A gene signature of inhibitory MHC receptors identifies a BDCA3+ subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro
Abstract
Dendritic cells (DC) are crucial gatekeepers in regulating immunity. Whereas mature immunostimulatory myeloid DC (DCims) potently promote immune responses, IL-10-induced myeloid DC (DC-IL-10) counteract T cell activation. To elucidate the molecular repertoire by which DC-IL-10 secure reduced T cell activation, comparative gene expression profiling was done using Affymetrix U133A microarrays. Among the genes overexpressed in DC-IL-10, eight immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing inhibitory molecules (ILT2, ILT3, ILT4, ILT5, DCIR, PILRA, FcγRIIB, SLAM) were found. Phenotypic analysis of DC-IL-10 defined an ILThigh DC subset further characterized by expression of CD14, TLR2, DC-SIGN, and CD123 and the lack of lymphocyte, monocyte/macrophage, and plasmacytoid DC markers such as CD3, CD8, and CD68. A unique feature of the ILThigh DC subset was expression of the novel DC marker BDCA3, which was not detected on monocytes, immature DC, DCims, or ILTlow DC-IL-10. While the allogeneic T cell proliferation induced by the entire DC-IL-10 population was approximately 30% of that stimulated by DCims, allogeneic MLR responses driven by the ILThigh DC subset were reduced to 8% of the allostimulatory capacity of DCims, although secretion of the inhibitory cytokine IL-10 and other Th1/Th2-associated cytokines was similar in these cells.
Abbreviations:
-
- DCims:
-
Immunostimulatory myeloid DC
-
- DC-IL-10:
-
IL-10-induced myeloid DC
-
- ILT:
-
Immunoglobulin-like transcript
-
- ITAM:
-
Immunoreceptor tyrosine-based activation motif
-
- ITIM:
-
Immunoreceptor tyrosine-based inhibitory motif
1 Introduction
Dendritic cells (DC) are gatekeepers of the immune system that may initiate or modulate immunity depending on their derivation and differentiation 1, 2. DC may be derived from CD34+ hematopoietic stem cells or from peripheral blood monocytes and are widely distributed in lymphoid as well as non-lymphoid organs as immature DC (e.g. splenic marginal zone DC, epidermal Langerhans cells, and interstitial tissue DC). At an immature stage, DC act as sentinels in the peripheral tissues, continuously capturing and processing antigens by phagocytosis, pinocytosis, or receptor-mediated endocytosis. Given the appropriate signals via pathogens (e.g. LPS, unmethylated CpG-containing oligonucleotides, double-stranded viral RNA) or T cells (e.g. CD154, IFN-γ), immature DC mature into immunostimulatory myeloid DC (DCims) characterized by expression of the DC maturation marker CD83 and up-regulation of MHC class II and costimulatory molecules (CD80, CD86, CD40). Mature DCims favor induction of Th1 cells through the release of proinflammatory cytokines such as IL-12 3, 4.
There is increasing evidence that in addition to DCims, other DC populations exert anti-inflammatory functions. Plasmacytoid DC2 cells are defined by their lymphoid origin and lack of expression of myeloid antigens (CD11c, CD13, CD33, mannose receptor), while they express high levels of CD123 as well as lymphoid markers (CD2, CD5, CD7) 5, 6. A second population comprises myeloid DC defined by their stage of maturation, with either an immature 7 or a semi-mature phenotype 8. A third population of anti-inflammatory DC consists of DC of myeloid origin generated in vitro by natural immunomodulatory mediators. With respect to this third population, myeloid DC induced by IL-10 (DC-IL-10) in addition to the usual maturation-inducing combination of proinflammatory cytokines have been most thoroughly examined 9–11. As compared to DCims, DC-IL-10 show moderate MHC class II levels and reduced expression of costimulatory molecules and CD83 10, indicating that DC-IL-10 might induce T cell hyporesponsiveness due to a lack of T cell receptor activation and costimulation.
Similar to this concept of defective immunization by DC, it was previously proposed that Th2-associated, anti-inflammatory macrophages might just be classical effector macrophages silenced by the inhibitory effects of IL-4 or glucocorticoids. In contrast, we and others have shown that alternative activation of macrophages is an active process accompanied by enhanced antigen uptake, processing, and presentation and by de novo expression of a Th2-associated molecular repertoire 12–15. We therefore reasoned that induction of DC-IL-10 might be accompanied by de novo expression of a specific molecular repertoire, some genes of which might be directly involved in the functions of these specialized DC.
In order to identify genes differentially expressed by DC-IL-10, microarray analyses on Affymetrix U133 gene chips was used to compare DCims and DC-IL-10 generated from immature myeloid DC by stimulation with a defined maturation cocktail of TNF-α, IL-1β, IL-6, and prostaglandin E2 16, with or without addition of IL-10, respectively. We identified a subset of DC-IL-10 that expresses high levels of immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing inhibitory MHC receptors of the immunoglobulin-like transcript (ILT) gene family together with BDCA3 and CD14 and exhibits considerably reduced allostimulatory capacity.
