Diabetogenic T cells are primed both in pancreatic and gut-associated lymph nodes in NOD mice
Abstract
Activation of an islet-specific immune response is an early yet essential step in autoimmune diabetes. The immune cells and antigen(s) involved in this early step and its anatomical site remain incompletely understood. To directly evaluate the site where islet-specific and diabetogenic lymphocytes are activated, we isolated lymphocytes from spleen and from pancreas-draining, gut-associated and subcutaneous lymph nodes of diabetic NOD mice and of young NOD mice, and transferred these into NOD scid/scid recipients devoid of endogenous islet-specific immune responses themselves. Although spleen lymphocytes from diabetic NOD mice induced diabetes more rapidly than lymphocytes from any other lymphoid tissue, spleen lymphocytes from young NOD donors were not superior to other lymphocytes from the same donors. At a donor-age of 6 weeks, the most-diabetogenic lymphocytes were found in pancreas-draining lymph node whereas gut-associated lymph nodes and the spleen were sources of intermediate diabetogenic activity. Lymphocytes from peripheral lymph nodes were only weakly diabetogenic at this age, and also remained the least efficient later. Surprisingly, lymphocytes isolatedeven from 3-week-old NOD mice had diabetogenic potential. However, such cells were almost exclusively found in gut-associated lymph nodes. This suggests that initial priming of diabetogenic cells takes place in the gut whereas pancreas-draining lymph nodes may serve as the site of amplification of the autoimmune response.
Abbreviations:
-
- GAD:
-
Glutamic acid decarboxylase
-
- GALT:
-
Gut-associated lymphoid tissue
-
- MAdCAM-1:
-
Mucosal addressin cell adhesion molecule-1
-
- MLN:
-
Mesenteric lymph node
-
- PaLN:
-
Pancreatic lymph node
-
- PeLN:
-
Peripheral lymph node
1 Introduction
Activation of an islet-specific immune response is an early yet essential step in autoimmune diabetes. For understanding the pathogenesis of autoimmune diabetes in detail, it is important thatthe immune cells and antigen(s) involved in this early step and the anatomical site where such activation occurs are further characterized. Although B lymphocytes also play a role as APC in NOD mice, DC are believed to be essential in the initial activation of islet-specific T cells 1. DC capture foreign antigen in tissue via endocytosis, then migrate into the draining lymph node and present their antigens to T cells in the lymph node. Besides foreign antigens, DC also present self-antigens derived from apoptotic cells. Depending on the activation status of DC and on other local factors, this presentation either leads to T cell activation or induction of tolerance. In type 1 diabetes, islet-specific T cells are activated to orchestrate destruction of β cells. Several molecules have been identified as their target antigens, including (pro)insulin 2, 3, glutamate acid decarboxylase ([GAD]65 and GAD67) 4, 5, tyrosine phosphatase IA-2 6, 7, heat-shock protein 65 8, 9 and carboxypeptidase H 10. The reason for the T cell activation is not yet fully understood although, apparently, antigens must be released from β cells for presentation by DC in pancreas-draining lymph nodes. This could happenas a consequence of local inflammation or other stressful conditions, or due to physiologic β cell turnover 1, 11.
The site where islet-specific lymphocytes are first activated in the NOD mouse has received relatively little attention. Activation of transgenic, islet-specific CD4 T cells (BDC2.5 cells) wasshown to take place in pancreas-draining lymph nodes of NOD mice from the age when islet-autoimmunity starts to develop 12. In a recent study, surgical removal of pancreas-draining lymph nodes at an early age abrogated the development of diabetes in NOD mice almost completely 13 and demonstrated a critical role for these lymph nodes in activation of diabetogenic T cells. However, these data do not rule out the possibility that diabetogenic lymphocytes could be activated primarily in other lymph nodes. A long-thought possibility is that an immune response towards islets could be triggered by an environmental antigen through molecular mimicry. In this scenario, environmental antigens structurally similar to β cell components would be able to activate T cells that would then also target islet β cells. In fact, amino acid sequences in several environmental antigens have been shown to share critical MHC anchor and/or TCR contact residues with diabetes-associated autoantigens 14.
Experimental evidence indicates cross-reactivity (at the T cell level) between GAD and cytomegalovirus 15, and GAD and rubella virus 16, 17. T cells that recognize the P2-C protein of coxsackie B3 have been proposed to also recognize GAD 18, 19. In children genetically predisposed to type 1 diabetes, early exposure to cow's milk formula can induce the production of antibodies to bovine insulin that cross-react with human insulin 20, and GAD-reactive T cells derived from peripheral blood of diabetic patients express the mucosal homing receptor α4β7-integrin 21. Expression of both isoforms of GAD in the gut has also been demonstrated 22. We have previously found that, in NOD mice, islet-infiltrating lymphocytes express α4β7-integrin 23 and that mucosal addressin cell adhesion molecule-1 (MAdCAM-1) is required for the development of diabetes in NOD mice 24. These data suggest that enteral antigens and immune responses arising in gut-associated lymphoid tissue (GALT) may be able to target islet β cells for destruction.
