CD69 down-modulation and inhibition of thymic egress by short- and long-term selective chemical agonism of sphingosine 1-phosphate receptors
Abstract
Thymic development requires proliferation, selection, maturation and release of mature single-positive CD4 and CD8 T cells into the periphery. In mice, non-selective sphingosine-1 phosphate (S1P) receptor agonists, active on four of the five known S1P receptors, alter thymocyte phenotype and egress. Here, we show that down-modulation of CD69 occurs acutely and transiently at a discrete and late stage of medullary development after a single-dose administration of S1P1 receptor-selective agonist, which induces long-term tonic receptor activation in the absence of receptor degradation. In addition, agonist acutely inhibited egress of mature thymocytes into peripheral lymphoid organs, suggesting that both the phenotype and migration of medullary thymocytes are regulated simultaneously and coordinately by agonism of S1P1 alone. Long-term dosing shifted the early/late medullary thymocyte ratio with an expansion of the late medullary compartment, as mature CD69– thymocytes were retained within the thymus. Therefore, chemical agonism of S1P1 accelerates medullary phenotypic maturation and inhibits egress, leading to the expansion and accumulation of the recent thymocyte emigrant population in the medulla. However, chemical agonism fails to replicate the S1P1-null CD69hi late medullary phenotype, suggesting that agonism and gene deletion operate by distinct mechanisms, and that functional receptor antagonism may not be required for lymphocyte sequestration.
Abbreviations:
-
- AAL-(R):
-
2-amino-4-(heptyloxyphenyl)-2-methylbutanol
-
- DN:
-
double negative
-
- DP:
-
double positive
-
- ILN:
-
inguinal LN
-
- i.t.:
-
intrathymic
-
- MLN:
-
mesenteric LN
-
- PI:
-
propidium iodide
-
- RTE:
-
recent thymic emigrants
-
- S1P:
-
sphingosine 1-phosphate
-
- SP:
-
single positive
Introduction
T cell development within the thymus begins with the initial entry of hemopoietic precursors via cortico-medullary blood vessels. Maturation proceeds with the generation of CD4– 8– (double negative, DN) cells, expansion and selection from CD4+ 8+ (double positive, DP) cells, culminating in a small population (relative to total thymocyte numbers) of CD4+ 8– or CD4– 8+ (single positive, SP) medullary thymocytes, which egress from the thymus into the bloodstream before populating peripheral lymphoid organs 1–3.
Many aspects of cortical development, such as commitment of hemopoietic cells to the T cell lineage and the positive and negative selection processes shaping the T cell repertoire, have been heavily studied. In contrast, the later stages of development, spanning up to 14 days in the medulla, remain relatively poorly understood. Negative selection may proceed for SP medullary thymocytes near the cortico-medullary junction 4, and the surviving (positively selected) cells undergo expansion 5–7 and acquire a more mature surface phenotype 8, 9. This phenotypic maturation is characterized by down-regulation of CD69, a putative retention signal 10, 11, and up-regulation of CD62L and β7-integrin, which help facilitate the blood-tissue-lymph recirculation of mature T cells 12, 13. The final stage of intrathymic development is egress of mature T cells into the periphery via cortico-medullary blood vessels.
Migration of lymphocytes from thymus to blood, and from lymph node (LN) to efferent lymph, has been shown to be inhibited by acute obligate agonism of sphingosine 1-phosphate (S1P) receptors 14–16. S1P is a physiological high-affinity receptor agonist generated predominantly by platelets, and constitutively present in plasma at high (∼0.1 μM) concentrations in a protein-bound form 17. The phosphate-ester metabolite of the immunomodulatory drug FTY720, a structural analog of S1P, binds with picomolar or nanomolar affinity, and acts as a full agonist on four of the five known S1P receptors, namely S1P1 (formerly endothelial differentiation gene-1, EDG-1), S1P3 (EDG-3), S1P4 (EDG-6), and S1P5 (EDG-8), but not S1P2 (EDG-5) 14, 15. S1P receptors, a subset of a broad family of lipid receptors, are expressed within a diverse range of cell types and tissues including neuronal, cardiac and lymphoid tissue. S1P receptor agonism elicits a wide range of G protein-coupled signaling pathways affecting many physiological processes, such as vascular maturation, heart rate and cell migration 17, 18.
The role of individual receptors in immune function, however, is poorly understood. Recently, deletion of the S1P1 receptor in lymphocytes results in developmental changes in surface phenotype (elevated CD69 expression in both in both T cells and B cells) as well as retention in lymphoid organs 19, 20. Transgenic expression of S1P1 within lymphocytes also alters cell trafficking in vivo 21–23, and both S1P1 and S1P3 have been reported to play a role in the organization of the splenic marginal sinus 24, 25. S1P4 is largely expressed within lymphoid tissue 26, 27, but as with S1P2 and S1P5, scant evidence exists for receptor function in vivo.
