Expansion of Vα24+Vβ11+ NKT cells from cord blood mononuclear cells using IL-15, IL-7 and Flt3-L depends on monocytes
Abstract
Human Vα24+Vβ11+ NKT cells are a unique T cell population specifically and potently activated by α-galactosylceramide (αGalCer; KRN7000) presented by CD1d. Here, we present a simple and efficient method for expanding Vα24+Vβ11+ NKT cells from human cord blood mononuclear cells (CBMNC) using αGalCer in the presence of interleukin (IL)-15, IL-7 and Flt3-L. The addition of αGalCer from day 0, compared to its addition from day 8 or day 15, induced a greater expansion of NKT cells. The maximal expansion of NKT cells was observed after 15 days (2300-fold). Thereafter, the number of NKT cells decreased slowly, a decrease that was correlated with the diminution of CD1d-positive cells. NKT cell proliferation induced by αGalCer was not observed when CD1d-expressing monocytes were depleted from CBMNC, whereas B cell and dendritic cell depletions had no effect. Expanded NKT cells were CD4+CD8– and secreted both IL-4 and IFN-γ. In this system, CD3+ T cells and CD3–CD56+ NK cells were also expanded. However, the expansion of NKT cells had no significant functional effect on T and NK cells. This expansion method of CBMNC-derived NKT cells is simple and may be helpful for clinical use.
Abbreviations:
-
- αGalCer:
-
α-galactosylceramide
-
- βGalCer:
-
β-galactosylceramide
-
- CBMNC:
-
cord blood mononuclear cell
-
- CBT:
-
cord blood transplantation
-
- GVHD:
-
graft-versus-host disease
-
- GVL:
-
graft-versus-leukemia
Introduction
Natural killer T (NKT) cells correspond to a novel T cell population with an invariant TCR that recognizes glycolipids presented by CD1d, an MHC-like molecule well conserved between humans and mice 1–3. Many studies have described the potential anti-tumor effect 4–7 and the immunoregulatory function of NKT cells 8–11. Vα24+Vβ11+ NKT cells, the human counterpart of mouse Vα14+Vβ8.2+ NKT cells, are limited to a small lymphocyte subpopulation 12 that can be selectively activated by α-galactosylceramide (αGalCer; KRN7000) presented by CD1d 13, 14. Takahashi et al. and others showed that they proliferate in response to dendritic cells (DC) once pulsed by αGalCer 15, 16. Several cytokines were used in attempt to expand and activate NKT cells from human peripheral blood 17–19, and recently from human cord blood 20, 21. Interleukin (IL)-2 is one of the classical cytokines used to expand and stimulate NKT cells 22. Here, we describe a combination of IL-15, IL-7 and Flt3-ligand (Flt3-L) to expand NKT cells from human cord blood mononuclear cells (CBMNC). IL-15 is a newly cloned cytokine that promotes T cell proliferation and NK cell activation 23. In fact, in IL-15–/– and IL-15Rα–/– mice, NK and NKT cell counts are diminished 24, 25. IL-7 is a potent lymphoid growth factor that is important for T cell proliferation and survival as well as for NK cell function 26. Flt3-L induces hematopoietic stem cell proliferation and early lymphopoiesis. In addition, we have previously shown that a high concentration of IL-15 (50 ng/mL), together with 10 ng/mL of Flt3-L, efficiently expands CD3+ T cells derived from human CBMNC 27.
Cord blood is a source of stem cells currently used for transplantation. Several aspects of unrelated cord blood transplantation (CBT) differ from bone marrow or peripheral blood stem cell transplantation. As many as two HLA mismatches are acceptable in CBT for successful engraftment with only a mild graft-versus-host disease (GVHD) 28, because cord blood T cells are immature and naive compared to peripheral blood lymphocytes. The relationship between lower risk of GVHD and higher risk of leukemic relapse is well known, and a higher risk of relapse in CBT was expected. Surprisingly, unrelated CBT and unrelated bone marrow transplantation had a comparable relapse rate in adults as well as in children 29, 30. However, the major causes of death in CBT in adults were relapse of the disease and infections due to delayed hematopoietic recovery 30, 31. As Vα24+Vβ11+ NKT cells produce both Th1 and Th2 cytokines upon activation, they may have an immunomodulatory effect that could abolish GVHD without affecting the graft-versus-leukemia (GVL) effect 32, 33.
