Role of the αI domain in ligand binding by integrin αEβ7
Abstract
The I domain of integrin αE was modeled on the crystal structure of that in CD11b and mutated to produce an open (high affinity) or closed (low affinity) conformation. K562 transfectants expressing mutant αE and wild-type β7 were tested for adhesion to E-cadherin-Fc. Downward displacement of the C terminus of the αI domain with a disulfide bridge enhanced adhesion and Mn2+ dependency. Adhesion greatly exceeded that observed using wild type integrin under similar conditions. The closed integrin gave poor adhesion which was greatly improved by PMA-induced clustering. Blocking β7 function with a βI domain-specific antibody inhibited the wild-type but not the locked open integrin. Isolated open αI domain expressed on K562 cells showed strong Mn2+-dependent adhesion to E-cadherin, whereas the wild-type version was ineffective. αEβ7 was shown to bind to monomeric E-cadherin but to only one component of dimeric E-cadherin. Finally, we report that M290, a function-blocking antibody, bound to a conformation-sensitive epitope near the rim of the αI domain MIDAS and recognized wild-type and closed αI domain but not the open conformation. The results broadly support the paradigm for affinity regulation by conformational change that has been established for β2 integrins. Nevertheless, for αE, the fully open conformation may represent an extreme situation that does not occur physiologically.
Abbreviation:
-
- MIDAS:
-
Metal ion-dependent adhesion site
1 Introduction
Recently there have been major advances in understanding integrin structure and function. Crystal structures are now known for the I domains of integrin α chains α1, α2, αL, αM and αX 1–6 and for α2 and αL in complex with their respective ligands, collagen and ICAM-1 7, 8. These structures have provided persuasive evidence that αI domains undergo a major conformational change associated with ligand binding by the metal ion-dependent adhesion site (MIDAS). Nine of the eighteenintegrin α subunits do not contain an αI domain. For these, the recent crystal structures of integrin αVβ3 9, 10 have revealed a mechanism for ligand binding that involves contact sites on both α and β subunits. The β subunit also contains an I domain, with a MIDAS, which undergoes a similar conformational change to that in the αI domain 11, 12. A central focus of current integrin research is to understand how conformational changes in the I domains that accompany ligand binding are controlled by the cell and how the two types of I domain function together.
Cumulative evidence from several incisive studies, particularly on the αI domain-containing integrins αLβ2 (LFA-1) and αMβ2 (Mac-1) and on αVβ3, is beginning to provide a paradigm for the regulation of integrin affinity 8, 13–17. Allosteric changes initiated from inside or outside the cell are propagated along the integrin ‘stalk’ to modulate affinity and to link ligand binding with intracellular signaling pathways. I domains in the α and β subunits are in close apposition in the integrin holoreceptor and it has been proposed that interaction between the two domains serves to modulate the conformation of the ligand-binding site in the αI domain 18. One particularly helpful approach has been to bypass allosteric regulation of the αI domain by introducing mutations. Guided by crystal structures, it has been possible to lock the αI domain of αL, αM and αX into an open, active, conformation and to test for ligand binding activity using the complete integrin holoreceptor or the isolated αI domain. This strategy has greatly increased the affinity of the integrin for its ligand and, in the case of αLβ2, has demonstrated that the αI domain provides the sole contact site for ICAM-1 19–23. It has been proposed that this picture provides a paradigm for all αI domain-containing integrins 8. Nevertheless, several studies suggest that the situation with αMβ2 ismore complex, the βI domain being directly involved in recognition of certain ligands, but also performing a regulatory function on the αI domain 24, 25.
The subject of this study, integrin αEβ7, is an αI domain-containing integrin which contributes to mucosal retention of T cells and also plays a role in the effector phase of allograft rejection 26, 27. The only characterized ligand for this integrin is E-cadherin, which is engaged by αEβ7 through a binding site on the distal surface of the N-terminal domain (EC1) of the cadherin molecule. A persuasive model for αE I domain docking to this site has been proposed 28. E-cadherin is thought to be expressed on the cell surface as a cis-dimer formed by contacts between adjacent EC1 domains 29 and recent studies have suggested that dimerization may be required for recognition by αEβ7 30. This could be explained by the integrin engaging each component of the dimer via two or more contact sites. The present work was undertaken to investigate in more detail molecular contacts required for the interaction between αEβ7 and E-cadherin and to broaden the current perspective on regulation of αI domain-containing integrins beyond the β2 family. During this investigation we found that an αE function-blocking antibody, M290, recognizes an epitope in the αI domain, near the MIDAS, that is sensitive to conformational changes which modulate ligand binding. Antibodies which bind close to the MIDAS are unusual and could be useful in addressing a central question in integrin research, namely, whether the cell can regulate the conformation of the αI domain in advance of ligand binding through ‘inside out’ signaling.