Acute chemical agonism of the four S1P receptors described above leads to an altered thymic phenotype and inhibition of thymic egress 28. However, it is unknown whether distinct S1P receptors mediate the acute phenotypic change and altered migration of medullary thymocytes, or whether a single receptor affects both aspects of thymic function. Here we have used a recently characterized S1P1 receptor-selective agonist (SEW2871) as a chemical tool to probe for putative functions of S1P1 receptors within the thymus of developmentally normal mice. Unlike the non-selective agonist FTY720, stimulation with SEW2871 does not induce receptor down-regulation and degradation, but instead S1P1 receptors are transiently internalized before recycling to the cell surface 29. SEW2871 also provides long-term biochemical responses downstream of receptor including MAPK activation for >4.5 h at saturating ligand concentrations 29. Thus, SEW2871 may permit the analysis of receptor agonism in vivo without functional antagonism. Recently, inhibition of LN egress has been shown to require obligate S1P1 receptor agonism and is reversed by antagonism 16, hence understanding agonist-induced events in vivo would provide insight into S1P1 receptor function.
Using this pharmacological approach, we show that acute and selective agonism of a single S1P receptor, S1P1, affects both the phenotype and migration of medullary thymocytes. Long-term dosing revealed gross phenotypic changes within the medulla, similar to those previously reported for FTY720, suggesting that the immunosuppressive properties of FTY720 and SEW2871 may operate by a similar agonist-driven mechanism in vivo. We also found thymic phenotype, such as CD69 loss, observed after acute and long-term chemical agonism of S1P1 receptors, to be distinct from recent descriptions of S1P1 receptor-deficient mice. Possible implications for differences between pharmacological and genetic approaches are discussed.
Results
S1P1 receptor-selective agonist, SEW2871, down-modulates CD69 at a discrete and late stage of T cell development
We have recently reported changes in thymic biology after administration of the S1P receptor agonist 2-amino-4-(heptyloxyphenyl)-2-methylbutanol, [AAL-(R)] 28. AAL-(R), a chiral analog of FTY720, has little intrinsic receptor binding properties, but after phosphorylation becomes a low nanomolar full agonist for S1P1, S1P3, S1P4, and S1P5, but not S1P2 receptors 15. Phosphorylated AAL-(R), AFD-(R), induces 50% inhibition of thymic emigration and down-modulation of CD69 on CD69-intermediate (CD69int) SP T cells at doses of 10–15 μg/kg, where maximal total plasma concentrations of AFD-(R) are <2 nM, of which only 3.2% is free to interact with receptors 28. While FTY720 and AAL-(R) are potent agonists and useful biological probes, they show little receptor subtype selectivity. Identification of S1P receptor-selective compounds with agonistic or antagonistic properties would permit the elucidation of pathways regulated by distinct S1P receptors. Using high throughput screening, we recently identified a compound (SEW2871) showing selective agonism for a single S1P receptor, S1P1, in vitro and used this compound to investigate the role of S1P1 receptor agonism in peripheral lymphocyte trafficking 30. Maximal lymphopenia is achieved by 5–6 h after a single dose of SEW2871 with blood lymphocytes sequestered within LN, and a reciprocal relationship exists between plasma concentration and lymphocyte numbers in blood 30.
We investigated whether, as within the LN, thymic changes could be evoked with selective agonism of S1P1 receptors. Thus, mice were gavaged with a single dose of the S1P1 receptor-selective agonist, SEW2871, and thymocytes assayed by flow cytometry after 5 h. Similar numbers of thymocytes were found from mice treated with vehicle (99.5 × 106 ± 12.7× 106) or SEW2871 (107.8× 106 ± 15.3× 106; mean ± SD; n=3 at 20 mg/kg) and the distribution of the major thymocyte populations (CD4– 8–, CD4+ 8+, CD4+ SP and CD8+ SP) was also unaffected after treatment (Fig. 1A). However, lymphopenia is achieved in these mice 5 h post-gavage with micromolar levels of compound typically present in plasma 30. Similar frequencies of DN, DP and SP thymocytes are observed up to 24–48 h after treatment (not shown), a time when blood lymphocyte numbers have returned to normal and plasma levels of compound are no longer detected 30. Therefore, unlike long-term dosing with FTY720 or recent studies with S1P1-deficient mice 19, 20, 31, acute agonism of S1P1 receptors does not cause a gross perturbation of T cell development.