Here, we present a simple and efficient method for expanding Vα24+Vβ11+ NKT cells derived from human CBMNC. Moreover, we observed that monocytes are critical for Vα24+Vβ11+ NKT cell expansion. Expansion of T lymphocytes and NK cells was also observed. Expanded Vα24+Vβ11+ NKT cells had a CD4+CD8– phenotype and secreted both IL-4 and IFN-γ. Observations presented here may be helpful for a more targeted use of human cord blood in immunotherapy.
Results
Phenotypic analysis of lymphocytes in human cord blood
After separation by Ficoll density gradient centrifugation, CD3+ T cells varied in fresh CBMNC from 2.5 to 36.2% (mean 15.0 ± 9.6%, n = 10). Other cell compartments consisted of 19.9 ± 10.3% CD3–CD56+ NK cells, 10.2 ± 4.4% CD19+ B cells, 32.4 ± 7.6% CD14+ monocytes and only 0.04 ± 0.02% CD3–CD1a+ DC as shown in Table 1 (day 0). The majority of CD3+ T cells were TCRαβ+ (97.0 ± 2.1%) and 3.0 ± 2.1% were TCRγδ+. Of T cells, 75.8 ± 14.1% were CD4+CD8– (CD4 single-positive cells), 18.2 ± 9.5% CD4–CD8+ (CD8 single-positive cells), 2.5 ± 2.5% CD4+CD8+ (double-positive cells) and 1.1 ± 0.9% CD4–CD8– (double-negative cells) (Table 2, day 0). Only 0.04 ± 0.03% of CD3+ T cells were Vα24+Vβ11+ NKT cells (Table 2).
|
Cord Blood |
T cells (CD3+) |
NK cells |
B cells |
Monocytes |
DC |
|---|---|---|---|---|---|
|
Day 0 |
15.0 ± 9.6 |
19.9 ± 10.3 |
10.2 ± 4.4 |
32.4 ± 7.6 |
0.04 ± 0.02 |
|
Day 8 |
74.6 ± 8.1 |
20.7 ± 8.2 |
2.16 ± 0.01 |
0.16 ± 0.01 |
0.11 ± 0.01 |
|
Day 15 |
86.2 ± 8.6 |
13.1 ± 9.0 |
ND |
ND |
ND |
- a) Cells were cultured in the presence of 50 ng/mL IL-15, 10 ng/mL IL-7, 10 ng/mL Flt3-L and 100 ng/mL αGalCer from day 0. Data shown are the means ± SD of ten samples (% of CBMNC). ND: not determined.
|
|
TCRαβ+ |
TCRγδ+ |
CD4+CD8– |
CD4–CD8+ |
CD4+CD8+ |
CD4–CD8– |
Vα24+Vβ11+ NKT |
|---|---|---|---|---|---|---|---|
|
Day 0 |
97.0 ± 2.1 |
3.0 ± 2.1 |
75.8 ± 14.1 |
18.2 ± 9.5 |
2.5 ± 2.5 |
1.1 ± 0.9 |
0.04 ± 0.03 |
|
Day 8 |
95.2 ± 0.8 |
5.3 ± 1.4 |
66.1 ± 12.1 |
29.6 ± 10.7 |
0.6 ± 0.1 |
3.7 ± 1.8 |
0.8 ± 0.6 |
|
Day 15 |
91.9 ± 1.6 |
8.0 ± 0.9 |
60.8 ± 9.6 |
31.4 ± 11.2 |
4.4 ± 1.1 |
3.4 ± 0.5 |
3.7 ± 0.3 |
- a) Cells were cultured under the same condition as in Table 1. Data shown are the means ± SD of six samples (% of CD3+ T cells).
Cytokine combination for cell expansion
Because NKT cells, and especially Vα24+Vβ11+ NKT cells, represent a very small population of T cells, we chose to expand CBMNC rather than sorting Vα24+Vβ11+ NKT cells. Previously, Nagamura-Inoue et al. found that a high concentration of IL-15 (50 ng/mL), together with 10 ng/mL Flt3-L, efficiently expanded CD3+ T cells derived from CBMNC 27. In addition, IL-7 is one of the growth factors needed to expand and activate T cells 26. Therefore, we compared different combinations of these three cytokines (50 ng/mL IL-15, 10 ng/mL IL-7 and/or 10 ng/mL Flt3-L). Results are shown in Fig. 1. In the absence of cytokines or in the presence of Flt3-L alone, CD3+ T cells and NK cells diminished by day 15, and the remaining cells were CD3–CD56–. IL-7 at 10 ng/mL only resulted in 2.3-fold expansion of T cells. A significant synergistic induction of CD3+ T cells was observed with a combination of 50 ng/mL IL-15, 10 ng/mL IL-7 and 10 ng/mL Flt3-L (14.1-fold induction). However, even by combining these three cytokines, no increase of Vα24+Vβ11+ NKT cells was observed unless αGalCer was added.