2 Results
2.1 Downward displacement of the α7 helix in the αI domain enhances cell adhesion
Fig. 1a, b shows a model of the αE I domain in open and closed conformations with the α7 helix (yellow) in the ‘down’ or ‘up’ positions, respectively. The amino acids mutated to form disulfide bonds to lock the two conformations are highlighted (dark blue, pale blue, red). Two additional residues were mutated: F319 (purple) which is predicted to undergo a major increase in solvent accessibility in the open conformation 3 and D317 (green) which undergoes minor displacement. D317 was chosen because mutations to alanine in the same position in human αE (E325A) 28 or to lysine in αM (D273K) 3 were reported to enhance adhesion. Fig. 1c shows the levels of expressionof αEβ7 or isolated αI domain on K562 transfectants used for adhesion tests. Transfectants expressing αE also expressed the β7 subunit (not shown).
K562 cells were tested for adhesion to E-cadherin-Fc in the presence of Ca2+ and Mg2+, with or without Mn2+. Fig. 2a, b demonstrates that locking the domain open by displacing the α7 helix in the down position (F341C/A348C) greatly increased adhesion, F319R had a similar effect in a majority of experiments but was not investigated in such detail as the disulfide mutations. Sensitivity to Mn2+ was increased in the activated mutants. The locked-closed conformation (V343C/A348C) slightly inhibited adhesion and the mutation D317A had little effect compared with wild type integrin. Adhesion was completely blocked by antibody ECCD2 which is specific for the N-terminal domain of E-cadherin. In a separate experiment E-cadherin-Fc was titrated to demonstrate the effect of the two disulfide-locked conformations compared with wild type. Open and closed conformations increased or decreased adhesion, respectively, at all concentrations (Fig. 2b).
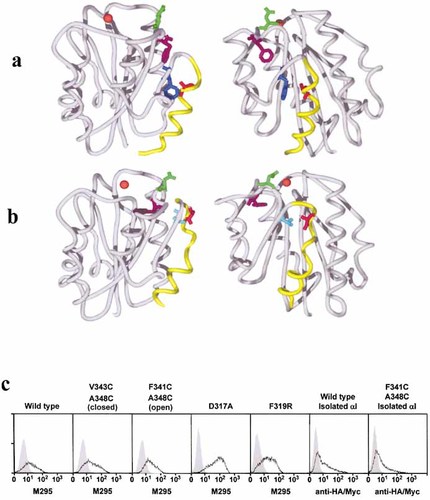
Mouse αE I domain in open (a) and closed (b) conformation modeled on CD11b αI domain structures. Images on the right are rotated anticlockwise through 900. The C-terminal α7 helix is in yellow. Mutated residues: dark blue, F341C; pale blue, V343C; red, A348C; purple, F319R; green, D317A. The orange sphere represents the divalent cation in the MIDAS of the open and closed structures of CD11b. (c) Expression of wild-type or mutant αEβ7 or isolated I domain on K562 cells. Cells were stained with the αE-specific antibody M295 or, for isolated I domain, with epitope tag antibodies. Shaded profiles represent negative controls with untransfected cells.