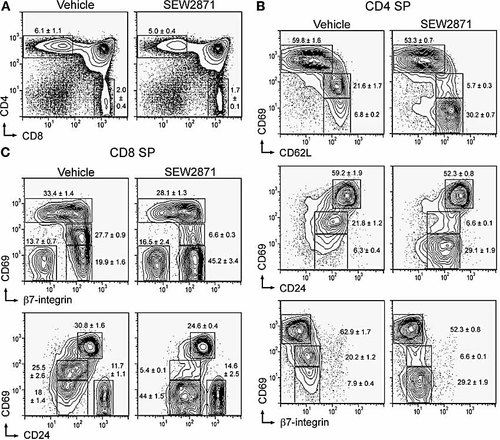
Thymic phenotype after acute and selective agonism of S1P1 receptors. Mice were gavaged with 10 mg/kg SEW2871 or vehicle control, and thymocytes analyzed after 5 h by flow cytometry. (A) PI– B220– cells were analyzed for CD4 and CD8 expression, with frequencies of SP populations indicated. The frequency of the CD4+ 8+ DP populations were 88.3 ± 1.7% and 90.1 ± 0.7%, with the CD4– 8– DN populations being 1.4 ± 0.1% and 1.3 ± 0.1% after treatment with vehicle and SEW2871, respectively. Phenotypic changes of medullary thymocytes are shown for PI– B220– CD4 SP cells (B) and CD8 SP cells (C).
Medullary thymocytes undergo phenotypic changes defining distinct stages of development prior to egress. This includes down-regulating CD69 and CD24, while up-regulating CD62L and B7-integrin as thymocytes mature in the medulla 8, 9. As agonism of S1P1, S1P3, S1P4 and S1P5, but not S1P2, receptors affects medullary stages of T cell development, we defined more clearly the population(s) of medullary thymocytes susceptible to acute S1P1 receptor-selective agonism using these markers (Fig. 1B, C).
Discrete phenotypic changes were readily detected within SP populations. Within the CD4 SP population, the CD69int cells, which are CD62Lhi CD24low and β7 integrinlow, were diminished from ∼20% to ∼5% after treatment with SEW2871. Concomitantly, the CD69– population (CD62Lhi CD24low β7 integrinlow) expanded from ∼5–10% to ∼30% after treatment. A similar effect was observed within the CD8 SP population with CD69int cells (CD24low and β7 integrin+) diminishing from 25–30% to ∼7% within treated mice with a simultaneous expansion of the CD69– population. The CD69hi population, which constitutes the early medullary thymocyte compartment in CD4 (CD62Llow CD24hi B7 integrinneg) and CD8 (CD24hi and acquiring β7 integrin) SP cells, was largely unaltered by SEW2871. However, in general, this population is largely unaltered by SEW2871 agonist treatment, consistent with previous studies with AAL-(R) 28. Therefore, CD69 was down-modulated on the cell surface at a discrete and late stage of medullary thymocyte development (restricted to CD69int SP thymocytes) after treatment with the S1P1 receptor-selective agonist, SEW2871.
We did not detect significant changes in the level of CD24, CD62L, β7-integrin or Qa-2 (not shown) within the 5-h time frame studied here with SEW2871 or with the non-selective agonist, AAL-(R) (unpublished data). The phenotypic change observed after acute treatment with selective or non-selective agonist contrast with the recent findings of S1P1-deficient lymphocytes where CD69, on both T cells and B cells, is significantly elevated 19, 20. Thus, while pharmacological and genetic approaches identify S1P1 as an important receptor in lymphocyte biology, phenotypic inconsistencies may reflect distinct mechanisms between the two in vivo models.
Potency of CD69 down-modulation induced by the S1P1-receptor-selective agonist, SEW2871
The dose response for down-modulation of CD69 was complete by 5 h, with similar dose responses observed for both CD4 and CD8 SP cells (Fig. 2). Doses of SEW2871 ⩾10 mg/kg induced complete CD69int to CD69– conversion for CD4 and CD8 SP medullary thymocytes, with an ED50 of 4.6 ± 0.2 mg/kg (CD4 SP; mean ± SEM) and 1.6 ± 0.1 mg/kg (CD8 SP) for the loss of the CD69int population, and similar dose responses were seen for the simultaneous increase within the CD69– population. The peak plasma concentrations at the ED50 values for the changes in medullary phenotype corresponded to 3 μM and 1.1 μM for decrease in the CD69int populations, and 1.6 μM and 0.9 μM for gain of the CD69– populations for CD4 SP and CD8 SP cells, respectively 30. The dose response for SEW2871-induced changes in cell surface CD69 on medullary thymocytes is similar to that for lymphopenia in potency 30. Therefore, the mechanism(s) of S1P1 receptor-selective agonists on thymic phenotype and lymph node egress are likely related.
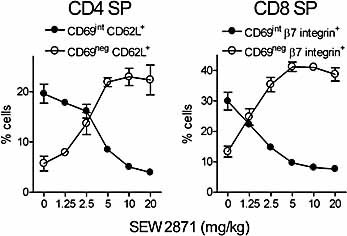
Dose response of medullary phenotypic changes induced by SEW2871. Dose response for changes in CD69int and CD69neg populations after gavaging with SEW2871 (mean ± SD; n=3). Zero represents vehicle treatment alone (n=6). Frequencies of indicated cell populations are shown (gating as in Fig. 1).