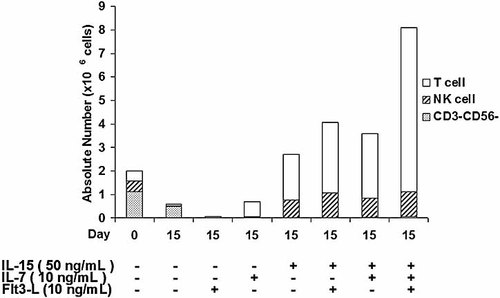
Comparison of cell compartments in fresh CBMNC (day 0) and after 15 days of culture with different combinations of 50 ng/mL IL-15, 10 ng/mL IL-7 and 10 ng/mL Flt3-L.
CD1d-expressing cells in human cord blood
Since Vα24+Vβ11+ NKT cell activation is CD1d dependent, we examined whether antigen-presenting cells like monocytes (CD14+), B cells (CD19+) and DC (CD3–CD1a+) expressed CD1d in fresh CBMNC. As shown in Fig. 2A, the majority of CD1d-positive cells were observed among CD14+ monocytes, whereas CD19+ B cells did not strongly express CD1d and only 0.01% of CD3–CD1a+ DC were CD1d+ among human CBMNC. By day 8 in the presence of IL-15, IL-7 and Flt3-L, CD1d+ cells decreased as shown in Table 1 (day 8). In addition, the remaining B cells, monocytes and the few DC no longer expressed CD1d (Fig. 2A, right panels).
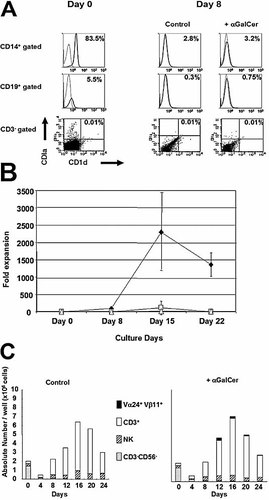
(A) CD1d expression on CD14+ monocytes, CD19+ B cells and CD3–CD1a+ DC in freshly isolated CBMNC (left panel, day 0) and after 8 days of culture with 50 ng/mL IL-15, 10 ng/mL IL-7 and 10 ng/mL Flt3-L in the presence or absence of αGalCer from day 0 (right panels, day 8). Isotype control is represented with a dashed line and CD1d-positive cells with a solid line for CD14+- and CD19+-gated cells. CD3– cells are gated in the dot blots and CD3–CD1a+CD1d+ cells represented only 0.01% of the CD3–CD1a+ cells. (B) Fold expansion of Vα24+Vβ11+ NKT cells. αGalCer (100 ng/mL) was added to the CBMNC from day 0 (♦), day 8 (□) or day 15 (▵) in the presence of 50 ng/mL IL-15, 10 ng/mL IL-7 and 10 ng/mL Flt3-L. The fold expansion represents the ratio of the absolute number of NKT cells per well after culture to the number at day 0. (C) Cell compartments of CBMNC in the presence or absence of αGalCer. CBMNC were cultured with 50 ng/mL IL-15, 10 ng/mL IL-7 and 10 ng/mL Flt3-L. αGalCer was added to the medium at a concentration of 100 ng/mL from day 0 (right panel). βGalCer was used in a control experiment (left panel).