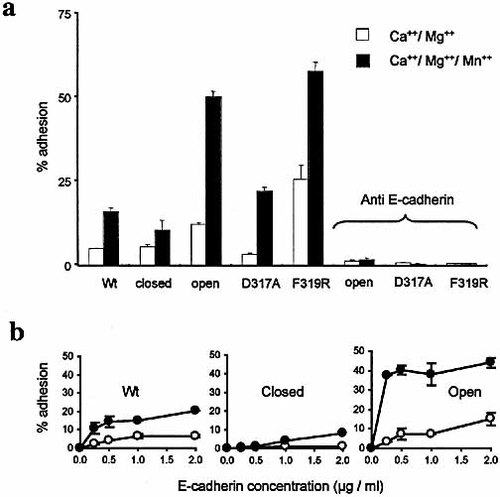
(a) Adhesion of K562 αEβ7 wild-type and mutant transfectants to E-cadherin-Fc (1 μg/ml). The α7 helix of the αI domain has been locked in the open (F341C, A348C) or closed (V343C, A348C) positions. Results with two additional mutations, F319R and D317A, are included. Divalent cations (1 mM) used in the assay buffer are shown. Inhibitory antibody (ECCD2) to E-cadherin demonstrates specificity of adhesion. (b) Wild-type, open and closed conformations are compared using a range of concentrations of E-cadherin-Fc (open symbols, Ca2+/Mg2+; closed symbols Ca2+/Mg2+/Mn2+).
2.2 Stimulation with PMA partly compensates for an unfavorable conformation in the αI domain
Activation of PKC with PMA is known to increase integrin avidity by promoting integrin clustering on the cell surface 31. To test whether clustering of αEβ7 can override the negative effect of the locked-closed conformation, we compared αEβ7 transfectants bearing locked-open and locked-closed αI domains for adhesion to E-cadherin-Fc. Fig. 3 shows that PMA improved adhesion of both mutants and narrowed the difference between the open and closed conformations.
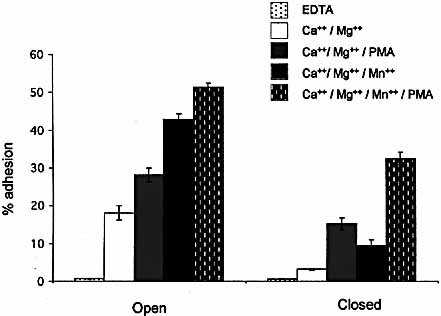
Effect of PMA on adhesion of K562 cells expressing open and closed conformations of αEβ7. Assay plates were coated with E-cadherin-Fc at 1 μg/ml. Divalent cations (1 mM) were included in the assay buffer as indicated.
2.3 Blocking the I domain of the β7 subunit
Antibodies specific for the I domain of the β7 subunit ablate the interaction between α4β7 and MAdCAM-1, VCAM-1 or fibronectin 32 and mutations in the β7 MIDAS had a similar effect on adhesion of αEβ7 to E-cadherin 28. Fig. 4 shows that blocking the β7 I domain with antibody Fib30 inhibited wild-type αEβ7 but had little or no effect on the locked-open form.
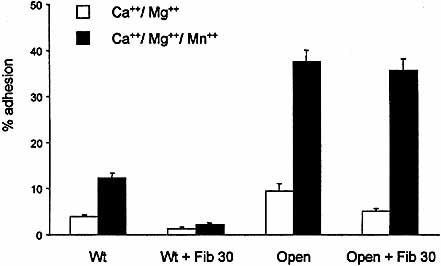
Effect of inhibiting the function of the β7 I domain with mAb Fib 30 on adhesion of K562 cells expressing wild-type and open conformations of αEβ7. Plates were coated with E-cadherin-Fc at 1 μg/ml. Divalent cations (1 mM) were included in the assay buffer as indicated.
2.4 Isolated αE I domain binds to E-cadherin
The αE I domain in the locked-open or wild-type form was expressed in isolation on K562 cells and tested for adhesion to E-cadherin-Fc. Fig. 5 demonstrates that wild-type αI domain showed essentially no adhesion, even in the presence of Mn2+. In contrast, cells bearing the locked-open form showed strong Mn2+-dependent adhesion. Activation of the cells with PMA had little or no additional effect. Adhesion of the open αI domain was shown to be ligand-specific because transfectants failed to adhere to R-cadherin-Fc (Fig. 5) and adhesion to E-cadherin-Fc was inhibited by ECCD2 (not shown).