2 Results
2.1 Differences in gene expression profiles of DC-IL-10 and DCims
In order to define the molecular changes induced in DC by IL-10, we hybridized DC-IL-10 mRNA from four unrelated individuals obtained after 9 days of culture onto Affymetrix U133A gene chips and compared the mRNA expression profile to that of DCims. A conservative analysis was performed, taking into account only genes that showed at least a two-fold up-regulation in either DC-IL-10 or DCims in all four donors. From the 22,283 genes represented on the Affymetrix U133A gene chip, 213 genes were up-regulated and 81 genes were down-regulated in DC-IL-10 as compared to DCims. The majority of genes up-regulated in DC-IL-10 belong to the groups of genes involved either in the immune response (26%) or in cell growth and maintenance (28%). The majority of genes up-regulated in DCims also belong to the group of genes involved in cell growth and maintenance (37%), whereas genes involved in the immune response (9.6%) have a lower representation in DCims as compared to DC-IL-10.
2.2 DC-IL-10 show enhanced expression of eight inhibitory immune receptors
When analyzing the differentially expressed immune response genes, special attention was given to identify molecules that might be involved in reduced DC-IL-10-mediated T cell activation. As summarized in Fig. 1, DC-IL-10 were found to overexpress a large number of MHC and Fc immunoreceptors that were absent in DCims. Among the MHC immunoreceptors, six members of the ILT gene family and two members of the C-type lectin superfamily and the paired immunoglobulin-like receptor alpha (PILRA) were detected. Eight genes that we found to be up-regulated in DC-IL-10, including ILT2, ILT3, ILT4, ILT5, DCIR (dendritic cell immunoreceptor), PILRA, SLAM (signaling lymphocyte activation molecule), and FcγRIIB, possess a cytoplasmic tail that contains ITIM and have therefore been assigned to immune inhibitory receptors. Next to ITIM-comprising genes, we found four activating immunoreceptors containing immunoreceptor tyrosine-based activation motifs (ITAM) overexpressed in DC-IL-10, namely DECTIN1, FcγRIIA, FcϵRIγ, and FcγRIIIA. Moreover, DC-IL-10 overexpressed components of the complement system that have been associated with protection from autoimmune diseases (C1s). Several anti-inflammatory mediators (formyl peptide receptor 1, interleukin 1 receptor antagonist) and B cell activation factors (signaling lymphocytic activation molecule, pre-B cell colony-enhancing factor) were also up-regulated in DC-IL-10. In addition to CD14, we found several factors that are involved in lipopolysaccharide (LPS) binding, signaling, and processing, including LPS-binding protein, acyloxyacyl hydrolase, triggering receptor expressed on myeloid cells (TREM 2), and CD18.
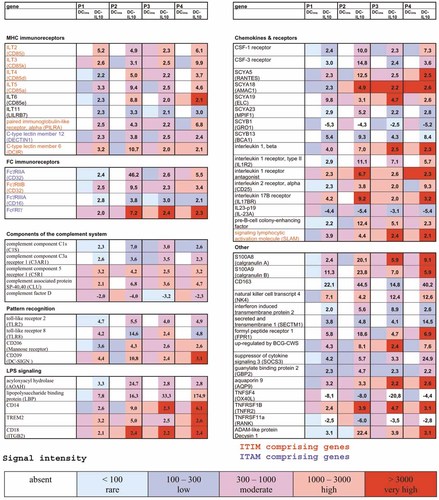
Differential expression of immune response genes in DC-IL-10 versus DCims. Expression profiles in four donors (P1 to P4) are given for MHC immunoreceptors, Fc immunoreceptors, components of the complement system, molecules involved in pattern recognition, molecules involved in LPS signaling, chemokines and their receptors, and other molecules. For each subgroup, the overexpressed genes (fold change ⩾2.0 between DCims and DC-IL-10) are shown in descending order with reference to DC-IL-10. The color range reflects the absolute level of mRNA expression for each individual gene.
2.3 Phenotypic profiles of DC-IL-10, DCims, and their progenitors
In order to confirm, on the protein level, the mRNA expression data obtained from the DNA chips and to further define the changes in protein expression between monocytes, immature DC, DCims, and DC-IL-10, we performed a thorough phenotypical FACS analysis with a number of available monoclonal antibodies (Fig. 2). According to their reactivity, we were able to classify the antigens recognized into those that were (1) up-regulated on DC-IL-10 as compared to DCims; (2) up-regulated on DCims as compared to DC-IL-10; (3) equally expressed on DCims and DC-IL-10; and (4) absent on both DCims and DC-IL-10.