Expansion of NKT cells from human cord blood
IL-15 is a cytokine that has been reported to induce monocyte differentiation into mature DC 34. In order to explore whether DC maturation could enhance Vα24+Vβ11+ NKT cell expansion, αGalCer was directly added to CBMNC cultured with IL-15, IL-7 and Flt3-L from day 0, day 8 or day 15. The addition of αGalCer from day 0 induced a significantly greater Vα24+Vβ11+ NKT cell expansion than the addition of αGalCer from day 8 or day 15 (Fig. 2B). After 15 days of culture, the absolute number of Vα24+Vβ11+ NKT cells pulsed with αGalCer was 2300 ± 1200-fold higher than that observed in fresh CBMNC (Fig. 2B). CD3+ T cells and NK cells were also expanded 27 ± 15-fold and 2 ± 1-fold, respectively, compared to the pre-cultured population (Fig. 2C). CD3+ T cells and NK cells represent 86.2 ± 8.6% and 13.1 ± 9.0%, respectively, of total cultured CBMNC on day 15 (Table 1, day 15). In control experiments, β-galactosylceramide (βGalCer) did not induce Vα24+Vβ11+ NKT cell proliferation, while the T cells or NK cells presented almost the same expansion rate. As described above, the CD3–CD56– fraction nearly disappeared on day 8 (Fig. 2C). Maximal expansion of Vα24+Vβ11+ NKT cells was observed after 15 days of culture. Thereafter, the absolute number of Vα24+Vβ11+ NKT cells slowly decreased, a phenomenon that followed the reduction of CD1d+ cells (Table 1, Fig. 2A).
Depletion of antigen-presenting cells
Because Vα24+Vβ11+ NKT cells could only be expanded in the presence of αGalCer, we next tried to detect which CD1d+ cells in the CBMNC were critical for the expansion of Vα24+Vβ11+ NKT cells. CBMNC were depleted of either CD14+ monocytes, CD19+ B cells, CD1a+ DC, or of all of these three antigen-presenting cells. Then, depleted cells were individually cultured in the presence of IL-15, IL-7 and Flt3-L as described above and pulsed with 100 ng/mL αGalCer from day 0. The absolute number of Vα24+Vβ11+ NKT cells was calculated as described in Materials and methods. When CD14+ monocytes were depleted, the Vα24+Vβ11+ NKT cells did not proliferate, as shown in Fig. 3. In contrast, depletion of CD19+ B cells or CD1a+ DC had no significant influence on the proliferation of Vα24+Vβ11+ NKT cells.
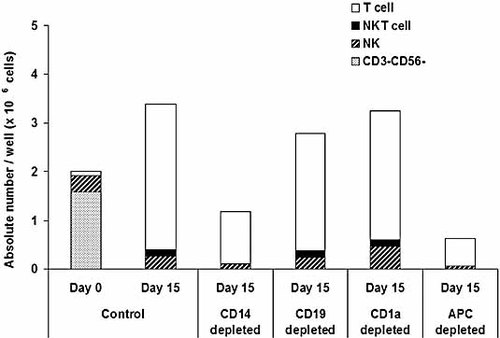
Depletion of CD1d-expressing cells. Either CD14+ monocytes, CD19+ B cells, CD1a+ DC or all of these three antigen-presenting cells (APC) were depleted from CBMNC using AutoMACS beads. After depletion, the remaining cells were cultured with 50 ng/mL IL-15, 10 ng/mL IL-7, 10 ng/mL Flt3-L and 100 ng/mL αGalCer for 15 days. One representative result from three independent experiments is shown.
Phenotypic analysis of expanded Vα24+Vβ11+ NKT cells and CD3+ T cells
CBMNC were cultured with IL-15, IL-7, Flt3-L and 100 ng/mL αGalCer. After 15 days, CD3+ T cells represented 86.2 ± 8.6% of the mononuclear cells and the remaining population was constituted by NK cells (13.1 ± 9.0%) (Table 1). Expanded Vα24+Vβ11+ NKT cells represented 3.7 ± 0.3% of the CD3+ T cells (Fig. 4A, lower part). Most of the Vα24+ NKT cells were positive for CD4 (95.6 ± 5.1%) and very few expressed CD8 (1.1 ± 1.1%) (n = 5). In addition, 1.8% Vα24+ NKT cells were double negative (CD4–CD8–). Of the other CD3+ T cells in cultured cells, 60.8 ± 9.6% were CD4+, 31.4 ± 11.2% CD8+, 4.4 ± 1.1% CD4+CD8+, 3.4 ± 0.54% CD4–CD8– (Table 2, day 15) and 23.8 ± 7.9% of CD3+ T cells were CD56+ (n = 6). In the absence of αGalCer, Vα24+Vβ11+ NKT cells were not detected, but CD3+ T cells showed the same phenotype as in the presence of αGalCer (data not shown).