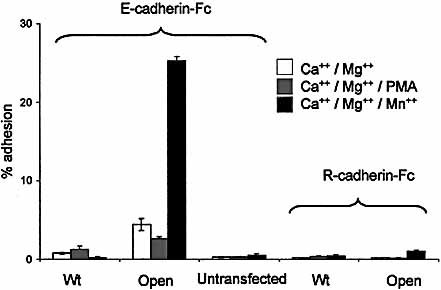
Adhesion of K562 cells expressing isolated αE I domain. Assay plates were coated with E-cadherin-Fc at 1 μg/ml. R-cadherin-Fc was used as a control to demonstrate specificity of adhesion.
2.5 αEβ7 binds to E-cadherin with 1:1 stoichiometry
E-cadherin is normally expressed on the cell surface as a dimer formed by contacts between the N-terminal (EC1) domains of two adjacent molecules 33. The dimerization mechanism is known to operate in E-cadherin-Fc fusion protein 30. To investigate the importance of dimerization in recognition by αEβ7 we tested K562 cells transfected with wild type αEβ7 for adhesion to monomeric E-cadherin or dimeric E-cadherin-Fc. In addition, cadherin dimers were used in which the critical contact residue for the αE MIDAS, E31, was mutated in one, both or neither E-cadherin chain. The dimers were prepared by fusing E-cadherin EC1–5 to an acidic/basic coiled coil leucine zipper rather than to IgFc (Fig. 6a). K562 cells transfected with wild-type αEβ7 were tested for adhesion to E-cadherin in monomeric or dimeric form, titrated to saturation. The monomer was shown to be strikingly more efficient than the dimer at equimolar levels of single cadherin chains (Fig. 6b). This suggests that only one component of the dimer functioned in adhesion. This interpretation was strongly supported by comparing wild-type and mutant zipped dimers (Fig. 6c). The results show that a single E31A mutation in the dimer had little or no effect on adhesion whereas the double mutation prevented it. Taken together, the data show that the αEβ7 interacts with monomeric cadherin and that in dimeric cadherin only one component of the dimer is engaged.
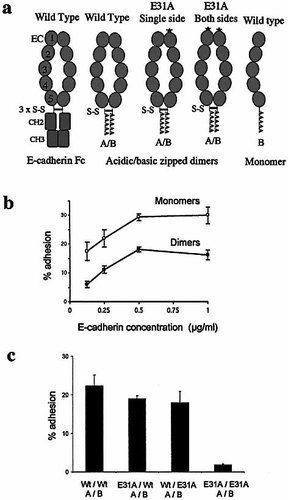
(a) Cartoon of E-cadherin fusion proteins showing the domain organization and the position of disulfide bonds in the Fc-fusion protein and the leucine zipped dimers. Dimerization of E-cadherin at the level of domain EC1 30 is indicated. (b) Adhesion of K562 transfectants expressing wild type αEβ7 to wild-type E-cadherin dimers or monomers attached to the assay plate via mAb DECMA-1 specific for EC4. (c) Adhesion of K562 cells expressing wild-type αEβ7 to acidic/basic (A/B) zipped dimers in which one, both or neither cadherin chain contained the mutation E31A. Assays were conducted in the presence of Ca2+, Mg2+, Mn2+ (1 mM) and PMA(15 ng/ml).
2.6 Antibody M290 binds to wild-type or closed αI domain but not to open conformations
K562 transfectants expressing αEβ7 or the isolated αI domain were tested for reactivity with the αE antibody M290. For comparison, the isolated I domain was detected with epitope tag antibodies and the αE subunit with a non-I domain antibody, M295. Fig. 7 shows that M290 is specific for the αI domain. In αEβ7, M290 reacted with the wild-type and locked-closed αI domain but not the open form nor the F319R mutant. Disulfide bond reduction of the locked-open I domain partially restored M290 reactivity (Fig. 7). The M290 epitope was mapped by introducing point mutations into regions predicted to be sensitive to conformational change, selecting mainly hydrophilic, exposed residues which were unlikely to influence overall conformation of the domain. Residues selected for mutation included some of the 11 amino acids which differ between mouse and rat αE because M290 is a rat mAb which is mouse specific. Mutants were prepared as αI domain-Fc fusion proteins and tested by ELISA for reactivity with M290. Fig. 8a shows that M290 binding was ablated by mutating Gly 291 to Arg, the equivalent residue in rat αE. This single mutation locates the M290 epitope near the MIDAS rim and explains the efficient blocking activity of M290. Our I domain models predict significant conformational change in this region, including 1.4Å displacement of F290 in the open conformation (Fig. 8b).