To evaluate more directly the site where early diabetogenic lymphocytes become activated in the NOD mouse, we isolated lymphocytes from different lymphoid compartments of female NOD mice at different stages of diabetes development, and compared their ability to transfer diabetes into NOD scid/scid recipients. Since the earliest diabetogenic cells were found in the gut, the role of mucosal immune system in the development of insulitis and diabetes was further characterized with a transgenic mouse model. The thorough phenotypic analysis that was performed suggests that the diabetogenic subpopulation represents an extremely minor cell population. The antigen-specificity of the early diabetogenic cells also remains elusive.
2 Results
2.1 In diabetic donors, lymphocytes capable of transferring diabetes reside in spleen and lymph nodes
To determine if diabetogenic lymphocytes in diabetic NOD mice preferentially accumulate in the spleen, and to evaluate the recirculation of such lymphocytes within mucosal and peripheral lymphoid compartments, we compared the efficiency of equal numbers of lymphocytes prepared from these sources in transferring diabetes to NOD scid/scid recipients. In recipients of spleen-derived lymphocytes, diabetes incidence reached 100% at 7 weeks post-transfer in comparison with 10 and 12 weeks post-transfer in recipients of mesenteric lymph node (MLN) lymphocytes and peripheral lymph node (PeLN) lymphocytes, respectively. The difference between recipients of MLN- and PeLN-lymphocytes was significant (p=0.028) (Fig. 1A). The number of regulatory CD25+CD4+ T cells and/or IL-4- or IL-10-producing cells was not different according to flow-cytometric analysis (data not shown), which suggests that diabetogenic lymphocytes preferentially accumulate in spleen of diabetic NOD mice and recirculate more actively within the mucosal than peripheral lymphoid compartment.
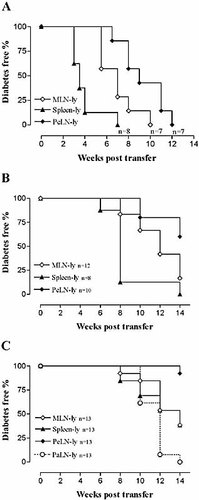
MLN lymphocytes (MLN-ly) of NOD donors transfer diabetes significantly more efficiently than PeLN-ly at all stages of the disease. (A) Transfer of lymphocytes from diabetic donors. (B) Transfer of lymphocytes from 10-week-old donors. (C) Transfer of lymphocytes from 6-week-old donors. Analysis of survival curves of MLN-ly and PeLN-ly transfers shows that MLN-ly transfer diabetes significantly more efficiently at all three different stages of the disease (A, p=0.028; B, p=0.036; and C, p=0.0032; Logrank test of survival).
2.2 The potential of lymphocytes to transfer diabetes correlates with the age of the donor
When using prediabetic NOD mice as lymphocyte donors, the final incidence of diabetes in recipient mice remained below 100%. However, the age of the donor and the potential of its lymphocytes to transfer diabetes were positively correlated. A 50% occurrence of diabetes was reached at 8 and 14 weeks post-transfer, respectively, when spleen lymphocytes were isolated from 10- and 6-week-old donors, whereas 50% occurrence was reached at 3.5 weeks post-transfer when lymphocytes were isolated from diabetic donors. This trend was also consistent with other lymphocyte populations (Fig. 1).
2.3 PeLN-lymphocytes do not transfer diabetes as efficiently as spleen-lymphocytes and MLN-lymphocytes
In 10-week-old donors, spleen-lymphocytes were still the most diabetogenic. At 8 weeks post-transfer, 7 out of 8 (88%) of spleen-lymphocyte recipients were diabetic, in comparison with 2 out of 12 (17%) recipients of MLN-lymphocytes. However, PeLN-lymphocytes transferred diabetes the least efficiently and the overall diabetes incidence in the recipients remained low. The final incidence in the recipients of PeLN-lymphocytes was only 40% (4 out of 10) after a 14-week follow-up time, in comparison with 100% (8 out of 8) in the recipients of spleen-lymphocytes and 83% (10 out of 12) in the recipients of MLN-lymphocytes. Statistical analysis of the survival curves indicated that the difference between the recipients of MLN-lymphocytes and PeLN-lymphocytes was significant (p=0.036) (Fig. 1B).