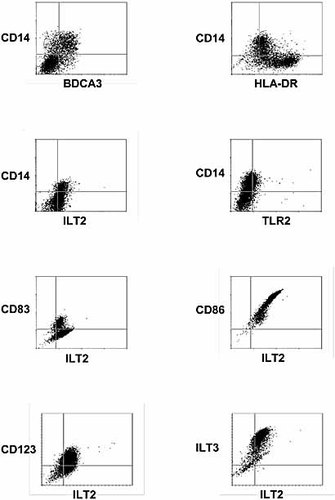
Comparison of the phenotypic changes induced in peripheral blood monocytes, immature DC (day 7), DCims, and DC-IL-10 (day 9) generated from peripheral blood monocytes. The cells were stained for analysis of molecules involved in antigen recognition and presentation (HLA-DR, CD1a, CD86, CD40, TLR2, CD206, DC-SIGN), MHC immunoreceptors (ILT2, ILT3), Fc immunoreceptors (CD16, CD32, CD64), markers of myeloidic cells (CD11b, CD11c), markers of DC (CD83, BDCA2, BDCA3, BDCA4), and further leukocytic markers (CD68, CD3, CD4, CD8, CD14, CD45, CD45RA, CD123) (filled histograms). Isotypic-matched antibodies were used as controls (empty histograms). The data shown are representative of four experiments with similar results.
As shown in Fig. 2, CD14, TLR2, CD123, BDCA3, ILT2, ILT3, CD32, and CD64 were clearly up-regulated on DC-IL-10 as compared to DCims. In addition, all of these markers showed a lower abundance on immature DC as compared to DC-IL-10. While present on monocytes and down-regulated on immature DC, CD14, TLR2, CD32, and CD64 were actively induced during the differentiation of DC-IL-10. In contrast, CD123 and BDCA3 were absent on monocytes and immature DC while being highly induced in DC-IL-10. ILT2 and ILT3 were slightly expressed on monocytes, up-regulated during the generation of immature DC, and further up-regulated after the induction of DC-IL-10. Interestingly, CD14, BDCA3, and ILT2 clearly divided DC-IL-10 into two subpopulations exhibiting either high or low levels of the respective marker. As expected, staining with antibodies against HLA-DR, CD86, and CD83 revealed higher levels on DCims as compared to DC-IL-10. In addition, these markers again divided DC-IL-10 into two subpopulations. In contrast, CD11c, CD11b, BDCA4, DC-SIGN, CD45, CD4, and CD206 were equally distributed on both DCims and DC-IL-10. While absent on monocytes, the markers BDCA4 and CD206 were up-regulated on immature DC. In addition, expression of DC-SIGN clearly indicated subdivision of DC-IL-10 into two subsets. CD1a, CD3, CD8, CD16, CD163, and CD45RA were absent on all DCims as well as DC-IL-10, while BDCA2 and CD68 were not expressed on approximately 90% of the cells. The presence of CD3 on monocytes might be related to the formation of conglomerates with CD4+ T lymphocytes directly after the Ficoll gradient. However, due to the observation that CD3 and CD8 were absent on immature DC, DCims, and DC-IL-10, we exclude a potential T cell contamination after plastic adherence-based separation of monocytes and T lymphocytes. The same low level of BDCA2 expression was detected only after maturation of immature DC into DCims and DC-IL-10, while minor expression of CD68 was already found on immature DC but not on monocytes.
DNA chip results and protein expression data were in good accordance for the majority of molecules analyzed; only for CD16 and BDCA4 were major differences seen. Gene array results revealed increased expression of CD16 in DC-IL-10, while CD16 was not detectable on the protein level in either DCims or DC-IL-10, suggesting post-transcriptional regulation. BDCA4 protein expression was strong in both DCims and DC-IL-10 despite minimal mRNA expression detected on DNA arrays. Considering that BDCA4 was strongly up-regulated during the induction of immature DC and that the mean fluorescence intensity of BDCA4 was slightly lower in DCims (112.7) and DC-IL-10 (123.7) as compared to immature DC (134.4), it seems possible that the down-regulation of BDCA4 expression after addition of the maturation-inducing cocktail is delayed on the protein level as compared to the mRNA level.
2.4 Identification of an ILThighBDCA3highCD14high DC-IL-10 subset
As phenotypic profiling with antibodies against CD14, BDCA3, ILT2, HLA-DR, CD86, CD83, and DC-SIGN had indicated that DC-IL-10 were divided into at least two subpopulations expressing either high or low levels of the respective marker, we used the combination of either CD14 or ILT2 with the remaining markers to further characterize these two subsets. This analysis allowed us to subclassify DC-IL-10 into a i) ILT2high ILT3high BDCA3high CD14high TLR2high HLA-DRlow CD86low CD83low DC-SIGNhigh CD123high CD11clow subset and a ii) ILT2low ILT3low BDCA3low CD14low/– TLR2low/– HLA-DRhigh CD86high CD83high DC-SIGNlow CD123low CD11chigh subset (Fig. 3). Depending on the blood donor, the ILThigh subpopulation represented between 20% and 45% of all DC-IL-10.
Phenotypic analysis of DC-IL-10 subsets. Day 9 DC-IL-10 were stained with combinations of monoclonal antibodies against CD14 or ILT2 and BDCA3, HLA-DR, CD83, CD123, and ILT3 as indicated in the dot plot representations. The data shown are representative of four experiments with similar results. Note that the most characteristic feature of the ILThigh DC-IL-10 subset is expression of BDCA3 and CD14.