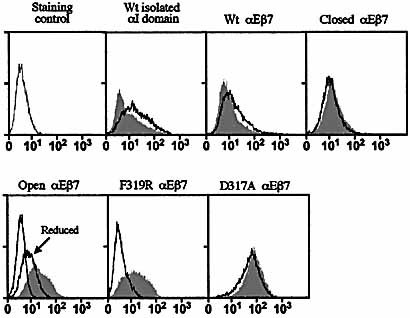
M290 recognizes wild-type and closed, but not open, αI domain conformations. K562 transfectants were stained with epitope tag antibodies for the isolated αI domain or with M295 for αEβ7 transfectants (shaded). Profiles are compared with those given by M290 (black line). Reduction partially restored M290 reactivity in the locked-open form. Reduction did not affect the M295 profile (not shown).
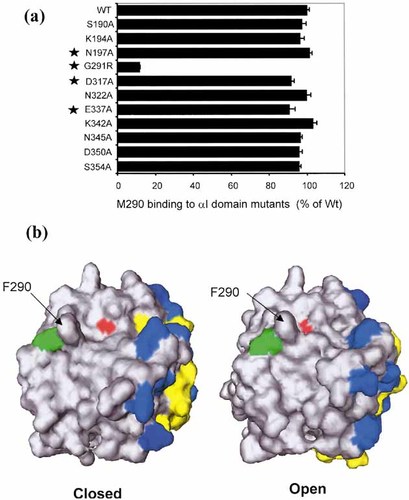
Mapping the M290 binding site. (a) Reactivity of M290 with αI domain mutants. The star symbols identify residues that differ between mouse and rat αE. (b) Surface models of the αI domain in open and closed conformation. The α7 helix is identified in yellow and the MIDAS cation in red. Glycine 291 is green. Blue residues identify point mutations in conformation-sensitive regions which have not affected M290 binding.
3 Discussion
The present results with the αE I domain agree, in general, with findings on β2 integrins 6, 8, 19–21. Nevertheless, differences have emerged which provide a broader perspective on the role of conformational change in integrin function. In addition, questions concerning the possible significance of E-cadherin dimerization in the interaction with αEβ7 have been answered.
First, we have demonstrated that locking the α7 helix of the αE I domain in the open or closed positions enhanced or diminished ligand-binding capacity, respectively. Efforts to obtain direct physicochemical evidence for the formation of the disulfide bonds were frustrated for technical reasons because the αE I domain contains two cysteine residues in the α1βB loop, distant from our mutations, that may form a disulfide bridge. Our molecular model predicted that the β carbon spacings for our cysteine substitutions were optimal for disulfide bond formation. This, considered together with the adhesion data and the antibody binding results, argues persuasively that the substitutions worked as anticipated. Our adhesion results show that downward displacement of the α7 helix increased ligand-binding capacity. There were, however, significant differences from results with β2 integrins. With αL and αM, locking the I domain open rendered the integrin insensitive to the divalent cation used in adhesion tests, Mn2+ offering no advantage over Mg2+ 19, 20. Importantly, in these studies the adhesive performance of the open mutants equalled that of wild-type integrin under conditions of maximal activation with Mn2+ or activating antibody. In our experiments, locking the αI domain open with a disulfide bond increased sensitivity to Mn2+ and produced adhesive activity exceeding the maximum obtained with wild-type under similar conditions. E-cadherin specificity was retained. Similarly, with the isolated αE I domain, adhesion was far more Mn2+-dependent than was the case for αL and αM 19, 20. Possibly the cation sensitivity of the locked-open αE I domain could reflect some flexibility in the region of the MIDAS despite the presence of the disulfide bonds locking the α7 helix. Thus, some conformational change in response to cations could occur. The explanation assumes that disulfide-locked αI domains of β2 integrins are more rigid structures which cannot respond in this way.