2.4 At an early age, lymphocytes from pancreas-draining lymph nodes are the most diabetogenic
Unlike the situation in diabetic and 10-week-old donors, in 6-week-old donors the spleen-lymphocytes were no longer the most diabetogenic. Instead, MLN-lymphocytes were as diabetogenic as spleen-lymphocytes, whereas PeLN-lymphocytes were hardly able to transfer diabetes at all (Fig. 1C). Notably, lymphocytes from pancreas-draining lymph nodes were the most diabetogenic: in the recipients of pancreatic lymph node (PaLN) lymphocytes, diabetes incidence reached 100% (13 out of 13) whereas in the recipients of either MLN- or spleen-lymphocytes, diabetes incidence reached only 62% (8 out of 13). These differences between the groups were significant (p=0.0097 for PaLN-lymphocytes vs. MLN-lymphocytes, and p=0.042 for PaLN-lymphocytes vs. spleen-lymphocytes) (Fig. 1C).
Consistent with the results in survival, the severity of insulitis in the recipients of spleen-lymphocytes and MLN-lymphocytes were essentially the same (0.76 and 0.91, respectively) and in comparison with PeLN-lymphocytes (0.35) a significant difference was observed for both (p=0.0026 and p=0.0056) (Fig. 2).
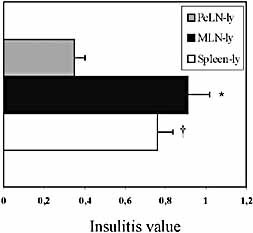
The stage of insulitis in the recipients of young (6-week-old) NOD donors' lymphocytes at 4 weeks post-transfer. Insulitis is significantly more severe in the recipients of MLN lymphocytes (MLN-ly) and spleen-ly in comparison with the recipients of PeLN-ly (the asterisk indicates p=0.0056, the dagger indicates p=0.0026; unpaired t-test with Welch's correction). Data are means ± SEM (n=5). A total of 284 (MLN-ly recipients'), 213 (spleen-ly recipients') and196 (PeLN-ly recipients') islets were analyzed.
2.5 At a very early age, diabetogenic activity is found preferentially in MLN
To investigate the site of diabetogenic activity in the very early stages of autoimmune diabetes, i.e. prior to insulitis, lymphocytes from 3-week-old donors were transferred. The lymphocytes of 3-week-old donors transferred diabetes very inefficiently, as expected. During the 14-week follow-up time, only 6 out of 22 recipients became diabetic. Interestingly, the diabetogenicity of MLN lymphocytes was clearly higher, as 38% (5 out of 13) of the recipients of MLN lymphocytes became diabetic in comparison with 11% (1 out of 9) of the recipients of PaLN lymphocytes. Furthermore, 3 out of 13 recipients of MLN lymphocytes already became diabetic by 12 weeks post-transfer, whereas in the recipients of PaLN lymphocytes the single case of diabetes onset was by 14 weeks post-transfer, i.e. at the very end of the follow-up time (Fig. 3A). Consistent with this result there was a significant difference in mean insulitis score between the recipients of MLN-lymphocytes and PaLN-lymphocytes (1.94 vs. 0.42; p=0.0032) (Fig. 3B). Based on the observed capabilities of transferred cell populations to induce diabetes and infiltrate islets, we have drawn a schematic graph to illustrate the diabetogenic activity of different lymphoid compartments at different stages of the disease pathogenesis (Fig. 4).
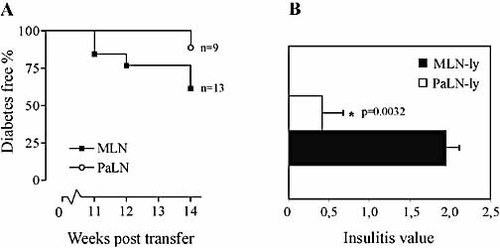
(A) Transfer of lymphocytes from 3-week-old NOD donors. The first MLN-lymphocyte recipients develop diabetes several weeks earlier than PaLN-lymphocyte recipients. The overall diabetes incidence among MLN-lymphocyte recipients is also higher (38% vs. 11%) during the 14-week follow-up time. (B) The stage of insulitis in the non-diabetic recipients of 3-week-old NOD MLN lymphocytes (MLN-ly) and PaLN-ly at 14 weeks post-transfer. The mean insulitis value is significantly higher in MLN-ly recipients than PaLN-lymphocytes recipients (p=0.0032; unpaired t-test with Welch's correction). Data are means ± SEM (n=4). A total of 205 (MLN-ly recipients') and 185 (PaLN-ly recipients') islets were analyzed.
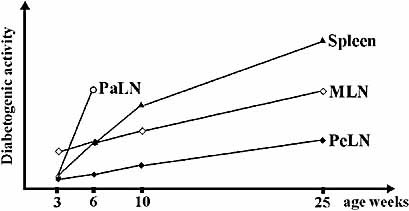
A schematic illustration of the diabetogenic activity in different lymphoid compartments at different stages of the disease based on the results from transfer experiments.
