Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection
Abstract
The present study was designed to elucidate the role of Vα14+ NKT cells in the host defense against pulmonary infection with Streptococcus pneumoniae using Jα281 gene-disrupted mice (Jα281KO mice) that lacked this lymphocyte subset. In these mice, pneumococcal infection was severely exacerbated, as shown by the shorter survival time and marked increase of live bacteria in the lung compared to wild-type (WT) mice. The proportion of Vα14+ NKT cells, detected by an α-galactosylceramide (α-GalCer)-loaded CD1d tetramer, increased in thelung after S. pneumoniae infection. This increase was significantly reduced in mice with a genetic disruption of monocyte chemotactic protein (MCP)-1, which was produced in the early phaseof infection in WT mice. In the lungs of Jα281KO mice, the number of neutrophils was significantly lower at 12 h than that in WT mice. In support of this finding, macrophage inflammatory protein (MIP)-2 and TNF-α synthesis in infected lungs was significantly reduced at 3 h and at both 3 and 6 h, respectively, in Jα281KO mice, compared to WT mice. In addition, treatment of mice with α-GalCer significantly improved the outcome of this infection. Our results demonstrated MCP-1-dependent recruitment of Vα14+ NKT cells and their critical role in early host protection against S. pneumoniae by promoting the trafficking of neutrophils to the site of infection.
Abbreviations:
-
- α-GalCer:
-
α-Galactosylceramide
-
- KO:
-
Knockout
-
- MCP:
-
Monocyte chemotactic protein
-
- MIP-2:
-
Macrophage inflammatory protein-2
-
- WT:
-
Wild-type
1 Introduction
Streptococcus pneumoniae, an extracellular gram-positive bacterium, is a leading causative agent of community-acquired pneumonia and frequently causes severe pneumonia and meningitis in elderly people 1, 2. This infection remains a serious problem, even in this era when antimicrobial chemotherapy is well developed, because of the emergence andspread of penicillin-resistant S. pneumoniae 3. Therefore, a more effective vaccine against infection with this organism has been sought, and for this purpose it is important to clarify the precise mechanism of host protection against this infection.
Neutrophils play a central role in eradicating extracellular bacteria via an oxygen radical-mediated killing mechanism 4. Pneumonia caused by infectious pathogens is characterized by massive accumulation of neutrophils in the alveolar spaces. Exacerbated pneumonia is observed under conditions of suppression of the CXC chemokine receptor CXCR2 and its ligands, such as macrophage inflammatory protein (MIP)-2 and lungkine 5–8, and pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 9–13, and is also associated with impaired recruitment of these inflammatory leukocytes to the site of infection 5–8, 11, 12. On the other hand, there have been few reports regarding the role of other innate immune cells in the host defense against these bacteria. Moore and co-workers 14 demonstrated that γ δ T cells are critical for survival and pro-inflammatory cytokine production during Klebsiella pneumoniae infection. NK cells were speculated to regulate the humoral immunity-mediated host protection against S. pneumoniae infection by decreasing the production of antibodies against T cell-independent antigens 15.
Natural killer T (NKT) cells, which express not only T cell receptors but also NK markers, have been identified as a novel lymphocyte population that acts in innate immune responses. These cells are characterized by an invariant TCR Vα chain, consisting of a Vα14-Jα281 gene segment and highly skewed Vβ chains, Vβ8.2, 7 and 2 in mice 16–18. Although the natural ligand for these cells remains to be defined, a synthetic glycolipid, α-galactosylceramide (α-GalCer), presented in the context of CD1d, has been demonstrated to induce the production of both interferon (IFN)-γ and IL-4 in a prompt manner 16–19. Previous investigations have provided three different findings with respect to the role of NKT cells in host defense to infections. Firstly, the clinical course of Mycobacterium tuberculosis and Salmonella choleraesuis infection is not much influenced by manipulations designed to deplete NKT cells, although activation of these cells by α-GalCer results in the enhanced host defense to the former pathogen 20–24. Secondly, infection with Listeria monocytogenes or Toxoplasma gondii was improved by suppressing the activity of NKT cells 25, 26. Finally, mice genetically lacking NKT cells were more susceptible to Leishmania major and Cryptococcus neoformans infection than control mice 27, 28.