Kinetics of CD69 down-modulation after single dosing with SEW2871
Early kinetic analysis showed that the onset of CD69 down-modulation could be detected within 3 h after gavage of a single dose of SEW2871 (10 mg/kg) with the loss of the CD69int population and increase in CD69– populations complete by 5 h (Fig. 3A). CD4 SP and CD8 SP populations were affected similarly. Thus, medullary thymocytes respond rapidly after systemic exposure to a S1P1 receptor-selective agonist.
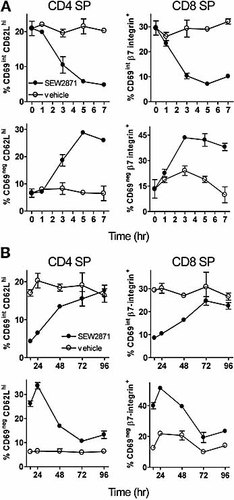
Kinetics of medullary phenotypic changes induced by SEW2871. (A) Onset of CD69 down-modulation after a single administration of 10 mg/kg SEW2871. Data are shown for CD69 and CD62Lhi populations (for CD4+ SP cells), or CD69 and β7-integrin+ populations (for CD8+ SP cells), as indicated in Fig. 1 (mean ± SD; n=3). Time point zero represents untreated mice (n=4). (B) Recovery of the same populations over time. The first data point in (B) represents 16 h.
Like LN egress inhibition, agonist-induced CD69 down-modulation was a reversible process with the altered thymic phenotype maintained through 16–24 h with medullary thymocyte populations returning to near normal distributions by days 3–4 (Fig. 3B). This slow resolution contrasts with the rapid recovery from lymphopenia, in which blood lymphocytes have returned to normal numbers by 16 h at this dose of agonist (not shown). While the maintenance of lymphopenia requires plasma concentrations of compound 30, this is not the case for maintenance of altered thymic phenotype whereby the delay in recovery may reflect the kinetics of the normal early-late maturation process in the medulla. However, we cannot exclude the possibility that recovery of medullary phenotype results from CD69 re-appearing on the thymocyte cell surface thereby reconstituting the CD69int compartment.
Emigration of T cells from thymus is inhibited by the S1P1 receptor-selective agonist, SEW2871
Acute agonism of S1P receptors alters lymphocyte trafficking including thymic egress into blood 14, 15, 28. Modulation of S1P1 expression either though receptor knockout 19, 20 or transgenesis 21–23 also alters cell trafficking, suggesting a role for S1P1 receptors for cell migration. Here, we investigated whether, like CD69 down-modulation, export of T cells from the thymus was regulated by acute agonism of S1P1 receptors. Mice were dosed orally with SEW2871 or vehicle and immediately received an intrathymic (i.t.) injection of FITC in PBS. Splenocytes, mesenteric (MLN) and inguinal (ILN) LN cells were isolated after 24 h and examined separately for the presence of FITC+ recent thymic emigrants (RTE) by flow cytometry (Fig. 4).
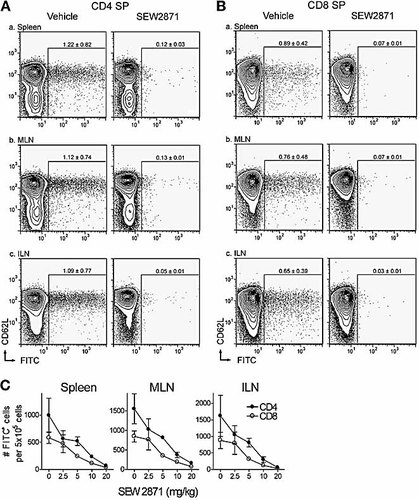
S1P1 receptor-selective agonist, SEW2871, inhibits thymic egress into peripheral lymphoid organs. Mice were gavaged with 20 mg/kg SEW2871 or vehicle control, followed by an i.t. injection of FITC, and thymic egress monitored after 24 h by flow cytometry by gating on CD4 SP cells (A) or CD8 SP cells (B) in peripheral lymphoid organs. RTE (FITC+ cells) are shown within spleen (a), MLN (b) or ILN (c) after treatment with SEW2871 or vehicle. FITC+ gating is based on untreated mice, containing <0.1% (mean; n=4) of FITC+ cells within SP populations (not shown). SP thymocytes were FITC labeled similarly from vehicle-treated (CD4 SP 46.7 ± 16.5%; CD8 SP 45.1 ± 16.7%) and SEW2871-treated (CD4 SP 56.5 ± 6.4%; CD8 SP 54.2 ± 6.9%; mean ± SD; n=4) mice. (C) Dose responses for the inhibition of thymic egress by SEW2871 are shown after normalization to correct for variations in thymic labeling (zero represents treatment with vehicle alone; mean ± SD; n=4).