Due to the potential of ILT to down-regulate proinflammatory immune responses, we further studied the expression of ILT2 and ILT3 on the two DC-IL-10 subsets over a period of 15 days. Anti-ILT2 and anti-ILT3 antibodies were used in combination with HLA-DR or DC-SIGN, respectively (Fig. 4). Both monocytes and immature DC abundantly expressed ILT2 and ILT3. DCims already showed decreased levels of ILT2 and ILT3 expression 2 days after the addition of the maturation cocktail. The absent mRNA transcription of ILT2 in day 9 DCims in three out of four donors was reflected by the observation that only ∼10% of DCims still expressed ILT2 on their surface. In the further course of the culture (day 11 and day 15), ILT2 became almost absent on DCims. As indicated by the results with the DNA chips, ILT3 is the only member of the ILT gene family that was highly transcribed in DC-IL-10 while exhibiting a residual transcription in day 9 DCims in all four donors. This was reflected by the fact that DCims did not totally down-regulate ILT3 expression on day 9, day 11, or day 15 of culture.
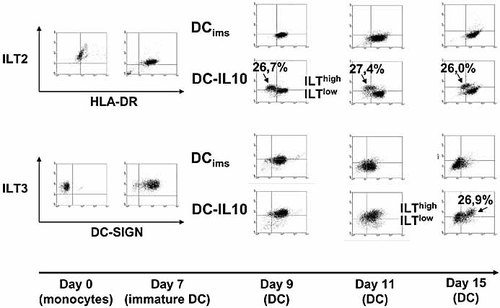
Phenotypic analysis of ILT expression in DC over 15 days. FACS analysis was performed on monocytes, day 7 immature DC, day 9 DC, day 11 DC, and day 15 DC using a combination of ILT2 and HLA-DR as well as ILT3 and DC-SIGN antibodies. Note the subdivision of DC-IL-10 into ILT2high/HLA-DRlow and ILT2low/HLA-DRhigh subsets as well as ILT3high/DC-SIGNlow and ILT3low/DC-SIGNhigh subsets on day 9 and day 15, respectively. The data shown are representative of the results of three separate experiments.
2.5 ILThigh DC-IL-10 exhibit reduced allostimulatory capacity
In addition to the phenotypical and molecular characterization of DCims and the two subsets of DC-IL-10, we were interested in analyzing the impact of ILThigh and ILTlow DC subpopulations on the allostimulatory T cell response. Thus, we sorted DC-IL-10 cells into the two DC-IL-10 subpopulations on day 9 of culture using CD14 as marker. Reanalysis of the sorted fractions confirmed the magnetic bead-based separation of the DC-IL-10 subpopulations (Fig. 5). The allostimulatory capacity of ILThigh and ILTlow DC-IL-10 was compared to unsorted DCims. In order to determine the impact of nonspecific T cell stimulation during the sorting process, we included DCims after CD14 negative selection as well as unsorted DC-IL-10 in the allogeneic MLR. Furthermore, we determined the viability of each DC preparation, which was consistently >90% as indicated by propidium iodide staining (data not shown). As shown in Fig. 6, we found that unsorted DC-IL-10 induced a noticeably reduced stimulatory response of CD4+ T cells as compared to DCims: depending on the ratio of DC and T cells, unsorted DC-IL-10 exhibited between 21% and 40% of the stimulatory capacity of DCims. Further analysis of sorted DC-IL-10 revealed that the stimulatory response of allogeneic CD4+ T cells was most strongly reduced after coincubation with the BDCA3+CD14+ILThigh DC-IL-10 subset (between 19% and 38% of the allostimulatory response detected after coincubation with unsorted DC-IL-10). In comparison, the proliferative response of CD4+ T cells after coincubation with BDCA3–CD14–ILT2low DC-IL-10 cells was clearly higher (between 63% and 84% of the allostimulatory response detected after coincubation with unsorted DC-IL-10) but lower than with unsorted and sorted DCims.
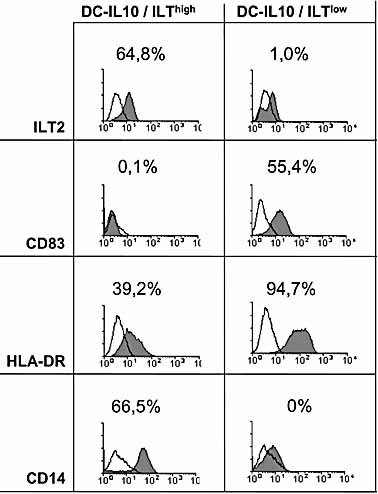
Sorting and phenotyping of the ILThigh and ILTlow subpopulations of DC-IL-10. ILThigh and ILTlow DC-IL-10 subsets were sorted on day 9 using FITC-labeled anti-ILT2 and PE-labeled anti-CD83 monoclonal antibodies. A sample of each sorted population was analyzed after the addition of PerCP-labeled anti-HLA-DR and allophycocyanin (APC)-labeled anti-CD14 monoclonal antibodies. The data shown are representative of five experiments with similar results.