The role of NKT cells in host defenses against extracellularly growing bacteria has not been elucidated, except for the recent report on Pseudomonas aeruginosa infection by Nieuwenhuis et al. 29. In the present study, we elucidated the role of NKT cells in host resistance to S. pneumoniae infection using Jα281 gene-knockout (KO) mice, which lacked Vα14+ NKT cells. We demonstrated that these mice were profoundly susceptible to this infection, probably due to reduced production of MIP-2 and TNF-α and impaired recruitment of neutrophils. We also addressed the mechanism of NKT cell increase in the lungs after intratracheal instillation of S. pneumoniae by testing the role of monocyte chemotactic protein (MCP)-1, which is thought to contribute to the accumulation of NKT cells 28.
2 Results
2.1 Impaired host resistance to S. pneumonia infection in Vα14+ NKT cell-deficient mice
In the first step, we defined the role of NKT cells in host protection against S. pneumonia by comparing the clinical course of infection caused by this microbial pathogen between wild-type (WT) and Jα281KO mice lacking Vα14+ NKT cells. As shown in Fig. 1A, whereas 75% of WT mice survived the pulmonary infection during the observation period, the survival time of Vα14+ NKT cell-deficient mice was significantly shorter and 87.5% of these mice were dead by day 7 post-infection. In addition, the number of live bacteria was 10,000- to 100,000-fold higher in Jα281KO mice than in WT mice on day 3 after S. pneumonia infection (Fig. 1B). These results clearly demonstrate that Vα14+ NKT cells play a critical role in the host defense to this microbial pathogen.
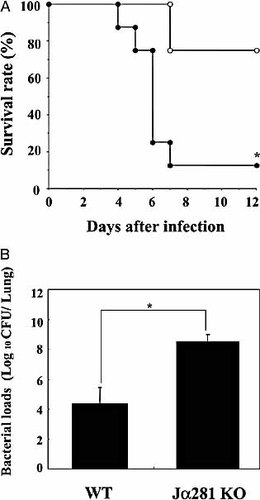
Clinical course of S. pneumoniae infection in Vα14+ NKT cell-deficient mice. WT and Jα281KO mice were infected intratracheally with S. pneumoniae. (A) The number of live mice was noted daily. Open circles, WT (n=8); closed circles, Jα281KO mice (n=8). (B) The number of viable colonies in the lungs was counted on day 3 post-infection. Each bar represents the mean ± SD of six mice. *;p<0.05, compared to WT mice.
2.2 MCP-1-dependent increase of Vα14+ NKT cells in the lung after infection with S. pneumoniae
In order to elucidate the mechanism of action of Vα14+ NKT cells in host defense, we examined whether these cells increased in the lung after infection with S. pneumoniae. As shown in Fig. 2, Vα14+ NKT cells, as shown by the cells bound to α-GalCer-loaded CD1d tetramer 30, comprised only 0.2±0.1% (n=5) of the lung lymphocytes before infection, but their proportion was increased to 0.7±0.2% (n=5) at 6 h post-infection. In contrast, S. pneumoniae infection did not increase the proportion of cells binding to α-GalCer-unloaded CD1d tetramer used as a control. On the other hand, NK1.1+ T cells, double-positive for TCRα β and NK1.1, increased from 0.6±0.2% (n=5) to 1.4±0.4% (n=5) in the lungs at 6 h post-infection (data not shown).
To define the contribution of MCP-1 to the increase of Vα14+ NKT cells, we compared the proportion of these cells in the lung at 6 h after infection with S. pneumonie between MCP-1KO and WT mice. As shown in Fig. 3, the proportion of Vα14+ NKT cells was significantly reduced in MCP-1KO mice compared to that in WT mice. These results demonstrated the contribution of MCP-1 to the increase of Vα14+ NKT cells at the site of infection with S. pneumoniae. In support of this conclusion, production of MCP-1 commenced to increase at 1 or 3 h post-infection, which almost paralleled the increase of NKT cells in the lung (Fig. 4).