RTE were readily observed within mice receiving an i.t. injection of FITC followed by immediate gavage with the vehicle alone. RTE comprised an average (n=4) of ∼1.2% and ∼0.9% of the CD4 SP and CD8 SP populations in the spleen, respectively (Fig. 3A, B). Similar frequencies of RTE were found in the MLN (∼1.1% of CD4 SP cells; ∼0.8% of CD8 SP cells) and ILN (∼1.1% of CD4 SP cells; ∼0.7% of CD8 SP cells) of vehicle-treated mice, with the vast majority of RTE being CD69– and CD62Lhi (Fig. 4A, B; and unpublished data), similar to earlier studies 28. However, splenocytes from mice treated with 20 mg/kg SEW2871 showed a dramatic reduction in the frequency of RTE (∼0.1% and 0.07% for CD4 and CD8 SP cells, respectively), suggesting that agonism of S1P1 receptors alone is sufficient to inhibit the egress of mature thymocytes into the periphery.
A similar reduction was observed within MLN cells where the frequency of RTE was 0.13% for CD4 SP cells and 0.07% for CD8 SP cells from SEW2871-treated mice. Likewise, the frequency of RTE within ILN cells of treated mice was also diminished, only comprising 0.05% and 0.03% of the CD4 SP and CD8 SP populations, respectively. Decreased numbers of FITC+ cells in the spleen are, therefore, a likely consequence of the inhibition of thymic egress, and unlikely to result from sequestration of RTE within LN associated with the peripheral lymphopenia.
After correction for variations in intrathymic FITC labeling, a single dose of SEW2871 at 20 mg/mL inhibited thymic egress of CD4 SP cells by 93.1 ± 1.5% to the spleen, 90 ± 0.9% to MLN and 96.0 ± 1.04% to ILN (Fig. 4C). Egress of CD8 SP cells was similarly perturbed with 93.9 ± 1.4% inhibition to spleen, 91.7 ± 1.2% to MLN and 96.9 ± 1.2% to ILN, over the 24-h time frame studied. Titration of SEW2871 revealed a similar dose-dependent inhibition for thymic egress to spleen and LN with an ED50 of 5.1 ± 0.3 mg/kg for CD4 cells and 4.7 ± 0.3 mg/kg (mean ± SEM) for CD8 cells. Plasma concentrations at the ED50 values are estimated as 3.2 μM and 3 μM, for CD4 SP and CD8 SP cells, respectively 30. Thus, selective and acute agonism of S1P1 receptors alone inhibits lymphocyte migration from thymus to peripheral lymphoid organs.
Thymic phenotype after long-term administration of SEW2871
Long-term dosing (5–20 days) with the non-selective S1P receptor agonist, FTY720, revealed gross changes within medullary thymocyte compartment, resulting from an accumulation of normal phenotype RTE in the medulla 31, in contrast to the accumulation of CD69+ cells seen in S1P1-null mice 19. We investigated whether long-term administration of the S1P1 receptor-selective agonist, SEW2871, affected thymic phenotype in a manner analogous to FTY720 treatment or instead whether it replicated the phenotype of S1P1-deficient mice.
Mice were administered daily with 10 mg/kg SEW2871, a dose which completely maintains CD69 down-modulation (Fig. 3C) and inhibits thymic egress by 76–86% over a 24-h period (Fig. 4C). Fig. 5 shows thymocyte phenotypes monitored by flow cytometry after 10 days of continuous dosing with compound. Unlike the subtle effects observed acutely (5 h) after treatment with agonist, striking changes were observed in thymocyte phenotypes after long-term treatment. Thus, the CD4 SP and CD8 SP populations increased from 6.5% (4 × 106 ± 0.3 × 106 cells/thymus; mean ± SD, n=3) to 9.5% (5.4 × 106 ± 0.4 × 106) and from 1.8% (1.1 × 106 ± 0.09 × 106) to 3.1% (1.8 × 106 ± 0.3 × 106) respectively, representing ∼30% and ∼60% expansion of the number of cells within these compartments. This change was reflected in a slight decrease in the DP population from 90.5% (55.6 × 106 ± 3.1 × 106) to 86.1% (49.2 × 106 ± 2.8 × 106), while the DN cells were unaffected in frequency and number (vehicle: 1.2% or 0.7 × 106 ± 0.07 × 106 cells; SEW2871: 1.3% or 0.7 × 106 ± 0.2 × 106 cells; analyzed using quadrant gating, not shown). Therefore, increased numbers of SP thymocytes were present in the thymi of SEW2871-treated mice. A similar effect was also observed by day 5 (data not shown).