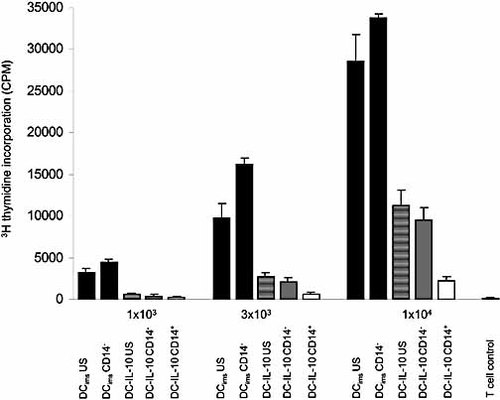
CD14+ILThigh DC-IL-10 exhibit reduced allostimulatory capacity. At day 9 of the DC culture, CD4+ T cells (1×105) were cocultured with various numbers (1×103, 3×103, 1×104) of allogeneic CD14+ILThigh DC-IL-10, CD14–ILTlow DC-IL-10, unsorted DC-IL-10 (DC-IL-10 US), and negatively selected DCims (DCims CD14–) as well as unsorted DCims (DCims US). T cell proliferation was assessed by addition of [3H]-thymidine. Data shown are representative of eight experiments for each subset. Results are given as mean ± SD.
As cytokines may regulate DC-driven immune responses, we analyzed secretion of IL-10 and other Th1/Th2-associated cytokines by DC subsets. As shown using the CBA assay (Table 1), DCims and DC-IL-10 showed no detectable secretion of TNF-α, IFN-γ, or IL-2. IL-4 and IL-10 were secreted in amounts below the assay sensitivity. In contrast, both DC populations produced considerable amounts of IL-6.
|
|
IFN-γ (pg/ml) |
TNF-α (pg/ml) |
IL-2 (pg/ml) |
IL-4 (pg/ml) |
IL-6 (pg/ml) |
IL-10 (pg/ml) |
|---|---|---|---|---|---|---|
|
DCims |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 |
1.4±3.1 |
42.1±44.2 |
0.4±0.9 |
|
DC-IL-10 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 |
1.6±2.5 |
37.6±49.3 |
0.9±1.3 |
|
Assay sensitivity |
7.1±0.1 |
2.8±0.2 |
2.6±0.2 |
2.6±0.2 |
3.0± 0.2 |
2.8±0.2 |
- a) Cells were washed on day 9 of culture and incubated in X-VIVO-15 medium (without cytokines) for an additional 2 days. On day 11 culture supernatants were harvested and cytokine content was assessed using the Cytometric bead array. Data shown are representative of four experiments. Results are given as mean ± SD.
3 Discussion
In this study, we have shown by gene expression profiling that IL-10-induced myeloid DC-IL-10 are characterized by a gene signature that features redundant expression of ITIM-containing inhibitory receptors of the ILT family, the C-type lectin superfamily, and the Fc immunoreceptor family. In support of our findings, it was previously shown that CD8+CD28- alloantigen-specific T-suppressor cells induce up-regulation of ILT3 and ILT4 on DC thereby rendering them tolerogenic towards CD4+ T helper cells 17. When we used anti-ILT2 and anti-ILT3 antibodies to paradigmatically probe for ILT protein expression, it became obvious that DC-IL-10 do not represent a homogeneous cell population. During development of DC-IL-10, immature DC concomitantly treated with maturation signals and IL-10 give rise to ILThigh and ILTlow DC-IL-10 subpopulations that can be further characterized as a i) ILT2high ILT3high BDCA3high CD14high TLR2high HLA-DRlow CD86low CD83low DC-SIGNhigh CD123high CD11clow subset and a ii) ILT2low ILT3low BDCA3low/– CD14low/– TLR2low/– HLA-DRhigh CD86high CD83high DC-SIGNlow CD123low CD11chigh subset, respectively.
With respect to the ILThigh DC-IL-10 subset, it could be argued that these cells are macrophage-like cells rather than true DC. However, it has been shown previously that monocyte-derived macrophages are only induced by IL-10 when added at the beginning of DC culture; when added after development of immature DC – as was also the case in our study – IL-10 did not reverse the DC differentiation process 18, 19. In accordance with the results of Allavena et al. 19 obtained with DC exposed to IL-10 on day 6 of the culture, we show here that both DC-IL-10 subsets resembled DC rather than macrophages. Despite the presence of CD14, neither DC-IL-10 subset expressed CD16 on the protein level and exhibited the same low amount of CD68 expression (∼10%) as DCims. Unique expression of the novel DC-specific marker BDCA3 also strengthens the notion that the DC-IL-10 generated in this study were DC. In addition, DC-IL-10 did not acquire the typical morphology of macrophages but displayed classical DC characteristics such as cytoplasmic protrusions and membrane ruffling and were non-adherent in culture (not shown).