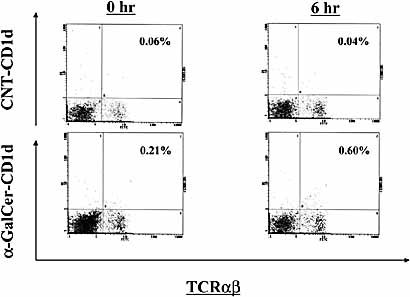
Increase of Vα14+ NKT cells in lungs after S. pneumoniae infection. WT mice were infected intratracheally with S. pneumoniae. The lung leukocytes prepared before and 6 h after infection were stained with FITC-anti-TCRα β and PE-α-GalCer-loaded or -unloaded CD1d tetramer. The lymphocyte population was analyzed by flow cytometry. Each histogram shows the representative staining profile of five mice. CNT-CD1d, unloaded CD1d tetramer; α-GalCer-CD1d, α-GalCer-loaded CD1d tetramer.
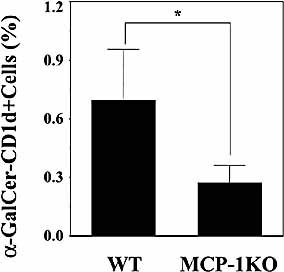
MCP-1-dependent increase of Vα14+ NKT cells in lungs after S. pneumoniae infection. WT or MCP-1KO mice were infected intratracheally with S. pneumoniae. The lung leukocytes were stained with FITC-anti-TCRα β and PE-α-GalCer-loaded or -unloaded CD1d tetramer at 6 h post-infection. The lymphocyte population was analyzed by flow cytometry. Each column represents the mean ± SD of five mice. α-GalCer-CD1d+ cells: cells stained with α-GalCer-loaded CD1d tetramer. *;p<0.05, compared to WT mice.
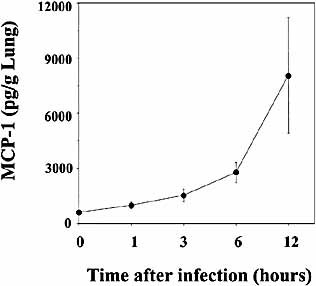
Production of MCP-1 in lungs after infection with S. pneumoniae. WT mice were infected intratracheally with S. pneumoniae. The lung homogenates were prepared before and 1, 3, 6 and 12 h after infection, and their concentrations of MCP-1 were measured. The results are expressed as the total amount/g lung. Each symbol represents the mean ± SD of six mice.
2.3 Reduced accumulation of neutrophils after infection with S. pneumoniae in Vα14+ NKT cell-deficient mice
Neutrophils play a central role in eradicating infection with S. pneumoniae 4, 31. To further clarify the mechanism of the contribution of Vα14+ NKT cells to host defense, we examined how the lack of these cells affected accumulation of neutrophils in the lung after this infection. As shown in Fig. 5, the number of accumulating neutrophils was significantly lower at 12 h in Jα281KO mice than that in WT mice.
MIP-2 is a major chemokine that attracts neutrophils to inflamed tissue 32, 33. TNF-α facilitates the adhesion of neutrophils to vascular endothelial cells by enhancing the expression of adhesion molecules, such as ICAM-1, vascular cell adhesion molecule (VCAM)-1 and E-selectin, which results in the increased accumulation of these cells 34, 35. Therefore, we compared the production of these cytokines in the lung after infection with S. pneumoniae, between Jα281KO and WT mice. As shown in Fig. 6A, MIP-2 production was detected as early as at 1 h and reached a peak level at 6 h in the lung homogenates of infected WT mice. Such production was significantly reduced in Jα281KO mice at 3 h, when compared to WT mice. Similar kinetics was observed in the production of TNF-α, and Jα281KO mice produced a significantly smaller amount of this cytokine at both 3 and 6 h than WT mice (Fig. 6B).
On the other hand, NKT cells are known to produce large amounts of IFN-γ and IL-4 in a very rapid manner when stimulated via antigen receptor 16–18. In the same series of experiments, production of these cytokines was not reduced by genetic depletion of Vα14+ NKT cells (data not shown).