2.6 Mononuclear cell subsets and expression of activation/homing markers on transferred T lymphocytes
To determine the possible differences in the phenotype of the transferred cell populations, immunofluorescence staining was performed on each transferred cell population, with a panel of mAb against a broad range of surface markers. Results of CD4, CD8, B220, β7-integrin, α4-integrin and L-selectin expression are shown in Table 1. As expected, the relative number of B lymphocytes in spleen is higher compared with lymph nodes. Consistent with a previous report 25, expression of mucosal homing receptors α4- and β7-integrin is almost identical in MLN- and PeLN-lymphocytes from 7-week- old NOD mice. PaLN-lymphocytes also had a very similar expression pattern but, at 10 weeks of age, there seems to be a slight increase in α4- and β7-integrin-expressing lymphocytes in comparison with other lymph nodes; however, its significance remains speculative. Apart from the surface markers shown in Table 1, the transferred cells were also stained for activation and memory markers including CD44, CD45RB, CD11a and CD25, but no significant differences could be observed at any stage of the disease (data not shown). A separate analysis with CD4+ and CD8+ T cells of 6- and 25-week-old donors was also performed with similar results. There was no difference in the number of CD25+CD4+ regulatory T cells (data not shown).
To test the possible differences in the relative number of APC, the transferred populations were stained for markers for macrophages and DC. Such cells were most frequent in the spleen (10–20% of the total number of cells) but no differences were seen in the number of these cells in MLN, PeLN or PaLN (Table 1).
|
|
Spleen |
PeLN |
MLN |
PaLN |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Antigen |
Age |
3wk |
6wk |
10wk |
25wk |
3wk |
6wk |
10wk |
25wk |
3wk |
6wk |
10wk |
25wk |
3wk |
6wk |
10wk |
25wk |
|
CD4 |
|
16 |
49 |
23 |
24 |
59 |
65 |
61 |
58 |
48 |
56 |
52 |
44 |
45 |
45 |
53 |
53 |
|
CD8 |
|
5 |
11 |
10 |
9 |
18 |
22 |
23 |
24 |
15 |
19 |
23 |
21 |
17 |
21 |
20 |
23 |
|
B cell |
|
51 |
24 |
48 |
19 |
10 |
8 |
16 |
11 |
18 |
13 |
22 |
14 |
18 |
40 |
22 |
12 |
|
Macrophage (F4/80) |
22 |
17 |
12 |
16 |
2 |
1 |
2 |
1 |
4 |
2 |
2 |
2 |
2 |
1 |
2 |
2 |
|
|
DC (33D1) |
4 |
3 |
4 |
N/T |
5 |
3 |
5 |
3 |
5 |
3 |
4 |
4 |
3 |
3 |
5 |
3 |
|
|
β7-integrin |
4 |
10 |
8 |
8 |
12 |
15 |
11 |
11 |
10 |
17 |
8 |
10 |
12 |
17 |
16 |
11 |
|
|
α4-integrin |
69 |
52 |
58 |
68 |
67 |
27 |
35 |
50 |
75 |
28 |
42 |
52 |
68 |
31 |
53 |
34 |
|
|
L-selectin |
56 |
75 |
76 |
76 |
80 |
90 |
85 |
70 |
73 |
80 |
55 |
66 |
70 |
68 |
80 |
54 |
|
- Numbers in the table are percentages of positive cells. The data are the means of 2–3 experiments. For each experiment, cells from three animals were pooled, stained for surface antigens and analyzed by flow cytometry. N/T, not tested.
2.7 Th1 vs. Th2 phenotype of the transferred lymphocytes
In NOD mice the pathogenesis of autoimmune diabetes is believed to be Th1-mediated, whereas Th2 cells are less harmful or may even act as regulatory cells delaying the onset of the disease. Therefore, possible differences between the transferred populations regarding Th1 vs. Th2 phenotype were investigated. Herein, intracellular cytokine staining was performed to detect expression of IFN-γ, IL-4 and IL-10 for both CD4+ and CD8+ cells after in vitro stimulation. IFN-γ was expressed on 5–10% of cells in each cell population tested whereas cells expressing IL-4 or IL-10 were equally rare in all samples (data not shown). To increase further the sensitivity of detecting Th1 vs. Th2 activity, mRNA levels of IL-4 and IFN-γ were measured by using quantitative PCR analysis (TaqMan). For these experiments total RNA was isolated from freshly isolated lymphocytes without stimulation. Spleen-lymphocytes clearly had the highest Th1-type reactivity at preclinical stages (6–10 weeks), which seemed to correlate with a better capability to transfer diabetes. However, contradictory to this, PeLN-lymphocytes, which were the least diabetogenic, had the second-highest Th1/Th2 ratio at all stages of disease. MLN-lymphocytes and PaLN-lymphocytes had a very similar Th1/Th2 pattern (Fig. 5).