Nevertheless, it could be argued that the two subsets of DC-IL-10 represent immature DCims frozen at different stages on their way to full maturation. Several findings, however, contradict this notion: (1) ILTlow DC-IL-10 exhibit expression levels of CD83, HLA-DR, CD86, and DC-SIGN equal to those of DCims; (2) gene expression profiling of DC-IL-10 revealed high expression of the ADAM-like protein decysin 1 (Fig. 1), which is not expressed in DC precursors or immature DC but gets expressed upon DC maturation 20, 21; and (3) most importantly, ILThigh DC-IL-10 show clear-cut de novo expression of CD14, which is down-regulated on immature DC 22, as well as BDCA3 and CD123, which are almost absent in immature DC generated according to our protocol.
In contrast to the linear maturation concept, we hold that our data favor a conception of multimodal DC differentiation with a considerable degree of overlap and plasticity. In support of this, te Velde et al. 23 have reported that mucosal DC markedly differ from in vitro-generated DCims that simultaneously express DC-SIGN, CD83, and IL-12; they show that mucosal DC can be subdivided into DC-SIGNhighCD83- and DC-SIGN-CD83high subpopulations. Kammerer et al. 24 analyzed DC in placenta; in addition to a CD83high subset, they specifically identified a CD14highDC-SIGNhighCD83- subset in this immunoprivileged organ. In addition, Langerhans cells, which are also viewed as immature tissue-resident DC, lack the typical immature DC markers DC-SIGN and DCIR 25, 26. We therefore propose that the ILThigh DC-IL-10 subset generated in vitro might well correspond to the DC-SIGNhighCD83- (CD14+) DC subset identified by te Velde et al. and Kammerer et al. in vivo.
Expression of BDCA2, a marker that was previously associated with CD11c-CD123bright plasmacytoid DC 27, was found to be moderately up-regulated in about 10% of DCims and DC-IL-10. As our cells showed greater than 99% CD11c positivity, we conclude that the BDCA2+ cells also express CD11c. In contrast to BDCA2, BDCA4 was highly expressed on immature DC and then down-regulated on both DCims and DC-IL-10 as indicated by FACS and DNA array analysis. In contrast to previous observations that found BDCA-3 expression in peripheral blood DC to be restricted to either CD11c+CD123- 27 or CD11clowCD14-HLA-DRhighCD86low DC 28, we found BDCA-3 together with CD123, CD11c, and CD14 on the ILThigh DC-IL-10 subset, which was HLA-DRlow and CD86high. Despite the phenotypical differences of BDCA3+ DC in vivo and in vitro, it is noteworthy that both DC populations are able to drive a Th2 cell profile 29, 30.
Several lines of evidence clearly show that inhibitory immune receptors are important for DC function. Chang et al. have directly shown a crucial role for ILT3 and ILT4 17. In addition, it has been shown for three of the inhibitory receptors up-regulated in DC-IL-10 (ILT2, ILT4, FcγRIIB) that concomitant engagement with activating receptors such as HLA-DR and the ITAM-containing Fc receptors (FcγRIIA, FcγRIIIA, FcϵRIγ), also present in DC-IL-10, results in a predominant inhibitory signal. Phosphorylation of the ITIM motifs of the inhibitory immune receptors leads to recruitment of the Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1), which is involved in negative signaling. Each of the up-regulated inhibitory receptors (ILT2, ILT4, and FcγRIIB) is able to inhibit activation processes induced by the immune activation receptors also up-regulated in DC-IL-10. While co-ligation of FcγRIIB inhibits FcϵRI- and FcγRIIA-dependent cell activation 31, ILT2 or ILT4 markedly reduces intracellular Ca2+ fluxes in monocytic cells after co-ligation with FcϵRI, FcγRIA, FcγRIIA, or HLA-DR 32. Moreover, ILT2 expression leads to inhibition of FcϵRI-induced serotonin release in basophilic granulocytes 33. Chimeric FcγRIIB or FcγRIIB/DCIR complexes down-modulate the B cell receptor (BCR)-mediated activation cascade, resulting in negative feedback regulation of IgG production 34.
In addition to co-ligation of activation receptors, SHP-1-mediated negative signaling occurs after the binding of ILT receptors to their ligands. In contrast to killer cell inhibitory receptors, which recognize only defined subsets of MHC allele products, at least two ILT receptors (ILT2 and ILT4) bind to a wide range of HLA-A and HLA-B alleles as well as to the monomorphic non-classical MHC class I HLA-G and HLA-F molecules 35. The ligands for the other members of the ILT family still remain elusive.
In contrast to ILT, which recognize MHC molecules involved in cellular immune responses, Fc receptors bind antibodies, protecting the organism from damage by removing antigen-antibody complexes from the circulation. In line with this, the low-affinity Fc receptors FcγRII and FcγRIII, which are responsible for binding and uptake of antibody-coated particles, are clearly up-regulated in DC-IL-10. FcγRII- and FcγRIII-mediated antigen uptake has been reported to occur in immature DC and to promote induction of DC maturation into DCims 36. Our observation that FcγRII and FcγRIII remain up-regulated in DC-IL-10 together with ILT receptors suggests that the immune system has chosen a state of readiness to react to penetrating pathogens of little to moderate danger potential by eliminating circulating antigen-antibody complexes without immune activation. Confirming this notion, it has been shown that autoimmune diseases may be caused by insufficient expression of intact Fcγ receptors or by defects within the inhibitory downstream signaling pathway such as allelic variants of FcγRIIB or SHP-1 deficiency 37, 38.