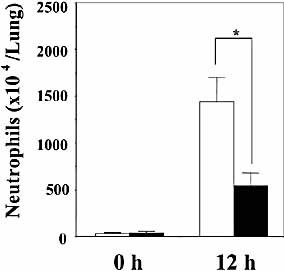
Accumulation of neutrophils in lungs after infection with S. pneumoniae. WT and Jα281KO mice were infected intratracheally with S. pneumoniae. The lung leukocytes prepared before and 12 h after infection were centrifuged onto a glass slide and stained using the May-Giemsa technique. The total number of neutrophils in lung was examined. Each column represents the mean ± SD of six mice. Open column, WT; closed column, Jα281KO mice. *;p<0.05, compared to WT mice.
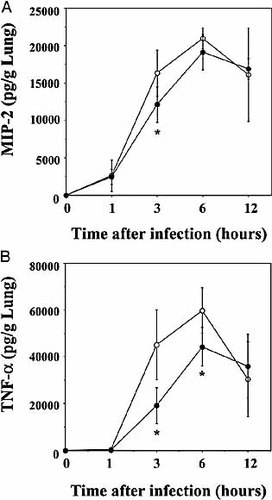
Production of inflammatory cytokines in lungs after infection with S. pneumoniae. WT and Jα281KO mice were inoculated intratracheally with S. pneumoniae. The lung homogenates were prepared before and 1, 3, 6 and 12 h after infection, and their concentrations of MIP-2 (A) and TNF-α (B) were measured. The results are expressed as the total amount/g lung. Each symbol represents the mean ± SD of six mice. Open circles, WT; closed circles, Jα281KO mice. *;p<0.05, compared to WT mice.
2.4 Protective effect of α-GalCer treatment against S. pneumoniae infection
The present findings clearly demonstrated the important role of Vα14+ NKT cells in host protection against S. pneumoniae infection. To further confirm this conclusion, infected WT mice were treated with α-GalCer, which specifically activates Vα14+ NKT cells 16–19, and its effect on the outcome of infection was examined. As shown in Fig. 7, α-GalCer treatment significantly reduced the number of live bacteria in lungs on day 3 after infection with S. pneumoniae, when compared to the control treatment. The protective effect of this treatment was not observed in Vα14+ NKT cell-deficient mice (data not shown), confirming the direct action of α-GalCer on this particular subset of NKT cells.
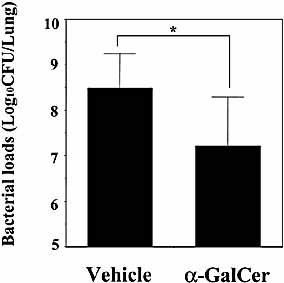
Effect of α-GalCer treatment on S. pneumoniae infection. WT mice were infected intratracheally with S. pneumoniae. The mice were injected once intraperitoneally with α-GalCer (2 μg/mouse) or vehicle on the day of infection. The number of viable colonies in the lungs was counted on day 3 post-infection. Each column represents the mean ± SD of six mice. *;p<0.05, compared to vehicle treatment.
3 Discussion
The role of NKT cells in host defense against infection differs from microbe to microbe 20–29. Among infections with extracellularly growing bacteria, Nieuwenhuis and co-workers 29 were the first group to report the important role of NKT cells in protecting mice from lung infection with P. aeruginosa. They observed impaired clearance of bacteria in CD1dKO mice that lacked whole subsets of NKT cells. In the present study, we obtained similar findings in a mouse model of pneumococcal pneumonia using Jα218KO mice lacking Vα14+ NKT cells, a major subset of these cells 16–18. Vα14+ NKT cell-deficient mice succumbed to S. pneumoniae infection with a marked increase in the lung bacterial loads compared with the control mice. These results clearly indicated the important role of Vα14+ NKT cells in the host defense to infection with S. pneumoniae. Furthermore, this conclusion was supported by our data showing that activation of Vα14+ NKT cells by α-GalCer treatment resulted in the acceleration of bacterial clearance from the infected lung.
We recently demonstrated that host protection against infection with C. neoformans, an intracellular fungal pathogen 36, was attenuated in Vα14+ NKT cell-deficient mice, as shown by the delayed clearance of this microorganism from the lung 28. Surprisingly, the impairment of host protection in Vα14+ NKT cell-deficient mice was much more profound against S. pneumoniae than against C. neoformans: the microbial loads in lung were 10,000-to 100,000-fold and less than 10-fold increased, respectively, when compared with those in WT mice. In P. aeruginosa infection, the number of live bacteria was reported to be ∼100-fold higher in CD1dKO mice than that in control mice 29.