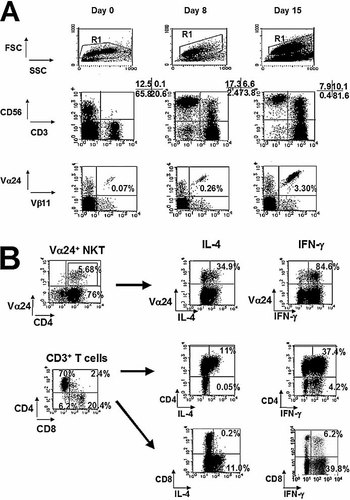
(A) Percentages of T and NK cells (R1 gated; middle part) and Vα24+Vβ11+ NKT cells (CD3+ T cell gated; lower part) at day 0 and after 8 or 15 days of culture with IL-15, IL-7, Flt3-L and αGalCer from day 0. (B) IL-4 and IFN-γ secretion by cultured Vα24+Vβ11+ NKT cells (CD4+ gated) and other T cell compartments. On day 15, the expanded CD4+ single-positive Vα24+Vβ11+ NKT cells produced IL-4 and also IFN-γ (upper part). The remaining post-cultured CD4+ T cells also produced IL-4 and IFN-γ, whereas CD8+ T cells secreted IFN-γ alone (lower part). Control experiment was made without stimulation with PMA and ionomycin.
Cytokine secretion profile of expanded Vα24+Vβ11+ NKT cells
Cytokine secretion of expanded Vα24+Vβ11+ NKT cells was investigated by examining the intracellular expression of IFN-γ as Th1 cytokine and of IL-4 as Th2 cytokine. On day 15, the expanded CD4+ single-positive Vα24+Vβ11+ NKT cells produced both IL-4 and IFN-γ (Fig. 4B). CD4+ T cells produced both IL-4 and IFN-γ, while CD8+ T cells secreted IFN-γ only. On the other hand, without expansion of CD4+ Vα24+Vβ11+ NKT cells by αGalCer, the CD4/CD8 ratio and the cytokine secretion of T cells did not change significantly (data not shown).
Cytotoxic activity against K562 cells
We evaluated the effect of Vα24+Vβ11+ NKT cells on the cytotoxic activity of the cultured CBMNC against K562 cells, an erythroleukemic cell line, as described in Materials and methods. Fresh CBMNC showed a minimal cytotoxic activity against K562 cells, while cells cultured for 15 days with αGalCer gained in cytotoxic activity. The CBMNC cultured with αGalCer had an enhanced cytotoxicity against K562 cells compared to the control, but had no significant additive effect on the cytotoxic activity of NK and T cells (p = not significant) (Fig. 5).
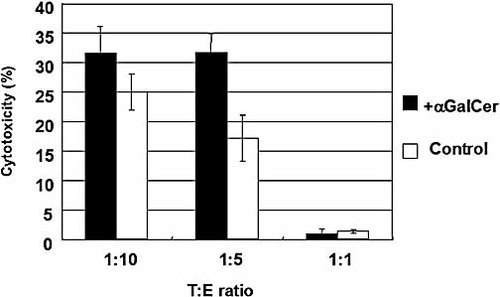
Comparison of the cytotoxic activity of the cultured CBMNC against K562 cells. On day 15, cells cultured in the presence (filled bar) or absence (open bar) of αGalCer were examined for their cytotoxic activity against K562 cells. The effector cells (E) and target cells (T) were mixed to obtain T : E ratios of 1 : 10, 1 : 5 and 1 : 1. One representative of three independent experiments is shown.
Discussion
In the present study, we succeeded in expanding Vα24+Vβ11+ NKT cells from human CBMNC without losing progenitor cells by cell sorting. The expanded Vα24+Vβ11+ NKT cells presented a CD4+ single-positive phenotype and secreted both IL-4 and IFN-γ.
Similarly to mouse Vα14+Vβ8.2+ NKT cells, the activation and proliferation of human Vα24+Vβ11+ NKT cells are CD1d dependent. Previous reports used DC as the strongest antigen-presenting cells for the expansion of Vα24+Vβ11+ NKT cells in adult peripheral blood 18, 19 and in cord blood 20. However, to avoid the loss of any cell compartments through additional manipulations, we describe here a method to easily expand Vα24+Vβ11+ NKT cells in the presence of IL-15, IL-7 and Flt3-L without DC induction.