Gene expression profiling indicates that ILThigh DC-IL-10 may also serve in the non-inflammatory removal of pathogens via expression of molecules involved in LPS processing. In addition to CD14, the receptor for LPS-binding protein (LBP), LBP itself is up-regulated in DC-IL-10. While Toll-like receptor 4 (TLR4), the pattern recognition receptor for LPS that activates inflammatory gene expression through NF-κB and MAPK signaling, was not expressed in DCims or DC-IL-10, acyloxyacyl hydrolase was up-regulated in DC-IL-10, suggesting that DC-IL-10 might inactivate and detoxify LPS by deacylation 39. Furthermore, CD14 is also expressed by a subset of dermal DC that inhibit T cell activation 40. Similarly, DC-SIGN, another broad pattern recognition receptor and an HIV coreceptor, is strongly expressed in ILThigh DC-IL-10 and down-regulates DC-mediated immune responses when engaged by mycobacterial cell wall components 41. By providing a link between noninflammatory removal of pathogens and reduced T cell activation, ILThigh DC-IL-10 are well placed at the interface of the innate and adaptive immune systems.
4 Materials and methods
4.1 Generation of DC
DC were generated from buffy coats as described before 16, 42. Briefly, peripheral blood mononuclear cells (PBMC) were obtained from buffy coats of healthy donors after Ficoll density gradient separation. Subsequently, monocytes were purified by plastic adherence on Costar 6-well plates (Corning, Wiesbaden, Germany) and cultured in 3 ml X-VIVO-15 (BioWhittaker, Vervier, Belgium) plus 1% heat-inactivated autologous plasma including 400 U/ml GM-CSF (Leucomax, Essex Pharma, Munich, Germany) and 500 U/ml IL-4 (PeproTech, London, England). At day 3, 1 ml of the medium was replaced with fresh medium enriched with GM-CSF and IL-4. At day 7, nonadherent cells were rinsed off, washed once in PBS, and transferred to Falcon 6-well plates (BD Bioscience, Heidelberg, Germany) at 1.0×106 cells in 3 ml X-VIVO-15 per well. For differentiation into DCims, a maturation cocktail consisting of 1000 U/ml TNF-α, 1000 U/ml IL-1β, 1000 U/ml IL-6 (PeproTech), and 1 µg/mL PGE2 (Sigma, Deisenhofen, Germany) was added at day 7. For the generation of DC-IL-10, IL-10 was added at a concentration of 40 ng/ml (20 U/ml, PeproTech) to immature day 7 DC 60 min after stimulation with the maturation cocktail. The time of addition of IL-10 as well as its final concentration was as given in the literature 10, 42. Day 9 DC were harvested for FACS, RNA extraction, and sorting experiments 48 h after the addition of the maturation-inducing cocktail.
4.2 Antibodies and FACS staining
Peripheral blood monocytes, immature DC (day 7), mature DCims (day 9), and DC-IL-10 subpopulations (day 9) were analyzed using direct or indirect labeling with the following antibodies: from BD Bioscience, ILT2 (CD85j, GHI/75), CD1a (HI149), CD3 (SK7), CD14 (M5E2), CD83 (HB15e), CD40 (5C3), CD206 (19.2), CD163 (GHI/61), HLA-DR (G46–6), CD11c (B-ly6), DC-SIGN (DCN46); from Beckman Coulter, ILT3 (CD85k, ZM3.8); from BioCarta, CD86 (BU63); from Ebioscience, TLR2 (TL2.1), CD123 (6H6); from ImmunoTools, CD16 (5D2), CD11b (B2), CD45RA (MEM-56); from Miltenyi Biotec, BDCA2 (AC144), BDCA3 (AD5–14H12), BDCA4 (AD5–17F6); from Caltag, CD32 (CIKM5), CD64 (10.1), CD68 (Ki-M7), CD45 (HI30); and from Chemicon, CD4 (DD42), CD8 (RTF-8γ).
Anti-HLA-DR and anti-DC-SIGN were used in conjunction with anti-ILT2 and anti-ILT3, respectively, to study the expression pattern on days 0, 7, 9, 11, and 15 of culture. Purified mouse IgG1 (MOPC-31C), IgG2A (G155–178), and IgG2B (27–35) (all from BD Bioscience) as well as rat IgG2A (02–04–2104, Biocarta) served as isotype controls. For cell sorting experiments, anti-ILT2 and anti-CD83 antibodies were purified using protein Ultrafree-MC columns (Millipore, Schwalbach, Germany).
