that has been used to generate numerous transgenic mouse lines. The effects of P + I stimulation on p56lck transgene expression is magnified by the inherent instability of the expressed protein and, in the case of BATF, its rapid turnover results in a complete loss of transgenic BATF protein within 24 h. The unique circumstances of this regulation were used to demonstrate the ability of BATF to induce a reversible block in T cell proliferation in vitro. While alterations in cell growth and differentiation are associated with increases in BATF expression in a number of model systems 23–29, the experimental approach identified here is ideally suited for future studies to identify the genetic targets of BATF transcription complexes that mediate these profound effects on cell growth.
Materials and methods
Transgenic mice and cell culture
FVB p56lckHA-BATF mouse lines 2 and 4 were described previously 13 and were backcrossed eight times to C57BL/6 mice. To generate CD2-HA-BATF mice, an EcoRI DNA fragment containing the cDNA encoding HA-tagged human BATF (HA-BATF) was cloned into the VACD2 transgenic expression vector 30 3′ to the human CD2 promoter. A SalI/NotI fragment containing the transgene was used to generate C57BL/6 transgenic mice which have been backcrossed extensively to C57BL/6. Three independent lines have been established. For genotyping, genomic DNA was analyzed by DNA blot and by PCR as described 13, 24. All experiments used 8–12-wk-old, sex-matched transgenic and non-transgenic (WT) littermates from the indicated mouse lines.
For in vitro assays, thymus tissue was dissociated into single-cell thymocyte suspensions, which were maintained at 37°C, 5% CO2 in primary T cell medium as described 24. Con A was used at a concentration of 2.5 μg/mL, PMA and 4αPMA were used at a concentration of 2.5 ng/mL, and ionomycin was used at a concentration of 125 ng/mL. The PKC inhibitor Ro320432 and the mTOR inhibitor rapamycin were purchased from Calbiochem-Novabiochem (San Diego, CA), and were used at a concentration of 0.1 μM and 10 nM, respectively, or at the varied concentrations indicated in Fig. 3C.
Cell proliferation assays
[3H]thymidine incorporation
Single-cell thymocyte suspensions, plated at 2×105 cells/well in 96-well plates, were activated using Con A or P + I. After 48 h, 1 μCi of [3H]thymidine (6.7 Ci/mmol; Amersham Pharmacia Biotech, Arlington Heights, IL) was added to each well. Cells were incubated for 18 h and [3H]thymidine incorporation into DNA was determined by scintillation counting.
CFSE dye dilution
Thymocytes at a density of 2.5×107 cells/mL in RPMI medium containing 0.1% FBS were incubated at 37°C for 10 min with 25 μM CFSE dye as described 31, 32. Cells were diluted in primary T cell medium 24 and incubated with DMSO (vehicle control) or with P + I. After 72 h, the cells were analyzed by FCM, and the number of divided cells calculated from the fluorescent intensities of individual cells in each sample.
Immunoblotting
Cells were lysed in RIPA buffer plus protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and protein concentration determined using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Equal amounts of protein were resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and processed as described 33. The following primary Ab were used at a dilution of 1:1000, or as directed by the manufacturer: mAb against cellular actin (clone C4; Chemicon International, Inc., Temecula, CA), mAb against the HA epitope tag (clone 3F10; Roche, Indianapolis, IN), mAb against Cre (Novagen, San Diego, CA), Ab against c-Jun (H-79), JunB (N-17) and JunD (329) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mAb against p70S6 kinase (H-9) and phospho-p70S6 kinase (A-6) (Santa Cruz Biotechnology), and Ab against ERK1/2 (9102) and phospho-ERK1/2 (9101) (Cell Signaling Technologies, Beverly, MA). Secondary Ab conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA) were used at a dilution of 1:5000. Signals were visualized by chemiluminescence using the Super Signal Reagent (Pierce, Rockford, IL) and autoradiography.
FCM and confocal microscopy
Thymocytes from WT and transgenic mice were blocked with 2.4G2 and stained with anti-CD4-PE (L3T4) and anti-CD8-PE-Cy5 (Ly-2) mAb (BD Pharmingen, San Diego, CA) as previously described 13. After washing with PBS, the cells were fixed and permeabilized using buffers and protocols recommended by eBioscience (San Diego, CA) and stained for HA-BATF by incubating at room temperature for 20 min with anti-HA-FITC mAb (clone 3F10; Roche). Following several washes with permeabilization buffer, the cells were suspended in PBS containing 2% formaldehyde and analyzed using a Coulter Epics Altra Flow Cytometer (Beckman Coulter, Miami, FL). For confocal microscopy, thymocytes stained as described above were sorted into DN, DP and SP populations by FACS, concentrated and spotted onto microscope slides. Images were aquired using an MRC-1024 (Bio-Rad, Hemel Hempstead, U.K.) with a Diaphot 300 inverted microscope (Nikon, Tokyo, Japan) and a 60× 1.4 NA lens.
Electrophoretic mobility shift assays
Nuclear extracts were prepared as described 34 and EMSA performed as described previously 13. Two micrograms of each nuclear extract were used per reaction. Double-stranded oligonucleotides containing consensus AP-1 and cyclic AMP response element binding sites were purchased from Promega (Madison, WI) and radiolabeled using [γ-32P]ATP (6000 Ci/mmol; Amersham Pharmacia Biotech) and T4 polynucleotide kinase (New England Biolabs). Unincorporated radioactivity was removed using a Sephadex G-25 spin column (Sigma). DNA-protein complexes were resolved using a non-denaturing 4% w/v acrylamide gel and visualized by autoradiography.
RNA analysis
Total RNA was isolated from cells using the TRIZOL reagent (Life Technologies). For RNA blots, 10 μg RNA from each experimental group were resolved on 1% denaturing agarose gels, transferred to a nylon membrane (Zeta-probe; Bio-Rad) and hybridized with DNA probes specific for the human BATF mRNA and the mouse GAPDH mRNA as described 23. For RT-PCR, 1 μg of RNA was reverse-transcribed using the Iscript cDNA Synthesis Kit (Bio-Rad) and PCR amplified as described 13 using Taq polymerase (Promega) and primers specific for the BATF transgene (5′ primer ATAAGAATGCGGCCGCATGGCTTCTAGCTATCCT, 3′ primer ATAGTTTAGCGGCCGCTCAGGGCTGGAAGCGC), Cre transgene (5′ primer CGATGCAACGAGTGATGAGG, 3′ primer GCATTGCTGTCACTTGGTCCT), mouse c-fos (5′ primer GTTTCAACGCCGACTACGAG, 3′ primer GATAAAGTTGGCACTAGAGACG), mouse β-actin (5′ primer AGAGGGAAATCGTGCGTGAC, 3′ primer CAATAGTGATGACCTGGCCGT), and mouse p56lck gene 35.
Acknowledgements
The authors thank Deborah Lane for technical assistance, the Purdue Cancer Center Transgenic Mouse Core Facility, and the Purdue Cancer Center Analytical Cytology Shared Resource for help with FCM and confocal microscopy. T.M.T. is a postdoctoral trainee of PHS CA-009634. This work was supported by PHS grant CA-78264 (E.J.T.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