Ueda et al. succeeded in expanding Vα24+Vβ11+ NKT cells by adding αGalCer directly to fresh CBMNC in the presence of IL-2 22. IL-15 has been recently reported as one of the cytokines involved in monocyte differentiation into mature DC, the strongest antigen-presenting cells 34, 35. In our system, we observed a 2300-fold expansion of Vα24+Vβ11+ NKT cells after 15 days, although DC did neither proliferate nor maturate.
According to the results of our depletion analysis, monocytes constituted the CD1d+ cell compartment critically involved in NKT cell expansion, whereas B cells and DC were not required. CD4+ Vα24+Vβ11+ NKT cells did not proliferate for more than 15 days in the presence of IL-15, IL-7, Flt3-L and αGalCer. This phenomenon seemed to be correlated with the decrease of the CD1d-presenting cells.
The complexity of the NKT cell phenotype in mice was emphasized by several classifications 36. Using CD1d tetramers loaded with αGalCer, four subsets of NKT cells were identified, differing in NK1.1 expression, TCR repertoire and CD1d-dependence 37, 38. These different subsets might explain the paradoxical properties of NKT cells, as CD1d-restricted NKT cells inhibited tumor growth in some cases, but could also suppress anti-tumor immunity in other experiments 12, 39. Although mouse CD1d-αGalCer tetramers stained human NKT cells, further studies are required to characterize human NKT cell specificities. In our system, expanded Vα24+Vβ11+ NKT cells were CD4 single positive and produced both IL-4 and IFN-γ. IL-7 up-regulates IL-4 production by NKT cells in mice 40, but we did not observe any Th2 polarization despite the presence of IL-7 in the culture medium. Lin et al. have tested different cytokines to expand NKT cells from peripheral blood mononuclear cells 16. IL-2 and IL-15 resulted in the best expansion rate, and IL-15 enhanced the cytotoxic activity of NKT cells against U937 cells after 7 days of culture. Both IL-7 and IL-15 preferentially expanded CD4–CD8– NKT cells, and the authors proposed to combine those two cytokines for an optimal anti-tumor application of NKT cells. However, in our study, CD4+ NKT cells were predominant when CBMNC were expanded with IL-15, IL-7 and Flt3-L, and they produced both Th1 and Th2 cytokines. Cord blood-derived NKT cells may have specific properties compared to peripheral blood-derived NKT cells.
Whether human Vα24+Vβ11+ NKT cells exhibit direct cytotoxic activity is still controversial 41. Because Vα24+Vβ11+ NKT cells produce huge amounts of cytokines rapidly after αGalCer stimulation, they may act preferentially by stimulating other effector cells such as NK or CD8 T cells. In our system, the cytotoxicity of CBMNC against K562 cells was enhanced after 15 days of culture. However, the Vα24+Vβ11+ NKT cell expansion did not enhance the cytotoxicity of NK cells. As CD4+ NKT cells produce both Th1 and Th2 cytokines, they may not influence the anti-tumoral response of NK cells. The culture of CBMNC with or without αGalCer did not change T and NK cell compartments either. The CD4/CD8 ratio remained stable, as was the cytokine profile of CD4+ and CD8+ T cells (data not shown).
The role of NK cells in acute GVHD and the GVL effect may be as important as that of T cells 42. In our system, the fact that CD8+ T and NK cells increased significantly after 15 days of culture might result in enhancing not only the GVL effect but also GVHD. The effect of activated NK cells and Vα24+Vβ11+ NKT cells on these CD8+ T cells remains to be further investigated.
In conclusion, our method for expanding Vα24+Vβ11+ NKT cells derived from CBMNC is easy to perform and may be helpful for future clinical use.
Materials and methods
Human umbilical cord blood
Human umbilical cord blood samples were obtained during normal full-term deliveries after obtaining informed consent. We used samples that were not processed by the Tokyo Cord Blood Bank for therapeutic use because of the small volume (less than 40 mL) or the time elapsed. Cord blood samples were stored at room temperature and processed within 36 h of collection.
αGalCer
αGalCer (KRN7000) was dissolved in DMSO and used at a concentration of 100 ng/mL for cell culture. Control experiments were done with βGalCer, an analogue of αGalCer. αGalCer and βGalCer were kindly provided by Kirin Brewery Co, Ltd. (Gunma, Japan).
Cytokines
Recombinant human IL-15, IL-7 and Flt3-L were purchased from Peprotech (London, UK) and used for cell culture at the indicated concentrations.