4.3 RNA isolation
Following addition of RNAlater RNA stabilization reagent, total RNA was extracted from 2.0×106 DC (DCims and DC-IL-10) from four donors by the isothiocyanate method (RNeasy RNA isolation kit, Qiagen). Subsequently, DNase treatment was carried out using DNase I (Ambion). RNA concentration and integrity was controlled using RNA 6000 Nano Assays performed on the Bioanalyzer 2100 Lab-on-a-chip system (Agilent Technologies).
4.4 Affymetrix DNA chip hybridization
Total RNA (5 µg) was converted into double-stranded cDNA using T7-(dT)24 primers and the Superscript Choice system for cDNA synthesis (Life Technologies). Biotin-labeled cRNA was prepared by in vitro transcription reaction using the BioArray HighYield RNA Transcript Labeling kit (Enzo Diagnostics) based on the manufacturer's protocol. The resulting biotin-labeled cRNA was purified, fragmented, and hybridized to U133A gene chips (Affymetrix). The hybridized gene chips were stained with streptavidin-phycoerythrin (MoBiTec) and scanned using the GeneArray scanner (Affymetrix). RNA quality was confirmed by spectrophotometric examination and by assessing 5′/3′ ratios of control genes provided on the Test3 array.
Data analysis was performed according to instructions provided by Affymetrix. To compare DCims with DC-IL-10, the baseline corrected data were imported into the Affymetrix Data Mining tool (DMT 4.0) using the publishing tool (MDB 3.0). Subsequently, the genes were filtered using Affymetrix statistical data analysis software (Affymetrix Microarray Suite version 5.0). The comparisons were based on a statistical analysis of probe sets consisting of 11 oligos recognizing different portions of the target gene. Probe sets were excluded if the detection call for both DCims and DC-IL-10 was absent, if the change call gave no change (NC) in comparison analysis, or if the signal log ratio between DCims and DC-IL-10 was between –1 and 1. Signal log ratio is used to describe the change between a target and a reference array. The change is expressed as log2 ratio. Thus, a signal log ratio of 1 equals a fold change (FC) of 2. Only genes that fulfilled the filtering criteria in all four blood donors were used for further analysis. Functional categorization of genes was based on ontological designations in the NetAffx Analysis Center (http://www.affymetrix.com), the AmiGO gene ontology database (http://www.godatabase.org), and gene descriptions in OMIM.
4.5 FACS sorting of ILT2high/CD83low and ILT2low/CD83high DC-IL-10
DC-IL-10 labeled with anti-ILT2 (FITC-conjugated, clone GHI/75, IgG2B) and anti-CD83 (PE-conjugated, clone HB15e, IgG1) (BD Bioscience) were sorted with the aid of a FACSCalibur-II (BD, Mountain view, CA) at a flow rate of approximately 2,000 cells/s. Data were accumulated from the histograms that correlated fluorescence intensity. From the analytical data generated, gates were selected to sort the cells into ILT2high/CD83low and ILT2low/CD83high populations. In order to exclude nonspecific stimulation of DC during the sorting process, sorted and unsorted DCims controls were included in the experiment. The individual populations were collected in separate tubes on ice and reanalyzed using anti-ILT2, anti-CD83, anti-HLA-DR, and anti-CD14 antibodies.
4.6 Th1/Th2 cytokine analysis
For determination of cytokine production, supernatants were collected from 2×105 DC after 2 additional days of culture in X-VIVO-15 medium without addition of cytokines. Amounts of TNF-α, IFN-γ, IL-4, IL-6, and IL-10 were determined with the Cytometric bead array according to the manufacturer's instructions (BD Biosciences).
4.7 Allogeneic mixed leukocyte reactions
CD4+ T cells were freshly purified from PBMC using the CD4+ T cell isolation kit as indicated by the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany; purity >98%). On day 9 of the DC culture, 1×105 allogeneic CD4+ T cells were added at various ratios to CD14-positive and -negative fractions of DC-IL-10 after magnetic bead-based separation using the CD14 magnetic bead kit as indicated by the manufacturer (Miltenyi Biotec). Unsorted DCims, DCims after CD14 negative selection, and unsorted DC-IL-10 were also included in the assay. T cells and DC populations were then cocultured in 200 µl X-VIVO-20 (BioWhittaker) for 4 days. On day 4, each culture was pulsed with [3H]-thymidine (Amersham International) and incubated for 16 h. The cultures were then harvested by blotting onto filter discs (PHD cell harvester, Cambridge Technologies Inc.) and counted using a liquid scintillation counter (Tri-Carb 2900 TR, Packard). Viability was determined before and after stimulation by propidium iodide staining.
Acknowledgements
This work was funded by a grant (B12/SFB405) from the Deutsche Forschungsgemeinschaft (DFG) to S. G. and a grant (932648.1) from the Forschungsfond der Fakultät für klinische Medizin Mannheim to F. W. V.. We wish to thank Dr. Kerstin Steinbrink, Dr. Thomas Wieland, and Edith Graulich for help with dendritic cell culture. We also thank Dr. Carl Thomas Nebe and Ingrid Brechtel for help with cell sorting.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