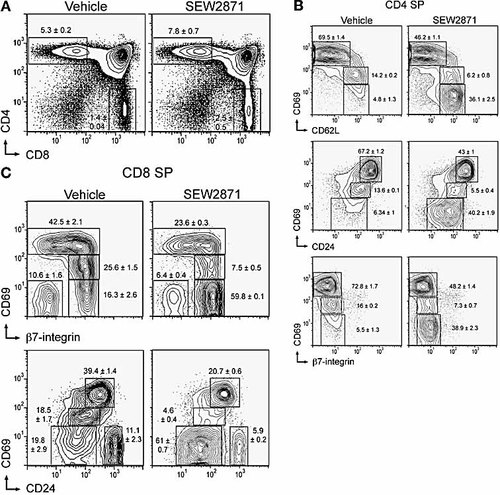
Thymic phenotype after long-term administration of SEW2871. Mice were gavaged daily with 10 mg/kg SEW2871 or vehicle control and thymocytes analyzed after 10 days by flow cytometry. (A) PI– B220– cells were analyzed for CD4 and CD8 expression, with frequencies of gated SP populations indicated (mean ± SD; n=3). Phenotypic changes of medullary thymocytes are shown for PI– B220– CD4 SP cells (B) and CD8 SP cells (C).
Within the medullary SP populations, we observed a dramatic decrease in the relative frequencies of early medullary thymocytes with CD69hi cells constituting just 46% and 22% of the CD4 SP and CD8 SP populations, respectively, representing a relative decrease of 34% and 46% of these compartments compared to vehicle-treated mice (Fig. 5B, C). However, the total cell numbers of the CD69hi populations were largely unaffected after treatment with vehicle (CD4 SP 2.3 × 106 ± 0.2 × 106; CD8 SP 0.35 × 106 ± 0.04 × 106) or SEW2871 (CD4 SP 2 × 106 ± 0.19 × 106; CD8 SP 0.33 × 106 ± 0.05 × 106). Concomitantly, the relative frequency and total cell numbers of the late medullary CD69– compartment expanded from 5% (0.15 × 106 ± 0.03 × 106) to 38% (1.6 × 106 ± 0.04 × 106) and from 18% (0.13 × 106 ± 0.01 × 106) to 60% (0.85 × 106 ± 0.14 × 106) within the CD4 SP and CD8 SP populations, respectively. The CD69int populations were maintained at low frequencies and reduced cell numbers within both CD4 SP (vehicle: 0.46 × 106 ± 0.03 × 106; SEW2871: 0.28 × 106 ± 0.05 × 106) and CD8 SP (vehicle: 0.22 × 106 ± 0.02 × 106; SEW2871: 0.11 × 106 ± 0.02 × 106) thymocytes, as observed during the acute phase of the response (Fig. 1B, C). As with acute treatment, no change in the expression levels of CD62L, β7-integrin or CD24 were detected within normal medullary populations. Thus, a disruption of the normal ratio of early/late medullary thymocyte populations is observed over this long dosing period with an expansion of the late medullary compartment. This reflects the accumulation of mature cells within the thymic medulla resulting from prolonged S1P1 receptor selective agonist-mediated inhibition of thymic egress.
Thymic histology was examined by hematoxylin and eosin staining of formalin-fixed paraffin-embedded tissue (data not shown). The distribution of cortical and medullary thymocytes was similar after long-term treatment with vehicle or SEW2871. Therefore, while SEW2871 induced an expansion of the late medullary thymocyte compartment, this was not reflected by a gross change in thymic histology.
These observations are similar, although less dramatic, to those reported previously for FTY720 31, suggesting the inhibition for thymic egress for FTY720 may be mediated through S1P1 receptor agonism. Some similarities are also shared by S1P1-deficient thymocytes which also accumulate in the medulla expressing a predominantly late medullary phenotype 19. However, S1P1-deficient thymocytes also express high levels of CD69, which is not observed after long-term treatment with SEW2871 (Fig. 5B, C) or FTY720 31 where mature CD69– thymocytes accumulate.
Discussion
This study documents the acute and chronic effects of a well-characterized selective agonist for S1P1 upon thymic function. SEW2871 recapitulates signaling pathways and migratory properties of S1P and, unlike FTY720, which degrades S1P1 receptors, allows receptors to recycle maintaining a functional receptor reserve 29. Rapid and reversible phenotypic changes (CD69 down-modulation) were observed at a discrete and late stage of development restricted to CD69int cells. Simultaneously, thymic egress was inhibited, and thus these two events were coordinately and rapidly regulated by agonism of single S1P receptor subtype, S1P1. Moreover, long-term dosing showed an accumulation of CD69– cells and, collectively, these observations suggest that agonist-induced sequestration occurs through a distinct mechanism from that within S1P1–/– mice, which accumulate CD69+ cells in thymus and spleen 19.