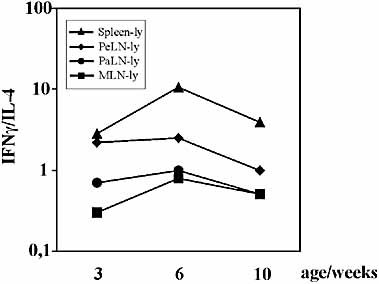
The Th1/Th2 (IFN-γ/IL-4) ratio of the transferred cell populations at preclinical stages of disease. Data are means of two separate experiments, in which two separate runs were performed.
2.8 T cell reactivity against GAD524–543 and insulin
To find potential associations between the diabetogenic potential of a donor cell population and T cell reactivity against identified autoantigens, we stimulated freshly isolated spleen lymphocytes and lymph node lymphocytes of donor mice with antigen (GAD524–543 or insulin) according to the standard methods described by Kaufman et al. 26. However, in NOD mice from our colony, no spontaneous proliferative responses to either of these antigens were measurable either in spleen lymphocytes or in other lymph node lymphocytes at any donor age. Lymph node cells from pre-immunized NOD mice showed weak, but significant proliferative responses (stimulation indices 2.0–2.6), indicating that antigens and culture conditions were appropriate (data not shown).
2.9 Lymphocytes activated in the GALT are able to migrate to pancreatic islets
Finally, we wanted to test the hypothesis that lymphocytes encountering their antigen in the GALT are able to migrate into the pancreatic islets and initiate β cell destruction if the antigen is also expressed on β cells. To test this, we used transgenic mice that express OVA as a model antigen under the insulin promoter (RIP-OVAlo mice) and that had received OVA-reactive CD8 T cells (OT-1 cells). Insulitis and often diabetes will develop only if immunization with OVA is performed, for example via oral administration 27, 28. When immunized orally, antigen-specific CD8 cells found in the lymphocyte infiltrates within the islets of these mice have been primed in the GALT. In our experiments, immunohistological staining of the islets of RIP-OVAlo mice ten days after feeding OVA shows co-localization of CD8 and mucosa-specific α4β7-integrin expression. This demonstrates that lymphocytes originally primed in the GALT are able to migrate to the pancreas and initiate inflammation leading to destruction of β cells (Fig. 6).
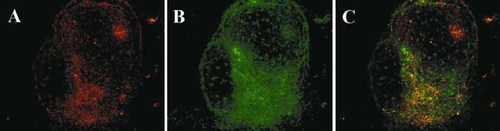
Immunohistological staining of a pancreatic islet from a transgenic RIP-OVAlo mouse ten days after induction of diabetes with OVA-specific CD8 T cells followed by oral priming with OVA. (A) α4β7-integrin; (B) CD8; (C) Superimposed images showing co-localization of α4β7-integrin and CD8 in yellow.
3 Discussion
The main purpose of this study was to find out the origin of diabetogenic cells in autoimmune diabetes. Because in vitro tests for T cell reactivity to autoantigens still achieve variable results and the earliest T cell response to islet-antigens may yet remain unknown, we committed ourselves to in vivo adoptive transfer experiments to evaluate the diabetogenic activity of T cells derived from different lymph nodes of NOD mice. T cells were transferred into immunodeficient NOD scid/scid mice that enabled us to identify even very subtle diabetogenic activity, characteristic of the earliest stages of the disease process. We have shown here that by transferring lymphocytes obtained from different lymph nodes of pre-diabetic mice, and even very young NOD mice, it is possible to induce diabetes in recipient NOD scid/scid mice and to compare the prevalence of diabetogenic T cells in different lymphoid organs at progressively earlier stages of the disease process.
PaLN are the default site where islet-reactive T cells should be activated and quite recently it was shown that the surgical removal of PaLN at 3 weeks of age almost completely abolishes the onset of diabetes in NOD mice, whereas if performed later the procedure has no effect. Reduction in the onset of diabetes was also seen in a cyclophosphamide-accelerated model in PaLN-excised mice but not after partial excision of MLN; based on this, it was concluded that initial priming of autoreactive cells takes place in the pancreas, not in the gut 13. These results suggest that generation of islet-reactive lymphocyte pools that lead to massive infiltration and clinical disease takes place in PaLN. However, as complete removal of MLN and other GALT cannot be achieved by surgical procedures, this still leaves the possibility that primary activation of diabetogenic T cells may take place in GALT.
To avoid tolerance, the presence of an inflammatory stimulus is probably required. Interestingly, at the age of 6 weeks, when there is no β cell destruction and yet subtle islet-inflammation exists, PaLN already contained T cells with high diabetogenic activity. It has been suggested that an inflammatory stimulus required for non-tolerogenic presentation of an islet-antigen could come from the islets either as a result of prior initiation of subtle insulitis, or local disturbance such as expression of retroviral neoantigen, local virus infection or a wave of apoptotic cell death 1, 29–31. Our findings support the former alternative, i.e. subtle priming of diabetogenic T cells in response to antigenic stimulus elsewhere, particularly in the gut, and selective migration of these cell into islets.