We have not made affinity measurements with our αE mutants and therefore cannot be precise about the default conformation of wild-type αE, i.e., whether it is nearer to the open or closed conformations. With αL the wild-type adopts a closed conformation having an affinity 104-fold lower than the open version 21 whereas the equilibrium with αM is much nearer to the open form 34. From our adhesion tests with αE it appears that the locked-open conformation represents an extreme situation that may not be achievable physiologically. It is interesting that in other αI domain-containing integrins the N terminus of the αI domain is tightly tethered to the β propeller domain 13. In αE, post-translational cleavage releases the I domain from this constraint 35 and it is possible that, in the wild-type integrin, the resulting freedom of movement of the domain diminishes affinity regulation by the so-called ‘bell rope’ mechanism 13.
The locked-closed αE conformation was less effective than wild-type in cell adhesion tests using K562 transfectants in the presence of Mg2+ or Mn2+ but this deficiency was partly offset when αEβ7 clustering was stimulated with PMA. In these circumstances the difference in adhesive performance between the locked-open and locked-closed integrin was quite small. This observation emphasizes the overriding importance of clustering in integrin regulation. That the locked-closed version worked at all and showed cation responsiveness could reflect residual flexibility in the MIDAS. It is notable that K562 cells transfected with β2 integrins are insensitive to PMA-induced clustering 36. This has been attributed to a single amino acid change in one of two consensus motifs in the cytoplasmic domain of the β2 subunit 37. Studies on the effects of αI domain conformation in β2 integrins have relied heavily on the use of K562 transfectants and, as far as we are aware, the effect of clustering locked-closed integrins has not previously been reported. The β7 subunit retains both the consensus motifs and, although it has been claimed that β7 integrins are refractory to PMA-induced clustering in K562 cells 36, this was clearly not the case in our experiments.
The prevailing view on the role of the β subunit I domain from studies on β2 integrins is that the βI domain directly contacts the αI domain, partly via the βI MIDAS, and that conformational changes are propagated from one domain to the other 13, 15. A report that mutations in the MIDAS of the β7 subunit in αEβ7 block recognition of E-cadherin 28 is consistent with this view but is also compatible with an alternative interpretation that the β subunit is directly involved in ligand binding. The present observation that blocking antibodies to the βI domain inhibit function of the wild-type αI domain, but not the locked-open form, supports the first interpretation and agrees with earlier findings on LFA-1 19
More direct evidence that the αE I domain provides the sole contact site for E-cadherin comes from our experiments comparing cadherin monomers and dimers. We show definitively that E-cadherin monomer supports αEβ7-mediated adhesion. The principal contact site for the αI domain of αEβ7 is at the tip end of E-cadherin and involves residue E31 in the BC loop. Our present results with acidic/basic leucine ‘zipped’ E-cadherin dimers show that the integrin engages only one of the two EC1 domains available in dimeric E-cadherin. This contrasts with results from similar experiments with LFA-1 and ICAM-1 which showed that dimeric ICAM-1 can bind two LFA-1 molecules 38. Our present finding is explained by a recent NMR study on E-cadherin dimerization 29. In the presence of Ca2+ adjacent EC1 domains interact so closely that steric constraints would preclude simultaneous binding of two αE I domains. The present results and the NMR study invite re-examination of previous findings which suggested that dimerization may be required for engagement of αEβ7 30. This earlier report showed that point mutations which prevent Ca2+ binding in the junction between EC1 and EC2, and ablate Ca2+-dependent dimerization, adversely affect αEβ7 binding, implying that dimerization is required. Crystallographic structures and chemical shift data of E-cadherin EC1 show that the point mutations or the loss of Ca2+ in the domain junction would have little orno affect on the integrin binding site 29, 39. However, with loss of interdomain Ca2+, the domain junction loses rigidity and permits a wide angle of free movement of EC1, relative to the rest of the molecule 29. This could compromise integrin binding and explain the apparent requirement for dimerization.
The finding that M290 binds a conformation-sensitive epitope in the αE I domain that is destroyed by activating mutations is unusual. There are very few function-blocking mAb that bind to sites on or near the α chain MIDAS 40, 41. That reduction of the disulfide bonds in the open conformation did not achieve complete restoration of the M290 epitope suggests that the α7 helix may have been displaced beyond its normal limits of movement. The incomplete effect of reduction cannot be explained by damage to the core of the domain caused byloss of the hydrophobic residue F341 because the single mutation F341C had no effect on M290 binding (data not shown).