Within the CD4 SP population, CD24hi cells have been shown to be susceptible to anti-TCR-induced apoptosis, suggesting negative selection may continue within the medulla 4. We found S1P1 receptor agonist susceptible CD69int population were CD24low in both CD4 SP and CD8 SP thymocytes, cells that are unresponsive to TCR-dependent apoptosis 4. S1P1 receptor agonist-induced CD69 down-modulation may, therefore, be restricted to a population either terminating or post-negative selection.
This pharmacological study is in partial agreement with recent studies using S1P1-deficient 19, 20 or -transgenic 21, 22 models in identifying S1P1 as an important S1P receptor in lymphocyte biology. However, phenotypic discrepancies exist between acute or chronic chemical agonism and genetic manipulation. Acute agonism led to the rapid phenotypic change within CD69int cells, whereas both chronic treatment with agonists and S1P1 deletion resulted in elevated numbers of SP cells in the medulla, and an alteration of the cortico-medullary balance within thymus. Additionally, using fetal liver reconstitution experiments, S1P1–/– SP cells expressing elevated levels of CD69 accumulated within the medulla, contrasting with the CD69int to CD69– conversion induced acutely by SEW2871 and AAL-(R) 28, and the accumulation of CD69– cells after chronic treatment with SEW2871 and FTY720 31. The effect of elevated CD69 expression in S1P1–/– chimeras is unclear, but may reflect a larger disruption in phenotype, development or function of S1P1–/– lymphocytes not observed in developmentally normal mice.
The link between S1P1 receptors and medullary expression of CD69 is unclear. While CD69 is a commonly used as an early marker of lymphocyte activation, the physiological function remains unknown with the nature of the ligand controversial 32–34. CD69-deficient mice have normal lymphocyte development and trafficking, yet mice with a thymic expressed CD69 transgene show an accumulation of SP thymocytes within the medulla, suggesting a retention function 10, 11, 35. S1P1 receptor agonism increases the frequency of CD69– cells acutely, yet this is not reflected by increased export of mature cells, rather the reverse situation occurs with thymic egress inhibited. Whether the acute down-modulation of CD69 (a phenotypic change) is related to an irreversible acceleration of maturation in the affected population is unclear. The fate and function of the newly formed CD69– population are currently being explored.
Chemical agonism and S1P1 deletion also have distinct affects upon lymphocyte trafficking. S1P1–/– T cells sequester in many lymphoid organs including spleen 19, 24. However, S1P receptor agonism inhibits egress from thymus and LN, but not spleen 14, 36, 37. Furthermore, splenic cell numbers decrease after agonist treatment with a redistribution of lymphocytes away from the spleen 36, 37, suggesting that a tissue-specific component also influences lymphocyte trafficking via S1P receptor agonism, which is not replicated within S1P1–/– mice.
After agonist treatment, lymphocyte-deficient sinuses are readily observed in LN with lymphocytes trapped on the abluminal side of sinus-lining endothelium 14, 30. Similar changes may occur within thymic vessels, although they are difficult to detect histologically (unpublished observations). Migration from lymph node and thymus may share a similar mechanism dependent on S1P1 receptor signaling, as the potency of SEW2871-mediated egress inhibition, as well as the FTY720 analog, AAL-(R), are similar for both lymphoid organs.
The molecular basis for the phenotypic and cell trafficking discrepancies between chemical and genetic approaches remains poorly defined. It has recently been proposed that FTY720 acts as a functional antagonist in vivo by down-regulating plasma membrane S1P1 receptors, thereby inhibiting cell migration to S1P 19, 38. This is considered to be analogous to gene deletion, and thus functional antagonism of S1P1 receptors has been suggested as the basis for lymphocyte sequestration. Moreover, a model has been proposed suggesting a concentration gradient of S1P promotes lymphocyte chemotaxis from lymphoid organs into blood or efferent lymph 39.
However, this is difficult to reconcile with the pharmacological data. Firstly, agonists such as AAL-(R) induce thymic phenotypic changes (CD69 down-modulation) and inhibit thymic egress rapidly (within 1 h) at extremely low (likely sub-stoichiometric) free compound concentrations (calculated to be 60 pM) with a steep concentration-inhibition curve more compatible with a true agonist effect 28. These agonists also activate S1P3 receptors, and are causally associated with an agonist-induced bradycardia via S1P3 activation that is sufficient to significantly impair cardiac function 30, 40. Lymphopenia is produced at significantly lower doses to bradycardia, and this reflects low fractional occupancies of receptor, functioning on the linear portion of the agonist curve 41. Secondly, S1P1-null lymphocytes are phenotypically distinct (CD69+) in vivo and sequester within the spleen 19, neither of which is observed after compound treatment. Thirdly, infusing S1P into the bloodstream inhibits egress from LN 14, contrary to the lymphocytic model which suggests that S1P promotes lymphocyte migration into blood or efferent lymph 39, 42. Fourthly, functional antagonism may be less likely for both SEW2871 and S1P where S1P1 receptors recycle rapidly to the plasma membrane, unlike the prolonged receptor internalization observed after binding with FTY720 29. Finally, recent work directly imaging LN medullary inhibition of sinus entry by S1P1 receptor selective agonists shows that obligate agonism is required, and that medullary lymphocyte arrest is reversed by S1P1 receptor antagonists 16.