After the finding that PaLN contain high diabetogenic activity soon after initiation of insulitis, it was important to try to localize possible diabetogenic activity prior to insulitis. Therefore, we transferred lymphocytes from even younger NOD donors in an endeavor to test for the presence of very early diabetogenic T cells. Based on the evidence of a clear diabetogenic activity in gut-draining lymph nodes (MLN) already at pre-diabetic stages (6 and 10 weeks), the potential of MLN-lymphocytes and PaLN-lymphocytes from 3-week-old donors to cause diabetes in NOD scid/scid recipients during a 14-week follow-up was finally investigated. According to our earlier experience (data not shown), transfer of naive lymphocytes from any lymphoid compartment is able to induce diabetes in NOD scid/scid recipients at 16–20 weeks post transfer. Apparently, this is due to the reconstitution of the immunodeficient NOD scid/scid recipient with the donor's naive immune system, which could be followed by the natural disease course similar to that in normal NOD mice.
Therefore, it was concluded that only those cells that are able to transfer diabetes before this "de novo diabetogenesis" would possess specific diabetogenic activity. Interestingly, during the 14-week post-transfer follow-up time, MLN lymphocytes from 3-week-old donors clearly had the highest diabetogenic potential, whereas PaLN lymphocytes seemed to have only a very weak ability to transfer diabetes. The finding was further supported by the analysis of pancreatic histology of normoglycemic mice at the end of these experiments, showing that recipients of 3-week-old MLN lymphocytes consistently had a heavy, and significantly more severe, inflammation in their islets, compared with a very modest insulitis in the recipients of PaLN lymphocytes. These results indicate that, at very early stage of autoimmune diabetes, the diabetogenic effector cells are found in the GALT rather than in the pancreas-draining lymph nodes.
Under physiological circumstances, the gut is a site of continuous immune responses against multiple antigens derived from microbial flora and food. Should the fortuitous activation of a T cell that is able to recognize some islet-antigen as well as a "foreign" antigen prove important in the early genesis of autoimmune diabetes, it could best be envisioned to take place in the gut. In the development of type 1 diabetes, the role of environmental factors is widely recognized by numerous studies in humans and animals 32, 33. We have previously demonstrated that molecules normally involved in mucosal homing are important in development of autoimmune diabetes in NOD mice 23, 24 In man, GAD-reactive peripheralblood mononuclear cells of diabetic patients were shown to express the mucosal homing receptor α4β7-integrin 21. Linking these facts together, it is conceivable that GALTmay be the site for primary activation of autoreactive lymphocytes, and thus may harbor a subset of lymphocytes capable of initiating inflammation in the islets.
A commonly accepted view is that type 1 diabetes is mediated by islet-reactive T cells predominantly of an IFN-γ-secreting phenotype (Th1), whereas the Th2-type cytokine IL-4 and particularly IL-10 have been shown to exert regulatory/protective effects (reviewed in 34). One explanation for the low diabetogenic potential of PeLN-lymphocytes could thus be the predominance of such IL-4/IL-10-secreting cells among the transferred cells. However, there was no correlation between diabetes-transferring potential and intracellular expression of these cytokines, or inTh1/Th2 ratios as detected with real-time PCR, indicating that a different relative number of Th1 effector cells or regulatory Th2 cells is not the explanation for the results of the transfer experiments presented in this work. Moreover, it has been reported that regulatory T cells appear less numerous or less efficient in PeLN than in spleen 35, thus excluding the possibility that the low diabetogenic potential of PeLN-lymphocytes we observed in this work was due to high numbers of regulatory cells. Consistent with this, we could not detect any difference in the number of CD25+CD4+ T cells in PeLN and MLN.
The phenotype and antigen-specificity of the early diabetogenic cells in MLN remain to be elucidated. Since it may take only few activated effector cells to initiate the inflammatory cascade within the islet environment, it is likely that these cells are also present in extremely low numbers among the lymphocyte populations studied. Therefore, with currently available methods it is difficult to identify any particular phenotype that would associate with diabetogenic cells originally capable of initiating insulitis. However, evidence based on our previous findings and the literature25, 36 allows us to speculate that these diabetogenic cells are most likely α4β7-integrinhighL-selectinlow and express common activation markers. Their antigen-specificity could not be identified in this work, consistent with a recent workshop report showing that most laboratories still experience difficulties in measuring T cell responses in NOD mice 37.