Cell culture
CBMNC were isolated by Ficoll-Paque (Sigma, 87. Louis, USA) density gradient centrifugation and washed twice with PBS. CBMNC (1 × 106 cells/mL) were suspended in complete RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 0.01 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin and 250 ng/mL amphotericin B, and cultured in 6-well plates (2 × 106 cells/well). IL-15 and/or IL-7 and/or Flt3-L were added at the indicated concentrations, and half of the medium was changed every 4–5 days with or without addition of αGalCer. The number of viable mononuclear cells was counted after Trypan blue staining. The absolute number of cells per well was calculated by multiplying the percentage of each subpopulation of CBMNC, measured by FACS analysis, by the total number of viable mononuclear cells.
Flow cytometric analysis
Cell surface markers were analyzed with a FACS Calibur using Cell Quest software (Becton Dickinson, Mountain View, CA). Fresh or cultured cells were suspended in PBS, incubated with mouse serum to block nonspecific binding, and stained for 20 min on ice with specific FITC-, PE- or allophycocyanin-conjugated mouse monoclonal antibodies (mAb). Anti-CD3, -CD19, -CD1a, -CD4, -CD8α and -TCRαβ mAb were purchased from e-Bioscience (CA, USA). Anti-Vα24 (C15) and anti-Vβ11 (C21) mAb were purchased from Coulter-Immunotech (Marseille, France). Anti-CD56, -CD14, -TCRγδ mAb and isotype controls were purchased from BD Pharmingen (CA, USA). PE- and FITC-conjugated anti-IL-4 and anti-IFN-γ antibodies were also from BD Pharmingen and were used for intracellular staining. Dead cells were stained by propidium iodide and gated out. The CBMNC were gated by forward scatter and side scatter, and then CD3+ cells were analyzed. CD3+CD4+CD8+ cells were considered as double-positive cells, but doublet formation had not been formally excluded.
AutoMACS depletion assay
Fresh CBMNC were stained with PE-labeled anti-CD14, anti-CD19, and/or anti-CD1a antibodies and were depleted using anti-PE beads in an AutoMACS magnetic sorting system (Miltenyi Biotec GmbH, Germany). Monocytes, B cells, DC or all of these three antigen-presenting cells were depleted, and the remaining cells were cultured with 50 ng/mL IL-15, 10 ng/mL IL-7, 10 ng/mL Flt3-L and 100 ng/mL αGalCer for 15 days as described above.
Intracellular staining
To detect intracellular expression of IL-4 and IFN-γ, cultured CBMNC were stimulated with 1 ng/mL PMA (Sigma, St. Louis, MO) and 2 µM ionomycin (Sigma) in the presence of 2 µM monensin (Sigma) for 4 h. Cells were then washed twice with PBS containing 0.5% BSA and 0.1% sodium azide (PBS/BSA/Azide) and stained for surface markers using fluorescence-conjugated mAb and ethidium monoazide bromide. Cells were fixed in 4% paraformaldehyde (Wako, Japan) for 20 min at room temperature, washed and permeabilized with PBS/BSA/Azide with 0.5% saponin for 10 min. Cells were then incubated with the anti-IL-4 or IFN-γ mAb for 30 min, washed twice and analyzed in a FACS Calibur.
Cytotoxic assay
Analysis of cytotoxic activity against K562 cells was performed with pre-cultured and post-cultured CBMNC using a non-radioactive cytotoxic assay, CytoTox 96 (LDH release methods; Promega, USA). The effector cells (E) and target cells (T) were mixed to obtain T : E ratios of 1 : 10, 1 : 5 and 1 : 1, and incubated for 4 h in 100 µL phenol red-free RPMI 1640 supplemented with 5% FBS in 96-well plates. Target cells were plated at 2 × 104 cells/well. Analysis and calculation were described elsewhere 27.
Statistics
Results are reported as means ± SD from more than three experiments. Significance levels were determined by analysis of variance (ANOVA) followed by the Dunett post-test for differences in means, using JMP software (SAS Institute Inc.).
Acknowledgements
We thank Dr. N. Watanabe for technical support and Pr. O. Spertini for helpful comments and suggestions on the manuscript. Dr. H. Okada's work in Japan has been supported by CHUGAI Pharma France. This work was supported by a Research Grant on Human Genome, Tissue Engineering (H17-O14) from the Japanese Ministry of Health, Labor and Welfare.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