In contrast, several investigators reported that CD1dKO and Jα281KO mice were not susceptible to infection with intracellular bacteria, M. tuberculosis and S. choleraesuis 20–23, indicating a less active contribution of NKT cells to the host defense against these bacteria. For other intracellular microorganisms, such as L. monocytogenes and T. gondii, the infection rate was reported to be improved by manipulations that suppress NKT cell activities 25, 26. These observations appear to suggest that NKT cells play a more important role in protecting mice from extracellular than intracellular microorganisms. However, it should be noted that activation of Vα14+ NKT cells by α-GalCer treatment has been reported to protect mice from M. tuberculosis infection 24. Further investigations are necessary to elucidate this possibility.
Both NK cells and NKT cells increased in the lung after infection with S. pneumoniae, similar to the observation in cryptococcal infection 28. Such an increase was observed as early as at 6 h post-infection, suggesting accumulation from the circulation, rather than local proliferation at the site of infection, as the mechanism to account for the S. pneumoniae-induced increase of these cells. Migration of NK cells is critically regulated by a variety of chemokines, including MCP-1, MCP-2, MCP-3, MIP-1α, regulated upon activation, normal T cell expressed and secreted (RANTES), IFN-inducible protein (IP)-10 and lymphotactin 37–42. In the present study, the increase in NK cells in the lung caused by S. pneumoniae infection was hampered in mice lacking MCP-1 production, which indicated the contribution of this chemokine. On the other hand, chemokines that recruit NKT cells have not been elucidated, with the exception of MIP-2 43. Recently, we identified MCP-1 as a chemoattractant for these cells by indicating that MCP-1KO mice showed a reduced increase of both total and Vα14+ NKT cells in the lung after infection with C. neoformans 28. Consistent with our earlier observations, S. pneumoniae infection attracted a smaller number of NKT cells and NK cells into the lungs of MCP-1KO mice than of WT mice, suggesting that a similar mechanism operated in the recruitment of these cells under infectious conditions by two microbial pathogens. In support of this conclusion, the kinetics of MCP-1 production paralleled the increase of NKT cells after infection, and the instillation of mouse recombinant MCP-1 into the trachea recruited both NK cells and NKT cells in the lung (data not shown). In a recent study, Kim et al. 44 demonstrated that human NKT cells expressed high levels of CCR2, a receptor for MCP-1, and migrated in vitro in response to this chemokine. Thus, in addition to MIP-2, MCP-1 is also a major chemokine that attracts NKT cells to the site of infection.
It has been well documented that neutrophils play a central role in eradicating extracellular bacteria, such as S. pneumoniae 4, 31. Neutrophils migrate from blood vessels, are recruited into the infected site, and phagocytose these bacteria. During this process, TNF-α facilitates the adhesion of neutrophils to vascular endothelial cells by enhancing their expression of adhesion molecules, such as ICAM-1, VCAM-1 and E-selectin 33, 34, and MIP-2 directly induces trafficking of these cells. In addition, TNF-α potentiates their killing activities against microbial pathogens 45–47. Many investigators have indicated the important role of these molecules in host protection against extracellular bacteria 6, 7, 9–11. In thepresent study, exacerbated infection by S. pneumoniae in Vα14+ NKT cell-deficient mice correlated well with reduced production of TNF-α and MIP-2 and hampered recruitment of neutrophils in the infected organ. Similarly, CD1dKO mice, which succumbed to P. aeruginosa infection, produced less MIP-2 and showed poor accumulation of neutrophils in the infected lung 29. These results suggest that neutrophil dysfunction caused the exacerbation of S. pneumoniae infection under conditions of a lack of Vα14+ NKT cells.