The results of adoptive transfer experiments presented in this work demonstrate that diabetogenic cells already exist in NOD mice at a very young age. More importantly, at all stages of the disease, gut-associated lymphocytes have higher diabetogenic potential than PeLN lymphocytes, indicating a clear distinction between these two lymphoid compartments in their role in the development ofinsulitis and autoimmune diabetes. Although diabetogenic cells become frequent in PaLN concomitantly with insulitis, prior to development of insulitis diabetogenic activity is almost exclusively found among gut-associated lymphocytes. These results support our earlier findings in which blocking of the gut-associated mucosal homing receptor, MAdCAM-1, in NOD mice was able to prevent both the priming of diabetogenic cells and their migration to the islets, and consequently also prevent the onset of autoimmune diabetes in these mice. Consistent with the results presented in the current work, MAdCAM-1 blocking was effective only if the treatment was started at a very early age and no effect on diabetes incidence or level of insulitis could be seen if it was started later. In conclusion, all these findings suggest an association between GALT and early islet-specific T cell activation in the pathogenesis of autoimmune diabetes in NOD mice.
4 Materials and methods
4.1 Mice
Local colonies of NOD and NOD scid/scid mice were used. Both of the colonies were housed and bred in the central animal laboratory of Turku University under specific pathogen-free conditions. The cumulative incidence of diabetes in the NOD colony by the age of 35 weeks is 80% in females and 15% in males. No spontaneous diabetes occurs in NOD scid/scid colony. All animal experiments were approved by the Ethical Committee of the University of Turku.
4.2 Antibodies
Hybridomas for monoclonal anti-CD4 (TIB-207), anti-B-cell (TIB-146), MAC-1 (TIB-128), F4/80 (HB-198), anti-DC (TIB-227, 33D1), anti-CD11a (TIB-217), anti-CD44 (TIB-241), anti-α4-integrin (CRL-1911) and anti-CD25 (TIB-222) were purchased from the American Type Culture Collection. Anti-L-selectin (anti-CD62-L, MEL-14), anti-β7-integrin (FIB-504) and anti-human CD44 (9B5) hybridomaswere kind gifts from E. C. Butcher (Stanford University, CA, USA). All the antibodies were concentrated from cell hybridoma supernatants with ammonium-sulfate precipitation and purified with Protein G columns (Pharmacia). When necessary, antibodies were FITC-conjugated using FITC (Sigma) or biotinylated by using N-hydroxy-succinimide (NHS)–biotin (Calbiochem). FITC- and R-PE-conjugated anti-CD8 (Lyt2.2), anti-CD34, anti-CD45RB, anti-α4β7 integrin (DATK32), anti-IL-4, anti-IL-10, anti-IFN-γ and their isotype-matched control antibodies were purchased from BD/Pharmingen. All the antibodies were used at a concentration of 10 μg/ml or at the concentration suggested by manufacturer.
4.3 Isolation of lymphocytes and immunofluorescence staining for surface antigens
Lymphocytes were isolated aseptically from lymphoid organs by homogenizing the organs manually in a glass homogenizer and then filtering the tissue suspension through a sterile nylon mesh. Forlysing erythrocytes from spleen cell suspension, the cells were incubated in hypotonic saline for 30 s. For flow cytometric (FACScan, Becton Dickinson) staining, lymphocytes from different lymphoid organs were isolated. The lymphocytes of three animals were pooled and stained either with FITC- or PE-conjugated mAb against selected surface markers or with unconjugated mAb and then with FITC-conjugated goat anti-rat-IgG (Sigma) secondary antibody (1:100). For biotinylated primary antibodies, PE-conjugated streptavidin (Becton Dickinson) was used at 1:40 as a second-step reagent. To block nonspecific binding, 5% normal mouse serum was used along with the second-step antibody incubation. For analysis, CellQuest® software (Becton Dickinson) was used.
4.4 Detection in the pancreas of lymphocytes primed in the gut
Transgenic RIP-OVAlo mice express chicken OVA in their β cells. In these mice, diabetes can be induced by introducing OVA-specific CD8 T cells (OT-1 cells) in the system followed by e.g. oral administration of OVA 27, 28. In these experiments 2×106 OT-1 cells were purified from transgenic OT-1 mice that express a transgenic TCR specific for OVA-peptide and H-2Kb. Isolated OT-1 cells were adoptively transferred to RIP-OVAlo mice that were then fed with 3 mg of OVA. Frozen sections of the pancreas were made ten days after feeding OVA. The sections were stained with PE-conjugated anti-α4β7-integrin (DATK 32) or isotype-matched control mAb, and FITC-conjugated anti-CD8 in for 20 min at room temperature. The sections were washed twice with PBS to remove excess mAb. The analysis and the imaging of the islets were performed under a UV-microscope (Olympus BX60) with a digital camera, using Analysis software.
4.5 Intracellular staining of isolated lymphocytes for Th1 and Th2 cytokines
Lymphocytes were isolated and pooled from two animals in each age group as described above. Cells (10–20×106) were incubated at +37 °C in 3 ml of RPMI-1640 medium with 10% FCS, Hepes, ionomycin (0.5 μg/ml; Calbiochem) and phorbol myristate acetate (1.0 ng/ml; Sigma). After 2 h, 3 μl of brefeldin A (10 mg/ml; Epicentre Technologies) was added for an additional 2 h. Cells were first stained for CD4 and then fixed and permeabilized, and stained for intracellular cytokines (IL-4, IL-10 or IFN-γ, or their isotype-matched controls) according to manufacturer's instructions (Pharmingen). Each staining was repeated three times.