The central issue of whether conformational change in integrin αI domains precedes ligand binding as a response to inside-out signaling or, alternatively, is a consequence of it, is yet tobe adequately answered. If the latter is true, the role of the β subunit could be to release the αI domain from structural constraints so as to permit conformational change rather than to enforce it. In either case, our data suggest that the change in conformation that occurs in the wild-type αE I domain after maximal activation may be more subtle than that in our engineered version. We anticipate that studies with M290 may help to reveal the extent of conformational change, if any, that can be induced by intracellular signaling. Initial studies show that treatment of αEβ7+ cells with Mn2+ or divalent cation-free medium has no effect on M290 binding, but a more extensive analysis, including stimulation with chemokines, is required to address this point adequately.
4 Materials and methods
4.1 Modeling the αE I domain
The αE I domain was modeled on the open and closed structures of CD11b (PDB 1IDO and 1JLM, respectively) using SWISS-MODEL accessed via Swiss PDB viewer 42: http://www.expasy.org/spdbv/. The αE I domain shows 39% amino acid identity to that of CD11b, 22% conservative differences and two additional residues. Threading energy was minimized manually and the sequence then submitted for modeling. There were no significant steric clashes in either the open or closed models of αE. RMSD values for fitting the open and closed models to the respective CD11b structures were 0.18 Å for the open version and 0.34 Å for the closed version. Cysteine mutations to form disulfide bonds to lock the C-terminal α7 helix in the up or down position (F341C, V343C, A348C) gave β-carbon distances of 3.67 Å and 4.17 Å, respectively, which are highly favorable for disulfide bond formation. Amino acids are numbered according to their position in full-length αE sequence, without the signal peptide. Molecular images were generated using Deep View PDB viewer (http://www.expasy.org/spdbv/).
4.2 DNA constructions
cDNA for mouse αE and β7 were cloned into pcDNA3 for stable expression in K562 cells. The QuikChange mutagenesis kit (Stratagene) was used to make the following mutations in the αIdomain: F341C/A348C (open), V343C/A348C (closed), D317A, F319R. To express isolated αI domains, αE I domain cDNA was cloned into pDisplay (Invitrogen), which provides an Igκ leader sequence, epitope tags HA and myc to flank the I domain and a platelet-derived growth factor receptor transmembrane domain. The αE I domain contained four additional αE residues on the N-terminal side and 25 additional residues on the C-terminal side and was prepared by PCR using the following primers: forward 5′-GGGGAGATCTGAGGAGGAAGATGGCACTG; reverse 5′-CAAGGTCGACCTGCCCCTT.
E-cadherin for use in cell adhesion tests comprised the five extracellular domains of E-cadherin, EC1–5, prepared as C-terminal fusion proteins. Three versions were used: dimeric E-cadherin-Fcpreviously described 30, an E-cadherin dimer in which the E-cadherin chain was fused to an acidic/basic coiled coil leucine zipper 38, 43 and,E-cadherin monomer in which EC1–5 was fused to the basic component of the zipper. DNA for the basic and acidic sides of the zipper, was prepared by annealing and filling in two overlapping oligonucleotides. Basic side, sense: 5′-GCTGCTCAGTGCAAAAAGAAATTGCAAGCACTGAAGAAAAAGAACGCTCAGCTGAAGTG; antisense: 5′-CTACTGGGCGAGTTTCTTCTTGAGGGCTTGAAGTTTCCACTTCAGCTGAGCGTTCTTTTTCTTCAGTGC; acidic side, sense 5′-GCTGCTCAGTGCGAAAAAGAGCTCCAGGCCCTGGAGAAGGAAAATGCACAGCTGGAATG; antisense 5′-CTACTGAGCCAGTTCCTTTTCCAGTGCTTGCAACTCCCATTCCAGCTGTGCATTTTCCTTCTCCAGGGC. The annealed and filled in products were amplified by PCR using primers with a Kpn1 site at the 5′ end and an Xba1 site at the 3′ end: forward basic, 5′-GGGGTACCGCTGCTCAGTGCAAAAAGAAATTG; reverse basic, 5′-GCTCTAGACTACTGGGCGAGTTTCTTCTTG; forward acidic, 5′-GGGGTACCGCTGCTCAGTGCGAAAAAGAGCTC; reverse acidic, 5′-GCTCTAGACTACTGAGCCAGTTCCTTTTCC. E-cadherin EC1–5 was then amplified by PCR from the E-cadherin-Fc construct starting at the ATG of the signal peptide, introducing a Not1 site at the 5′ end and an Kpn1 site at the 3′ end with primers: forward, 5′-CATAAGAATGCGGCCGCATGCCGCTGCTGCTACTGCTG; reverse 5′-CCGGTACCTGTAACTTGCAATCCTGCTGCCACG. The E-cadherin PCR product and the acidic and basic zipper products were digested with Kpn1 and ligated. Finally, the ligated product was amplified by PCR using the E-cadherin forward primer and the reverse acidic or basic primers and the product ligated into pCDNA3(+). In this construct the cadherin sequence finishes at V553 and is followed by a linker sequence TGTAAQC common to basic and acidic components. A disulfide bond formed by the cysteine coded by this sequence stabilizes the leucine zipper which extends C-terminally.