If chemical agonists operated by functional receptor antagonism in vivo, pharmacological and genetic approaches would be expected to evoke qualitatively similar biological responses. As chemical treatment is unable to replicate S1P1-deficient lymphocyte phenotype or trafficking, it cannot be extrapolated that chemical agonists work solely by functional antagonism. Resolution of these differences requires selective deletion of S1P1 in peripheral lymphocytes and the use of antagonists in vivo.
The mechanism of action of chemical agonists is further complicated by the abundant expression of S1P1 27, 43. It remains unclear as to whether agonist-induced changes in phenotype and recirculation are intrinsic properties of lymphocytes, or whether the changes are dependent upon other cell types within thymus, lymph node and Peyer's patch. Lymphocytes express S1P1 mRNA and manipulation of receptor expression within lymphocytes affects trafficking, suggesting a direct effect of S1P upon T cells being the primary mechanism for sequestration, while denying a role for non-lymphocytes or stromal cells in this process 19–23. However, S1P1 receptors are also expressed by endothelial cells and S1P regulates barrier integrity through changes in vascular permeability 44, 45. Thus, S1P or chemical agonists may influence lymphocyte egress indirectly via effects upon endothelium or other vascular-associated cells, controlling lymphocyte access to blood or efferent lymph. Whether such processes occur in addition to any direct effects upon T cells is currently under study 42.
Using a recently characterized chemical tool, we showed co-ordinate phenotypic and migratory changes of thymocytes after acute agonism of a single S1P receptor, S1P1. While discrepancies between the pharmacological and genetic approaches are apparent, collectively these studies suggest sensing mechanisms exist within lymphoid organs whereby innate inflammatory mediators, such as S1P, simultaneously regulate T cell maturation and lymphocyte recirculation through S1P1 receptors. Exploitation of the S1P system through receptor-selective agonists or antagonists may be useful in the design of clinically beneficial immunosuppressants.
Materials and methods
Mice and compound
Female C57BL/6J mice, 8–12 weeks old, were bred and maintained within TSRI. Procedures used throughout this study were approved by the Institutional Animal Care and Use Committee.
The structure, biological and pharmacological properties of compound SEW2871 (Maybridge) are published 30. SEW2871 was administered as described 30.
Flow cytometry
Lymphocytes were isolated by gently disrupting thymus, spleen, MLN and ILN in 5% FBS in PBS on ice and passing through a 40-μm nylon mesh. Subsequently, 2 X 106–4 × 106 cells were stained with combinations of the following antibodies in the presence of 2.4G2 (BD Bioscience) and 5% FBS/PBS: CD69-biotin (clone H1.2F3; Southern Biotechnology) revealed with streptavidin-PE, CD24-FITC (M1/69), β7-integrin-FITC (M293), CD8-PE-Cy5 (53–6.7), B220-PE-Cy7 (RA3–6B2), CD62L-allophycocyanin (MEL-14), CD4-allophycocyanin-Cy7 (GK1.5) (all from BD Biosciences). Propidium iodide-negative (PI–) events were acquired with a FACSVantage SE using CellQuest software (Becton Dickinson) and analyzed with Flowjo software (Treestar Inc., San Carlos, CA). Profiles of a single mouse from experimental groups are shown as contour plots with 5% confidence intervals with numbers indicating frequency (%) of indicated cell populations (mean ± SD).
For thymic emigration studies, intrathymic injections were performed under anesthesia as described previously with 10 μl FITC (1 mg/mL in PBS) given per lobe 28. Weakly labeled thymi (<30% FITC+ for both CD4 and CD8 SP thymocytes) were excluded from analyses. When comparing compound- and vehicle-treated groups in Fig. 4C, the percentage of FITC+ thymocytes from individual mice were normalized to the mean thymic FITC labeling within this study (46.36 ± 12.3% for CD4 SP; 44.74 ± 13.45% for CD8 SP; mean ± SD, n=20) and this multiplication factor applied to the percentage and number of FITC+ CD4 or CD8 SP cells within corresponding spleens and LN. Lymphocyte phenotypes and patterns of thymic migration from vehicle-treated mice were indistinguishable from untreated mice.
Plasma concentrations of SEW2871 were estimated by referring to a standard curve where a linear relationship exists between the injected dose of compound and its concentration in plasma 30.
Acknowledgements
We thank members of the MGM-W laboratory for flow cytometry expertise and Dusko Trajkovic for histology. This work is supported by R01 AI055509 to H.R.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