4.6 Disease induction by adoptive cell transfer
Transferred cells were isolated aseptically from spleens, MLN, PaLN, and other PeLN including cervical, axillar and inguinal lymph nodes of female NOD mice. Donors at different stages of disease were used. These stages included: 3-week-old mice representing the naive situation when no lymphocytes have yet infiltrated the pancreas; 6-week-old mice representing the very early stage of the disease pathogenesis when some lymphocytes can be seen in the peri-islet region; 10-week-old mice representing the stage where the β cell destruction and clear lymphocyte infiltration within islets can be seen; and diabetic mice representing the end stage of the disease with fully developed insulitis. The cell suspensions were made as described above and 20×106 cells were injected into the tail vein of recipient NOD scid/scid mouse. The recipient mice were monitored for urinary glucose (Glucotest, Boehring) twice a week and blood glucose was measured (MediSense, UK) from glucosuric mice. Mice with blood glucose over 240 mg/ml (14.3 mmol/l) were considered diabetic.
4.7 RNA isolation and cDNA synthesis
The cells were isolated from three animals as described above, pooled and total RNA was isolated from single cell suspension using Ultraspec® RNA isolation reagent (Biotecx Laboratories) according to the manual. The purity was confirmed on agarose gels. Total RNA (2 μg) was treated with DNase I (Amplification Grade, Gibco-BRL, Life Technologies). The OLIGO(dT)-primed first-strand cDNA synthesis was carried out in a total volume of 20 μl with Superscript II RNase H reverse transcriptase according to the manufacturer's protocol (Superscript Preamplification System for First strand cDNA synthesis; Gibco-BRL). A volume of 1 μl of produced cDNA was used for TaqMan measurements.
4.8 RT-PCR (TaqMan) and cytokine quantification
The primers and probes for quantitative detection of IL-4 and IFN-γ mRNA levels with TaqMan were designed according to Casteels et al 38. Primers were purchased from CyberGene and probes from Eurogentech. TaqMan Universal PCR Master Mix (PE Biosystems) was used to prepare the reaction solution for each target gene. Each reaction was done as duplicate for every TaqMan run. Two separate runs were performed for each sample. For calculations, the average of the resulting readings was used. Each sample was normalized to β-actin. The obtained values were further processed by calculating the Th1/Th2 ratio by dividing the values of IFN-γ signal with the values of IL-4 signal. These ratios were then compared between transferred cell populations during the disease pathogenesis.
4.9 Histology and grading of islet infiltrates
The state of insulitis was graded from each pancreas according to the following criteria: 0= no insulitis; 1= peri-insulitis; 2= insulitis covering <50% of the islet; and 3= insulitis covering ≥50% of the islet. The insulitis value of given pancreas was calculated by dividing the sum of graded islets with the total number of islets analyzed. An average of 46 islets was graded from each pancreas. The mean insulitis values from each group were calculated.
4.10 Proliferation assay of donor cells for reactivity against autoantigens
Lymph node cells were plated at 2×105 cells per well in RPMI-1640 medium supplemented with 10% FCS, 20 mM L-glutamine and 5×10–5 2-ME on round-bottomed 96-well plates (Nunc), and spleen cells at 5×105 cells per well in HL-1 medium (Bio Whittaker) on flat-bottomed 96-well plates (Nunc) with the following antigens: GAD524–543 (at 20 μg/ml) or porcine insulin (100 μg/ml; Sigma) or, as controls, equal concentrations of OVA257–264 or OVA (Grade VII, Sigma). Plates were incubated for 72 h at +37°C and pulsed with 1 μCi [3H]thymidine for the last 6 h. Plates were harvested using a semi-automated cell-harvester (Tomtech MACH III, Fisher Scientific, Orange, CT, USA). In parallel, proliferation tests were performedfor draining lymph node lymphocytes of NOD mice that had previously been immunized with either GAD524–543 or insulin (s.c. in emulsified CFA) to ensure that antigens and conditions used were optimal.
4.11 Statistical analysis
For statistical analysis, GraphPad Prism® software was used. The tests used in the analysis of different experiments are indicated in the figure legends.
Acknowledgements
This work was supported by the Finnish Academy (grant # 47864), the Sigrid Juselius Foundation, the Technology Development Center of Finland, the Finnish Diabetes Research Foundation, the Finnish Cultural Foundation, the Juvenile Diabetes Research Foundation and the Turku Graduate School of Biomedical Sciences. Ms Maritta Pohjansalo is thanked for technical assistance in TaqMan analyses and Mrs Anne Sovikoski-Georgieva for secretarial assistance.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