The disulfide bond position relative to EC5 of E-cadherin, is closely similar to that in the Ig hinge of E-cadherin-Fc, so both dimers are structurally comparable.
To identify the epitope for mAb M290, the αE I domain was cloned into the vector Signal pIg-Tail (R & D Systems) for preparation of an IgG1Fc fusion protein. The following mutations were made using the QuikChange mutagenesis kit: S190A, K194A, N197A, G291R, D317A, N322A, E337A, K342A, N345A, D350A, S354A.
The fidelity of all constructs was confirmed by sequencing.
4.3 Transfection
Stable transfectants of αEβ7 and isolated αI domain were made in K562 cells (a myeloid leukemia line) by electroporation and G418 selection. Cells were cloned by limiting dilution.Cos7 cells were transfected with E-cadherin constructs using DEAE dextran. Single constructs were transfected to produce IgG1Fc fusion proteins or E-cadherin monomer with a basic tail and, for zipped dimers, basic and acidic constructs were co-transfected in equal proportion. The cell supernatants were harvested after 7 days and the E-cadherin concentration was measured by ELISA 30. Monomeric E-cadherin was confirmed to be monodisperse by fractionation in 50 mM Tris HCl pH 7.4, 150 mM NaCl, 1 mM CaCl2 using a Superdex 200 PC3.2/30 column with a Pharmacia SMART (microbore HPLC) system.
4.4 Flow cytometry
Expression of αE on K562 cells was determined using rat mAb M295 or M290. Co-expression of the β7 subunit was verified using M298. Isolated I domain was detected with mouse mAb to HA and myc tags, 3F10 and 9E10, respectively, used in combination. Secondary antibodies were FITC-labeled sheep anti-rat or anti-mouse IgG (Serotec). For reduction of the locked-open integrin, K562 transfectants were stained at 0oC with M290 in the presence of 2% mercaptoethanol in Dulbecco's modified Eagle's medium containing 1% FCS and 0.1% sodium azide.
4.5 Cell adhesion tests
The cell adhesion test has been described previously 30. Ca2+, Mg2+ and Mn2+ were used at 1 mM each and in some experiments PMA (15 ng/ml) was present. Acidic/basic leucine zipped dimers were attached to assay plates using antibody 2H11 38, 43 which is specific for the dimerized zipper. For experiments comparing monomeric and dimeric E-cadherin, both species were attached to the assay plate using mAb DECMA-1 which is specific for E-cadherin EC4. Inhibitory antibodies ECCD2 and Fib30 were used at 20 μg/ml and 10 μg/ml, respectively and were pre-incubated for 15 min with the assay plates or the K562 cells and were present throughout the assay. Adhesion assays were conducted in triplicate or quadruplicate and results are presented as means ± SEM.
Acknowledgements
We thank Mr. G Morgan for his help with cell sorting and the Bioinformatics Group, The Babraham Institute, for advice with protein modeling. Part of this work was funded by Project Grant 202/GTH12498 from the Biotechnology and Biological Sciences Research Council, UK.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