Previous investigations have shown that NKT cells are strongly associated with the differentiation of Th1 and Th2 cells via their production of IFN-γ and IL-4 16–18. Vα14 TCR-transgenic mice showed elevated serum levels of IgE and IL-4 48, and activation of Vα14+ NKT cells by α-GalCer induced a T cell response to ovalbumin (OVA) polarized toward the Th2-dominant condition 49. In contrast, activation of Vα14+ NKT cells by α-GalCer led to the rapid production of IFN-γ by themselves and other bystander cells, such as NK cells, in vitro 50 and suppressed in vivo the Th2 differentiation and subsequent IgE synthesis induced by OVA immunization or infection with Nippostrongylus brasiliensis through the induction of IFN-γ production 51. In our recent study, Vα14+ NKT cells were found to play a critical role in the induction of Th1 responses, such as IFN-γ production and delayed-type hypersensitivity response, during infection with C. neoformans. Interestingly, in the very early phase (until 12 h) of S. pneumoniae infection, Vα14+ NKT cells were not likely to alter the balance of Th1 and Th2 responses, because production of IFN-γ and IL-4was not much different between WT and Jα281KO mice. However, further investigations are required to define the role of these cells in the differentiation of Th1 and Th2 cells in the later phaseof this infection.
In conclusion, in the present study we demonstrated the MCP-1-dependent accumulation of Vα14+ NKT cells in the lungs after infection with S. pneumoniae and their important roles in host resistance to this infection. This particular subset of NKT cells was suggested to promote both recruitment of neutrophils into infected sites and their killing activity against thisbacterial pathogen through the enhancement of TNF-α and MIP-2 production. At present, the mechanism(s) involved in the synthesis of these cytokines in Vα14+ NKT cells remain(s) to be elucidated. Our present findings about the functional interaction between NKT cells and neutrophil-mediated host defense to S. pneumoniae infection have novel implications for the development of more effective vaccines against invasive pneumococcal diseases.
4 Materials and methods
4.1 Animals
Vα14+ NKT cell-deficient mice (Jα281KO mice) were established by targeted deletion of the Jα281 gene segment 52 and backcrossed eight times with C57Bl/6mice. MCP-1-deficient mice (MCP-1KO mice) with a genetic background of C57BL/6 mice 53 were kindly provided by B. J. Rollins (Harvard Medical School, Boston, MA). These mice werebred in a pathogen-free environment in the Laboratory Animal Center for Biomedical Science, University of the Ryukyus. C57BL/6 mice were purchased from Charles River Japan (Osaka, Japan) and used as control WT animals. All mice were used at 8–15 weeks of age. All experimental protocols described in the present study were approved by the Ethics Review Committee for Animal Experimentation of our university.
4.2 Bacteria
A clinical strain of S. pneumoniae, designated as URF918, was established from a patient with pneumococcal pneumonia. The bacteria were cultured in Todd-Hewitt broth (Difco, Detroit, MI) at 37°C in a 5% CO2 incubator, harvested at a mid-log phase and then washed twice in phosphate-buffered saline (PBS). The inoculum was prepared at 2×107–6×107 CFU/ml based on its turbidity. To induce pulmonary infection, mice were anesthetized by intraperitoneal injection of 70 mg/kg of pentobarbital (Abbott Lab., North Chicago, IL) and restrained on a smallboard. Live S. pneumoniae were inoculated at 50 μl per mouse by insertion of a 25-gauge blunt needle into and parallel to the trachea. In every experiment, quantification culture was performed to confirm the inoculation dose.
4.3 Preparation of pulmonary intraparenchymal leukocytes
Pulmonary intraparenchymal leukocytes were prepared as described previously 54. Briefly, the chest of the mouse was opened, and the lung vascular bed was flushed by injecting 3 ml of chilled physiological saline into the right ventricle. The lungs were then excised and washed in physiological saline. The lungs, teased with the stainless mesh, were incubated in RPMI 1640 (Gibco BRL, Grand Island, NY) with 5% of fetal calf serum (FCS; Cansera, Rexdale, Canada), 100 U/ml penicillin G, 100 μg/ml streptomycin, 10 mM Hepes, 50 μM 2-mercaptoethanol, and 2 mM L-glutamine, containing 20 U/ml collagenase (Sigma Chemical Co., St. Louis, MO) and 1 μg/ml DNaseI (Sigma).
After incubation for 60 min at 37°C with vigorous shaking, the tissue fragments and the majority of dead cells were removed by passing through the 50-μm nylon mesh. After centrifugation, the cell pellet was resuspended in 4 ml of 40% (v/v) Percoll (Pharmacia, Uppsala, Sweden) and layered onto 4 ml of 80% (v/v) Percoll. After centrifugation at 600×g for 20 min at 15°C, the cells at the interface were collected, washed three times and counted with a hemocytometer. About 1×105 cells were centrifuged onto a glass slide at 800 rpm for 3 min using Auto Smear CF-12D(Sakura Co., Tokyo, Japan), and stained using the May-Giemsa technique. To analyze the leukocyte fraction, at least 300 cells were examined photomicroscopically.
4.4 Flow cytometric analysis
Cells were pre-incubated with anti-FcγRIII mAb, prepared by a protein G column kit (Kirkegaard & Perry Laboratories, Gaithersburg, MD) from the culture supernatants of hybridoma cells (clone 2.4G2), on ice for 15 min in PBS containing 1% FCS and 0.1% sodium azide, stained with FITC-conjugated anti-TCRα β mAb (clone H57–597; PharMingen, San Diego, CA) and PE-conjugated α-GalCer-loaded or -unloaded CD1d tetramer (kind gifts of M. Kronenberg, La Jolla Institute for Allergy and Immunology, San Diego, CA) 30 in the presence of 5 μg/ml propidium iodide (PI; Molecular Probes, Eugene, OR) for 25 min and then washed three times in the same buffer. Isotype-matched irrelevant antibodies were used for control staining. In some experiments, cells were similarly stained with FITC-conjugated anti-TCRα β mAb and PE-conjugated anti-NK1.1 mAb (clone PK136; PharMingen). The stained cells were analyzed using an EPICS XL flow cytometer (BeckmanCoulter Inc., Fullerton, CA). Data were collected from 15,000 to 20,000 individual cells using parameters of forward scatter and side scatter to set a gate on the lymphocyte population and of PI staining togate out the dead cells.
4.5 Treatment with α-GalCer
α-GalCer was provided by Kirin Brewery Co. (Gunma, Japan) and prepared as described previously 55. The stock solution of α-GalCer (220 μg/ml in 0.5% polysorbate 20 in normal saline) was diluted to 10 μg/ml with normal saline. Polysorbate 20 solution (0.02% in normal saline) was used as a control vehicle solution. α-GalCer or control solution was injected intraperitoneally at 200 μl per mouse on the day of infection.
4.6 Enumeration of viable S. pneumoniae
Mice were sacrificed on day 3 after infection, and lungs were dissected carefully and excised, and then separately homogenized in 10 ml of half saline by teasing with a stainless mesh at room temperature. The homogenates, appropriately diluted with half saline, were inoculated at 100 μl on 3% sheep blood Mueller-Hinton agar plates and cultured for 18 h, followed by counting the number of colonies.
4.7 Measurement of cytokines
The lungs were homogenized in 2 ml of PBS, and the concentrations of MCP-1, MIP-2, TNF-α, IFN-γ and IL-4 were measured by respective ELISA kits (MCP-1: BioSource International Inc., Camarillo, CA; MIP-2 and TNF-α: R&D Systems, Minneapolis, MN; IFN-γ and IL-4: Endogen Inc., Cambridge, MA). The detection limits of assays for MCP-1, MIP-2, TNF-α, IFN-γ and IL-4 were 9, 1.5, 5.1, 15 and 5 pg/ml, respectively.
4.8 Statistical analysis
Analysis of data was conducted using Statview II software (Abacus Concept Inc., Berkeley, CA) on a Macintosh computer. Data were expressed as mean ± standard deviation (SD). Statistical analysis between groups was performed using the ANOVA test with a post-hoc analysis (Fisher PLSD test). Survival data were analyzed using the generalized Wilcoxon test. A p value less than 0.05 was considered significant.
Acknowledgements
The authors thank Dr. B. J. Rollins (Harvard Medical School, Boston, MA) for the kind gift of MCP-1-deficient mice and Dr. M. Kronenberg (La Jolla Institutefor Allergy and Immunology, San Diego, CA) for the kind gift of α-GalCer-loaded and -unloaded CD1d tetramers. This work was supported in part by Grants from the Ministry of Health and Welfare, Japan.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




